Abstract
Endectocides, or macrocyclic lactones, are veterinary parasiticides used globally to control nematodes and arthropods affecting livestock. Cattle treated with these products fecally excrete residues that are toxic to dung-inhabiting insects, including species that accelerate dung degradation. Concerns have been raised that use of endectocides may reduce insect diversity and cause the accumulation of undegraded dung on pastures. This article synthesizes the results of studies performed to assess the nontarget effects of endectocide use in Canada. Residues reduce insect activity in dung of treated cattle for weeks to months after application. The duration of effect is influenced by several factors, including insect species and product. For example, in terms of toxicity, doramectin > ivermectin ≈ eprinomectin >> moxidectin. Reduced insect activity may retard dung degradation. Within the framework of regional conditions and management practices, endectocide use in Canada is unlikely to pose a significant widespread threat to the environment. Nevertheless, nontarget effects may be of concern to individual cow–calf operators, particularly those treating cattle in the spring. This synthesis, the first assessment of the nontarget effects of endectocide use in Canada, emphasizes the importance of presenting findings within an appropriate context.
Résumé
Les endectocides, ou lactones macrocycliques, sont des parasiticides vétérinaires utilisés pour enrayer les nématodes et arthropodes affectant le bétail. Les bovins traités avec ces produits excrètent dans leurs fèces des résidus qui sont toxiques pour les insectes retrouvés dans les bouses, incluant les espèces qui accélèrent la dégradation des bouses. Des inquiétudes ont été soulevées quant au fait que les endectocides puissent réduire la diversité des insectes et causer une accumulation de bouses non dégradées dans les pâturages. Cet article résume les résultats d’études effectuées pour évaluer les effets non ciblés de l’utilisation des endectocides au Canada. Les résidus diminuent l’activité des insectes dans les bouses des bovins traités pendant des semaines à des mois suite à leur application. La durée de l’effet est influencée par plusieurs facteurs, incluant les espèces d’insecte et le produit. À titre d’exemple pour la toxicité : doramectin > ivermectin ≈ eprinomectin >> moxidectin. La réduction de l’activité des insectes peut retarder la dégradation des bouses. À l’intérieur du cadre des conditions générales et des pratiques de régie, il est peu probable que l’utilisation des endectocides au Canada représente une menace significative généralisée pour l’environnement. Néanmoins, les effets non ciblés peuvent être une préoccupation dans les opérations individuelles vache-veau, particulièrement pour ceux qui traitent les animaux au printemps. Cette synthèse sur l’évaluation des effets non ciblés de l’utilisation des endectocides au Canada met en évidence l’importance de présenter les trouvailles à l’intérieur d’un contexte approprié.
(Traduit par Docteur Serge Messier)
Introduction
Endectocides, or macrocyclic lactones, are veterinary parasiticides that include the avermectins (abamectin, doramectin, eprinomectin, and ivermectin) and the milbemycins (moxidectin). They have a broad spectrum of activity against nematodes and arthropods, are convenient to use, and generally have low mammalian toxicity. Endectocides have achieved global popularity for these reasons.
Endectocides are excreted primarily in the feces of the treated animal. Hence, application to reduce numbers of internal and external parasites of livestock can also reduce numbers of insects in their dung. Reduced numbers of several species of pest flies have been reported in dung of livestock treated with various endectocides and can be viewed as an additional benefit of application. However, only a small percentage of the insect species associated with cattle dung in North America are pests, albeit some are of significant importance; for example, horn fly (Haematobia irritans [L.]) and face fly (Musca autumnalis De Geer [Diptera: Muscidae]).
The potential for endectocide residues to adversely affect nonpest species in dung has raised several concerns. Residues may be toxic to dung-dwelling insects, including species of threatened or endangered status. No such species are listed in Canada, but several rare species of dung-breeding insects have been reported for Britain (1). Reductions in the feeding and tunneling activities of dung-dwelling insects may delay dung degradation. Undegraded dung pats provide sites for pest flies to complete development, harbor nematodes parasitic in livestock, reduce available grazing area, and represent a loss of soil nitrogen in pastures (2). Reduced numbers of dung-breeding insect species can also affect other aspects of the pasture ecosystem; for instance, coprophilous insects pollinate plants and provide food for vertebrates.
Numerous studies have assessed these concerns, with variable results (3). Fecal residues typically are toxic to flies (Diptera) in the suborder Cyclorrhapha but innocuous to flies in the suborder Nematocera. Activity of beetles (Coleoptera) may be unaffected in dung of treated cattle or be suppressed in fresh dung of cattle treated weeks or even months previously. Dung of treated animals may degrade in a similar fashion, degrade more slowly, or degrade more quickly than dung of untreated animals.
The interpretation of results from these studies has occasionally been ambiguous and subject to rancorous debate. The authors of a study in Britain concluded that increased use of ivermectin by the livestock industry was likely to pose a serious threat to at least some dung-breeding species of insects and could increase the amount of pasture fouled by cattle dung (4). Scientists representing the manufacturer of ivermectin disputed these concerns, partially because they were based on use of an experimental bolus formulation of ivermectin (5,6). The formulation was subsequently registered in Britain, in 1992 (7), but is no longer marketed. A 2nd British study failed to find any effect on dung decomposition of ivermectin applied to cattle either as an injectable or a bolus formulation, which suggests that insects either had little role in dung decomposition or were relatively unaffected by fecal residues (8). These findings were criticized on several grounds, including the absence of data on insect abundance (9,10). Subsequent reviews concluded that use of avermectins would have little impact on nontarget populations of dung-breeding insects or on rates of dung degradation (11,12), although there were dissenting views (13–15). Herd (13) stated the following: “None of the published studies performed or sponsored by industry have concluded that drug residues exert a significant effect on the rate of dung degradation in temperate regions. By contrast, independent studies in Denmark, the UK, and the USA arrived at the opposite conclusion.”
Debate on the nontarget effects of endectocide use is considered by this author to have had at least 2 beneficial outcomes. First, it has spurred efforts to harmonize guidelines to assess the environmental risks associated with use of veterinary products. As part of this initiative, representatives from industry, regulatory agencies, and research institutions met in 2001 to form the group DOTTS (Dung Organism Toxicity Testing Standardisation). This official affiliate of the European Branch of the Society for Environmental Toxicology and Chemistry is developing standard protocols to assess the toxicity of veterinary parasiticides to coprophilous flies and dung-breeding beetles (3). Second, the debate has illustrated the importance of viewing results within an appropriate context. The nontarget effects of endectocide use are affected by formulation and dose, route and frequency of administration, and regional and seasonal differences in climate and dung fauna. Hence, conclusions derived from studies in a single region may be inappropriate elsewhere without consideration of regional differences.
Only recently have the nontarget effects of endectocides been studied in Canada (16–23). The absence of such previous studies reflects classification of endectocides for use in animals as “veterinary drugs,” for which environmental assessments are not needed for Canadian registration. Hence, there is no requirement for manufacturers to test for nontarget effects of endectocides in fecal residues in pastures. However, registration of new products, coupled with growing awareness of potential nontarget effects reported in other countries, prompted research in the mid-1990s to assess the nontarget effects of endectocide use in Canada. The findings and the identification of confounding factors are predicted to have heightened significance with ongoing efforts to develop and adopt international standards for the registration of veterinary products.
This article reviews the nontarget effects of endectocide application in cattle on coprophagous insects and on dung degradation in Canada. Within this broad framework there are 3 objectives.
To synthesize the Canadian data for the benefit of researchers and regulators involved with the registration of new veterinary products. Previous reviews of nontarget effects generally have emphasized studies performed in Australia or Europe (3).
To develop a suitable context for Canada within which nontarget effects should be assessed. This context would contribute to nonambiguous interpretation of the data and aid in the design of appropriate experiments for future research.
To raise awareness of this topic in a broader audience. Most of the relevant literature is in entomologic journals, such that many veterinarians may be unaware of the nontarget effects of endectocide use.
Other classes of veterinary parasiticides also are fecally excreted and may adversely affect pasture ecosystems (3). However, data on the nontarget effects of these products are limited, and no studies have been conducted in Canada. Hence, this article is restricted to discussion of endectocides while recognizing that many of the issues may have relevance to other classes of parasiticides.
Cattle production and patterns of endectocide use
The beef industry can be separated into cow–calf and feedlot operations. Most cattle are bred in June and July, with calving in March and April. Cows and calves typically graze on pasture from May to October. Stocking rates are dictated by the type of pasture and range condition. Average stocking rates for a cow–calf pair in Alberta range from 0.4 to 1.3 hectares per month on native pastures and from 0.1 to 0.5 hectares per month on tame pastures (24). Calves are sent to feedlots in autumn. Heavier animals enter finishing feedlots and are fed a silage/grain diet until they reach slaughter weight. Lighter animals enter backgrounding feedlots, where they are fed a forage diet until they reach mature size, and then they are sent to a finishing feedlot.
The beef industry is concentrated in the prairie regions of Alberta, Saskatchewan, and Manitoba. This region is characterized by hot, dry summers, long, cold winters, and low levels of precipitation (25). Mean annual summer and winter temperatures, respectively, range from 14°C to 16°C and from −12.5°C to −8°C. Annual precipitation levels range from 250 mm in southwestern Saskatchewan and southeastern Alberta to almost 700 mm in southern Manitoba. Dry soil conditions are exacerbated by high winds, which accelerate water evaporation.
Climatic conditions affect parasite populations and consequent use of endectocides. Low stocking rates and periods of extreme cold or of hot, dry weather usually maintain infections of internal parasites in pastured cattle at subclinical levels. Hence, endectocides in Canada are applied most often in autumn, when cattle are moved from pastures into feedlots. Application at this time targets both external and internal parasites that are common pests of feedlot cattle during winter months. Respective examples include sucking lice (Haematopinus eurysternus [Nitzsch], Linognathus vituli [L.], and Solenopotes capillatus Enderlein) and chewing lice (Bovicola bovis [L.]) and gastrointestinal nematodes (Ostertagia and Cooperia) and cattle grub (Hypoderma spp.). Spring application of endectocides in cattle being turned out on pasture is much less common but can help reduce the buildup of nematodes in pastures and provide a measure of control against horn fly (26).
The normal practice of single autumn applications in Canada contrasts sharply with practices in Europe, where high stocking rates and more benign climates promote the spread of internal parasites on pasture. A survey in England identified that 80% of dairy producers were using anthelmintics on their young stock 2 to > 4 times per year (27).
Formulation, metabolism, and excretion of endectocides
Four endectocides are registered in Canada for use in cattle: doramectin, eprinomectin, ivermectin, and moxidectin. Ivermectin was introduced onto the world market in 1981, moxidectin in 1990, doramectin in 1993, and eprinomectin in 1997 (7). Each is available in 1 or more formulations and sold under various trade names (Table I). Injectable formulations are injected subcutaneously. Topical, or pour-on, formulations are poured along the dorsal midline of the animal. Product absorbed through the skin enters the circulatory system to control internal parasites or is retained on the hide to control external parasites. Sustained-release (SR) bolus formulations are applied orally as a reservoir of active ingredient that lodges in the rumen and releases a constant dose of medicine for weeks to months. An SR bolus formulation of ivermectin was registered for use in Canada but discontinued in June 2004 (28).
Table 1.
Endectocide products (avermectins and milbemycins) registered for use in cattle in Canada (28)
| Product class and active ingredient | Formulation and trade name |
|---|---|
| Avermectins | |
| Doramectin | Pour-on: Dectomax |
| Subcutaneous injection: Dectomax | |
| Eprinomectin | Pour-on: Eprinex |
| Ivermectin | Pour-on: Ivomec, Megamectin, Noromectin, Unimectrin |
| Subcutaneous injection: Ivomec, Noromectin, Unimectrin | |
| Sustained-release bolus:a Ivomec | |
| Milbemycins | |
| Moxidectin | Pour-on: Cydectin |
| Subcutaneous injection: Cydectin | |
Discontinued in June 2004
Endectocides are slightly to moderately metabolized in the treated animal and then excreted in the feces for weeks to months (29). The pattern of excretion is affected by formulation and other factors. Peak excretion usually occurs in the first week with injectable or topical formulations of ivermectin (17,30–32) and doramectin (32). Peak excretion of moxidectin occurs 1 to 2 d after application of an injectable formulation (29,33) but is delayed by 9 to 10 d after topical application (29 [Table 4.8]). The concentration of residual endectocide declines rapidly thereafter, but product can still be detected in dung up to 58 d after application (32). Use of ivermectin in a bolus formulation results in a relatively stable level of fecal excretion for up to 120 d (34). Differences in diet can cause a 4-fold difference in the peak concentration of residues in dung of cattle treated with ivermectin in an injectable formulation (31). Self-grooming by cattle treated topically can increase 10-fold the amount of ivermectin excreted in feces (35). Grooming between cattle may transfer endectocides to untreated cattle (36).
The distribution and fate of excreted residues in the environment depends on whether the treated animals are on pasture or in feedlots. Excreted residues bind tightly to the fecal material. On pasture, residues remain highly localized within the dung pat, such that their immediate effects are limited to dung-dwelling species (3). The limited data indicate that there is little degradation of residues until the dung is incorporated into the soil. Ivermectin residues in cattle dung exposed on pastures in Denmark and Tanzania showed no appreciable degradation after 45 and 14 d, respectively (30). Reported half-lives in soil are 61 to 79 d for doramectin (37), about 64 d for eprinomectin (38), and about 60 d for moxidectin (39) and in soil and soil/manure mixtures from 14 to 56 d for ivermectin (40). In feedlots, dung of treated cattle is scattered and trampled to form a relatively solid mat that accumulates during winter months. The level of degradation of fecal residues in the matted material during this time is unknown. Dung is removed from pens in the spring and composted or spread directly onto cropland. Incorporation into the soil before seeding, coupled with aerobic degradation and photo-degradation, further reduces residue concentrations (41).
Residues in pastures are assumed to pose the greatest risk to the environment and have been the subject of most research. Although restricted to a very localized area (the dung pat), residue concentrations can be relatively high in dung deposited by cattle shortly after treatment and potentially affect dozens of species of organisms that may colonize dung in pastures during summer months. In contrast, trampling makes dung deposited in feedlot pens unattractive to most insects. Further, most dung in feedlots is deposited during late autumn or winter, when insect activity is low or nonexistent.
The dung pat community
To assess the effect of endectocide residues in dung of pastured cattle, information is needed on the abundance and seasonality of dung insects. Coprophilous insects are represented by more than 450 species in North America (42) and by at least 80 species in western Canada (18).
Fresh dung is colonized almost immediately by adult flies, which feed, mate, and lay eggs that produce a new generation of flies in about 2 to 3 wk. Fly numbers rapidly decline after a few hours, by which time crust formation on the pat has reduced the release of odor attractants. Dung-feeding beetles arrive shortly thereafter to feed and oviposit, with peak colonization finished by the end of the 1st week after deposition. In contrast to flies, the egg-to-adult development time of dung-feeding beetles often takes weeks to months. Parasitic wasps and predaceous beetles arrive concurrently with the flies and dung-feeding beetles to feed on immature insects developing in the dung pat or to oviposit. There is very little additional colonization of dung by coprophilous insects 2 to 3 wk after deposition. Tunneling and feeding by insects accelerates pat degradation, which allows the pat to be more easily penetrated by vegetation and incorporated into the soil.
Earthworms can be a main agency of dung removal on pastures in Europe, but that is not necessarily so in North America. On pastures in England during summer months an estimated 1% to 2% of a dung pat’s initial dry weight may be removed via insect production, as compared with an estimated 50% to 60% via earthworm consumption (11). However, earthworms are largely absent from cattle-producing areas in Canada (M.J. Clapperton, Lethbridge Research Centre, Lethbridge, Alberta: personal communication; and personal observation) and in parts of the United States (43). This absence probably reflects a dry prairie climate, as well as eradication during past glaciation events. According to Fender (44), “nearly the whole of Canada and the northern edge of the United States was covered by an ice sheet which undoubtedly eradicated earthworms over vast areas.” This difference emphasizes the importance of regional knowledge of processes and species affecting dung degradation; that is, whereas earthworms may be more important than insects in degrading dung in Europe, the reverse is true in Canada.
Dung beetles of interest in Canada
Dung-feeding beetles (Scarabaeidae) are among the most prominent insects in cattle dung in terms of size, abundance, and role in dung degradation. Assessing their exposure to fecal residues of endectocides requires information on species composition, seasonal activity, and geographic distribution. Most such information for Canada derives from a 3-y study near Lethbridge (20). Of the 17 species of dung beetles recovered, most were of European origin; in descending order, Onthophagus nuchicornis (Linné), Aphodius prodromus (Brahm), A. distinctus (O.F. Müller), and A. fimetarius (Linné) dominated. The most common native species, A. vittatus Say, accounted for only 4% of the total collection.
European species of Aphodius are common in cattle dung throughout North America (20). Adults arrive at the pat and tunnel within to form brood chambers in which to lay eggs. At high densities, adult activity disrupts and aerates the dung pat. An average of 200 to 600 adult A. fimetarius have been reported to colonize fresh pats on California pastures (43), and an average of 853 A. distinctus were recovered from fresh pats on pastures in Illinois (45). More typically, dung degradation is associated with the feeding activity of larvae, which may consume 50% to 100% of their dry body weight (BW) per day (46). Larval feeding occurs for weeks or months and is complemented by the activity of other decomposers (fungi and bacteria) to convert the pat into a dry, granular material. This material is readily scattered by wind, penetrated by vegetation growing below, or worked into the soil by biotic and abiotic factors. Fragmented dung may lose 20% to 30% more weight in the first 50 d after deposition than unfragmented dung, presumably owing to a higher surface-to-volume ratio and the resultant increase in microbial activity (11). Hence, it seems reasonable to suggest that fecal residues of endectocides sufficient to reduce numbers of Aphodius beetles breeding and developing in cattle dung could also reduce rates of dung pat degradation.
Onthophagus nuchicornis illustrates the potential for insect communities in dung to change with time and for these changes to affect dung degradation. This beetle was accidentally introduced on the east and west coasts of North America during European settlement and subsequently expanded its distribution inland (20,47). First recorded in Alberta in 1961, O. nuchicornis is now among the most abundant species in southern Alberta (19,20). In contrast to species of Aphodius, O. nuchicornis degrades the pat primarily through adult activity, beginning within hours or days. Adults arrive at the pat to remove and bury small amounts of dung in the soil, into which eggs are laid; these “brood balls” nourish the developing larvae. Dung removal disrupts the pat, tunneling improves soil aeration and water filtration, and the nitrogen in remnant brood balls enhances soil fertility. Although relatively effective in disrupting small, shallow pats during spring and early summer, O. nuchicornis appears to be ineffective in disrupting larger, thicker pats (48). Laboratory experiments suggest that dung burial by O. nuchicornis has little effect in reducing numbers of pest flies breeding in cattle dung on pasture (48) but may contribute significantly to soil fertility (49).
Timing of field studies in Canada
Information on seasonal insect activity helps to identify the appropriate timing of field studies. Adult beetles are active in Canada from mid-March to mid-November, with peak activity in spring and again in autumn or only once, in spring to mid-summer (20). The peak in spring and early summer corresponds to the emergence of overwintered beetles, which colonize fresh dung to feed and lay eggs. Dung degradation is most rapid during this time owing to the tunneling of egg-laying adults and the feeding of hatched larvae. The autumn peak corresponds to the emergence of new adults, which have developed from eggs laid in spring. Hence, April through early June is the most appropriate time in Canada to test for the indirect effects of cattle endectocide treatment on dung-dwelling insects even though endectocides are usually applied in autumn.
Two studies illustrate the importance of assessing experimental results within a seasonal context. In Britain, dung deposited in July and in September showed no difference in degradation whether excreted by untreated cattle or by cattle treated with ivermectin in a bolus formulation (8). In Canada, dung deposited in July would contain few adult or immature dung beetles, and dung deposited in September would likely contain only adult beetles. Seasonal insect activity was not reported in the British study, but the activity of at least some Aphodius species in Europe is similar to that observed in Canada (20). Hence, the lack of a reported effect in the British study may be partially due to low numbers of dung beetles in the pats being monitored for degradation. Similarly, degradation of dung from untreated cattle and from cattle treated with an injectable formulation of ivermectin appeared to be similar when deposited on pasture during September through October in Texas (50). The numbers of O. gazella F. were reportedly similar for dung from the 2 groups of cattle, but no information was provided to indicate whether beetle activity would normally be expected to disrupt pats during the study period. The absence of this seasonal context makes it difficult to assess the relevance of the reported results.
Additional considerations
Information on species ecology clarifies how endectocide residues can affect dung-dwelling insects. For example, several Aphodius species common as adults in cattle dung may breed partially or primarily in adjacent cropland. Scarab larvae at densities exceeding 50/m2 were collected from cultivated fields near Lethbridge in 2000 and reared to adults subsequently identified as A. distinctus, A. granarius (Linné), and A. prodromus (unpublished data). Conversely, we rarely have reared A. distinctus from cattle dung, a finding reported by others (45). Hence, the presence of adult dung-feeding beetles in cattle dung does not necessarily imply that their progeny will be directly exposed to endectocide residues.
Differences in dung fauna, even over relatively short distances, can alter the outcome of experiments. Dung-baited pitfall traps were operated from March through November to compare dung beetle assemblages between 2 sites in southern Alberta separated by 12 km (20). Of the average of 2260 beetles per trap recovered at site 1, 94% were Aphodius and 6% were O. nuchicornis. Of the average of 9400 beetles per trap recovered at site 2, 50% were Aphodius and 50% were O. nuchicornis. Most of the Aphodius recovered (A. distinctus and A. prodromus) likely were breeding in surrounding fields and unlikely to have a large role in dung degradation. In contrast, O. nuchicornis is an obligate dung-breeder that can be relatively effective in disrupting pats (48). Hence, tests at site 2 would be more likely to show an association between reduced numbers of dung beetles and reduced rates of dung degradation. These data illustrate the importance of documenting the abundance and species of insects at study sites during experimental periods.
In summary
The preceding section provides context for concerns regarding the potential for endectocide use to adversely affect dung-dwelling insects and dung degradation. It does not provide experimental support for these concerns. Such evidence, from studies performed in Canada, is summarized in subsequent sections.
Lethal effects on coprophilous insects
Pest species
Because of their economic importance, species of pestiferous flies (Diptera: Muscidae) often are used in bioassays to assess the toxicity of fecal residues. Two such studies have been performed in Canada. The 1st study reported suppression of horn fly development in dung from cattle treated up to 20 d previously with a recommended topical dose of ivermectin (500 μg/kg BW) (16). The 2nd study assessed, in feces, the residual toxicity of 4 endectocides against horn fly, house fly (Musca domestica L.), and stable fly (Stomoxys calcitrans [L.]) (22). Recommended topical doses (500 μg/kg BW) of doramectin, eprinomectin, and ivermectin suppressed the development of horn fly in dung from cattle treated at least 4 wk previously and the development of house fly and stable fly in dung from cattle treated 1 to 5 wk previously. Moxidectin suppressed the development of horn fly in dung from cattle treated no more than 1 wk previously but did not suppress the development of house fly and stable fly. Overall comparison of the 4 products suggested that, in descending order of larvicidal activity, doramectin > ivermectin ≈ eprinomectin >> moxidectin. The results of studies on house fly in Hungary (51) and on bush fly (Musca vetustissima De Geer) in Australia (52) support this ranking.
Nonpest species
Two studies in Canada, with similar methodology, have assessed the toxicity of fecal residues to nonpest insects (18,23). Artificial dung pats were placed in the field for 5 to 11 d during peak periods of insect activity to permit natural colonization. Pats comprised dung from untreated cattle and dung from the same cattle after topical application of endectocide. To synchronize placement of all pats in the field, dung was collected fresh and then frozen until used. After exposure, the pats were removed from the field and held individually in indoor cages. Emergent adult insects were collected from the cages, counted, and identified as to species.
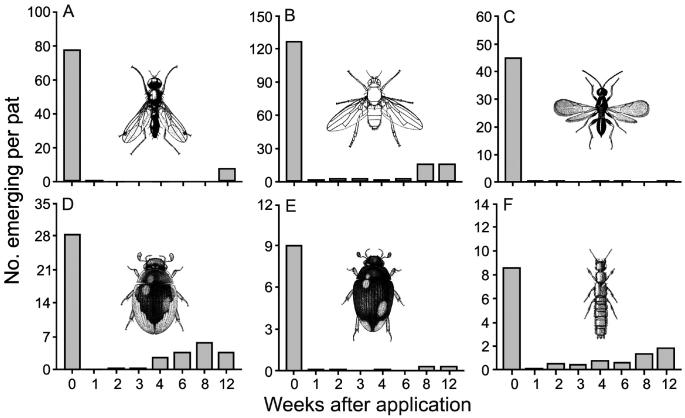
The 1st study compared insect emergence from dung of untreated cattle and dung collected from the same animals after a recommended topical application of ivermectin (18). Fewer dung-feeding flies, parasitic wasps, and both predacious and dung-feeding beetles emerged from the dung after treatment. Whereas some species appeared to be unaffected by fecal residues, the emergence of other species was reduced in dung excreted 12 wk after application (Figure 1). Generally consistent patterns were observed in each of 3 independent experiments. Tests using thin-layer chromatography detected ivermectin residues in dung of these cattle for up to 10 d after application, thus showing a direct link between the presence of residues and reduced number of insects (17).
Figure 1.
Emergence of insects developing in dung pats from untreated cattle (treated for 0 wk) versus dung from cattle treated 1 to 12 wk previously with a topical dose of ivermectin (500 μg/kg body weight). A, B, dung-feeding flies Sepsis spp. and Coproica mitchelli (Malloch), respectively. C, parasitic wasps (Eucoilidae). D, E, dung-feeding beetles Cercyon pygmaeus (Illiger) and C. quisquilius (Linnaeus), respectively. F, predaceous beetle Aleocharinae sp. Values are means for 12 pats per interval. Adapted from Floate (18); reproduced with the author’s permission.
The 2nd study compared insect emergence from dung of untreated cattle and dung collected from the same animals after a recommended topical dose of doramectin, eprinomectin, ivermectin, or moxidectin (23). In each of 3 independent experiments, reduced emergence of insects was associated with application of the first 3 products, but no effect was observed for moxidectin. When the data were combined across the experiments to increase sample sizes, reduced emergence of insects was observed for each compound, moxidectin having the least effect. Reductions in the numbers of several taxa were observed in dung from cattle treated up to 4 wk previously with doramectin, eprinomectin, or ivermectin. No effort was made to detect reductions beyond this period. Affected species included dung-feeding flies, parasitic wasps, and predaceous beetles. Results for dung-feeding beetles were ambiguous, suggesting a possible effect on A. vittatus and no effect on A. granarius. On the basis of the number of species affected and the duration of suppression, doramectin > ivermectin > eprinomectin >> moxidectin in descending order of adverse effect. This ranking was similar to that obtained in laboratory bioassays with pest flies (22).
Sublethal effects on coprophilous insects
Nontarget effects of endectocide residues in dung usually are underestimated, because most studies consider only reductions in insect number. However, insects surviving exposure to endectocide residues may have deformities, longer developmental times, delayed ovarian development, or reduced fecundity (3). Further, interactions between dung-dwelling insects (3) may result in endectocide residues from 1 group of insects indirectly affecting a 2nd group (parasitoids or predators).
Indirect effects on parasitic wasps
Several studies have reported reduced numbers of parasitic wasps developing in dung of endectocide-treated cattle (18,23,50). The reductions can be attributed to at least 2 routes of indirect effect. First, these wasps require fly pupae in which to complete development. Residues reduce the number of flies developing in dung. Hence, residues indirectly affect parasitoids by reducing the availability of host pupae. Second, sublethal exposure of fly pupae to residues may alter their suitability as hosts for parasitoids. This has been documented for house flies reared from egg to pupation in media with various concentrations of ivermectin residue and then exposed to parasitism by the wasp Muscidifurax zaraptor Kogan & Legner (Pteromalidae) (21). Fly pupae exposed to 0.25 parts per million (ppm) of ivermectin during development produced 63% fewer wasps than did control pupae. In contrast, pupae exposed to 0.01 ppm of ivermectin produced 23% more parasitoids than did control pupae. The mechanism behind these results is unknown.
Altered patterns of colonization
Residues can also affect the number of coprophilous beetles (Hydrophilidae, Scarabaeidae, and Staphylinidae) attracted to dung of treated cattle. In a 2-y study in southern Alberta, collections of beetles were compared between pitfall traps baited with dung from untreated cattle or cattle treated 1 or 4 wk previously with a recommended dose of ivermectin in a pour-on formulation (19). In year 1, significantly more A. fimetarius and A. distinctus at each of 2 sites were collected from the dung of treated cattle. In year 2, significantly fewer A. fimetarius and A. distinctus were collected from the dung of treated cattle. A change in cattle diet may have altered levels of fecal residues, thereby changing beetle preferences.
There was also a seasonal change in preferences exhibited by at least some species (19). Similar numbers of O. nuchicornis and A. fimetarius were collected in spring from the dung of treated or untreated cattle, but significantly more beetles of both taxa were collected in autumn from the dung of untreated cattle. In contrast, the preference of A. prodromus for dung from treated cattle was unchanged between spring and autumn. Hence, in addition to reducing numbers of insects in dung directly by insecticidal action, fecal residues may indirectly reduce insect numbers by lowering the frequency with which insects colonize the pat. Other studies have similarly shown fecal residues to alter the attractiveness to coprophilous beetles of dung from endectocide-treated animals (19). The mechanism for this phenomenon is unknown.
Insects and dung degradation
A key concern is the effect of endectocide use on dung degradation rather than on the dung-dwelling insects. To document the role of insects in degrading dung in southern Alberta, artificially formed control and treatment pats were monitored for 1 y on a pasture near Lethbridge (18). Control pats consisted of fresh dung excreted by untreated cattle. Treatment pats consisted of dung from the same cattle to which ivermectin (2 ppm of dung wet weight) was directly added. The pats were placed in the field in May to coincide with peak activity of dung-feeding beetles. At 20, 60, 80, and 340 d after deposition, 2 to 5 pairs of control and treatment pats were removed from the field and assessed for degradation, quantified as the portion of the pat (percentage of total dry weight) that had degraded to a granular consistency. The treatment pats formed hard, solid masses that showed no obvious signs of degradation after 340 d. The control pats were about 80% degraded after 80 d. Whereas the control pats contained numerous insects and obvious signs of tunneling, the treatment pats contained only a few dead adult dung beetles and no signs of tunneling.
In a subsequent study (23), degradation was compared in dung from untreated cattle and dung from cattle treated 1 wk previously with a recommended topical dose of doramectin, eprinomectin, ivermectin, or moxidectin. Artificially formed pats were placed on pasture in spring and removed in autumn the same year. Degradation was measured as described for the earlier study. The experiment was repeated in each of 3 y. In 1998, reduced degradation was detected after the addition of doramectin, eprinomectin, and moxidectin. In 1999, no effect on degradation was detected. In 2000, reduced degradation was detected after the addition of moxidectin. When the data from the 3 experiments were combined, delayed degradation was detected only after the addition of eprinomectin.
Few studies have assessed the role of insects in degrading dung in North America. One such study (53) is discussed here at length because of its relevance to research in Canada, its provision of an estimated cost for undegraded dung on pastures, and its illustration of the complex and interacting factors that affect dung degradation. The investigators compared the surface areas of artificially formed control pats and similar pats to which the insecticide lindane had been added. The study was performed in the foothills of northern California, which has a dung fauna and hot, dry summers similar to those on the Canadian prairie. Pats were placed in the field during various months of the year and replicated in 4 pasture ecosystems: naturally vegetated woodland range (NW), partially cleared woodland range (PC), totally cleared woodland range (TC), and totally cleared, cultivated, and irrigated (I) pasture. Depending upon the pasture type, month, and fauna, elimination of insect activity in pats with lindane delayed total pat degradation by 4 to 21 mo in NW and PC pastures, by 4 to 30 mo in TC pastures, and by 3 to 6 mo in I pastures. Total degradation of untreated pats deposited in May required about 5 to 16 mo, compared with 9 to 40 mo for untreated pats deposited in June to September.
Undegraded dung pats smother new vegetation in subsequent years, reducing the amount of available forage. Also, the rank forage immediately adjacent to degrading pats is avoided by cattle. For a hypothetical ranch of 2024 ha supporting 455 cattle, and under the conditions at their California study site, the authors estimated that the forage lost in this manner represented a loss of 2730 kg of beef in the 1st growing season. Additional losses of 628 and 112 kg of beef were estimated for the 2nd and 3rd growing seasons after dung deposition. Calculated in $US for the year 1984, this lost production was estimated to be $3822 after the 1st year and a cumulative $4858 after 3 y. This cost would occur for each group of 455 cattle grazed per year.
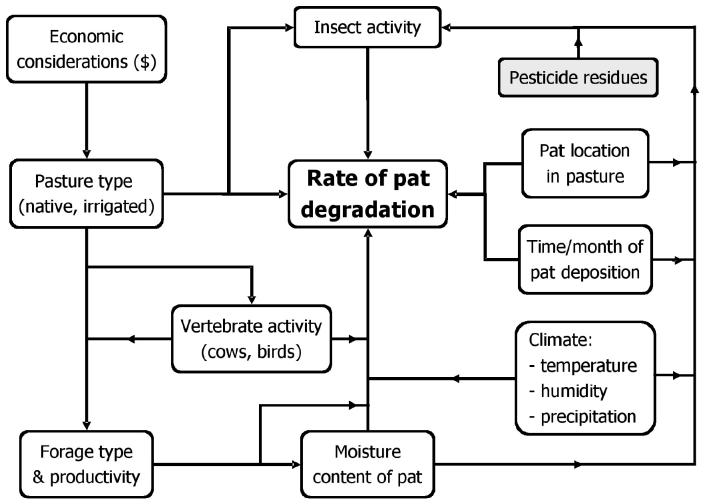
A key finding of the California study is that insects were less important than pasture type or month of deposition in determining the rate of dung pat degradation. This result emphasizes that the process of dung degradation reflects numerous factors working independently and in concert (Figure 2). Economics dictates the type of pasture maintained by the rancher (native, improved, or irrigated), pasture type determines forage productivity, productivity affects stocking rate, and stocking rate alters the likelihood that pats will be disrupted by trampling. Forage productivity influences the moisture content of the pat (which has a direct effect on dung degradation) and the number and species of insects colonizing the pat (which indirectly affect dung degradation). Time (day versus night), month (spring versus winter), and location of pat deposition (shaded woodland versus open grassland) directly and indirectly, through effects on coprophagous insects, affect the rate of pat degradation. However, all else being equal, dung pats degrade faster in the presence of insects than in their absence (53).
Figure 2.
Abiotic and biotic factors that influence the rate of degradation of cattle dung pats on pasture. Pesticide residues typically reduce insect activity in dung, which may slow degradation. Adapted from Merritt and Anderson (43); reproduced with the authors’ permission of the publisher.
Conclusions
This article has examined the complexity of factors that affect conclusions regarding the nontarget effects on dung-dwelling insects and dung degradation of endectocide residues in feces. This examination has emphasized that such conclusions should be considered in an appropriate context to reduce ambiguous interpretations, which in the past have led to heated debate, and to permit more meaningful comparisons across studies.
Findings from studies in Canada, supported by results of studies elsewhere, show the following.
Use of endectocides can reduce insect activity in dung of treated cattle.
Reductions in insect activity can occur in dung deposited by cattle treated weeks to months previously, depending upon formulation, insect species, and active ingredient.
Fecal residues of moxidectin are less toxic to dung-dwelling insects than are residues of doramectin, eprinomectin, and ivermectin.
Reduced insect activity can retard dung pat degradation.
These findings can be presented within the context of the livestock industry as a whole or within the context of the individual cow–calf producer. Within an industry context, the current pattern of endectocide use in Canada is unlikely to pose a significant widespread threat to pasture environments. Most treatments are applied in autumn to cattle entering feedlots. Residues degrade during winter months before being further diluted via incorporation into cropland, where they appear to degrade readily, according to available study results. Treatments in spring or early summer have the greatest potential to adversely affect dung-dwelling insects because of peak insect activity during this time.
Within the context of individual producers, use of endectocides may be of concern. I have been contacted by dozens of ranchers in Canada and the United States concerned with perceived reductions of dung beetle numbers on their properties. These reductions may reflect factors unrelated to endectocide use. Nevertheless, concerned producers can reduce exposure of dung-dwelling insects to insecticidal residues in cattle dung by minimizing endectocide use in spring and early summer. Alternatively, they can use endectocide products that produce fecal residues less toxic to dung-dwelling insects.
Fecal residues of endectocides in cattle dung affect components of the pasture ecosystem including dung-dwelling insects and the process of dung degradation. Discussions of these residues and their significance to pasture management have largely been between researchers and regulators. However, it is the individual cattle producer who ultimately decides on product use and time of application and who is affected by the outcome. This article is presented to provide producers with the best current information on which to base their management decisions and to foster continued research and constructive debate on the nontarget effects of fecal residues in pasture environments. Such efforts will be needed to assess the effects of future products, formulations, and management practices on pasture health.
Acknowledgments
The author thanks the funding agencies, technicians, and summer students who contributed to this research. Doug Colwell and Rosemarie De Clerck-Floate provided much appreciated comments on earlier drafts of this article. The author also thanks the 2 anonymous reviewers. This work is Lethbridge Research Centre Contribution Number (387) 03052.
References
- 1.McCracken DI. The potential for avermectins to affect wildlife. Vet Parasitol. 1993;48:273–280. doi: 10.1016/0304-4017(93)90162-g. [DOI] [PubMed] [Google Scholar]
- 2.Fincher GT. The potential value of dung beetles in pasture ecosystems. J Ga Entomol Soc. 1981;16:301–316. [Google Scholar]
- 3.Floate KD, Wardhaugh KG, Boxall ABA, Sherratt TN. Faecal residues of veterinary pharmaceuticals: non-target effects in the pasture environment. Annu Rev Entomol. 2005;50:153–179. doi: 10.1146/annurev.ento.50.071803.130341. [DOI] [PubMed] [Google Scholar]
- 4.Wall R, Strong L. Environmental consequences of treating cattle with the antiparasitic drug ivermectin. Nature. 1987;327:418–421. doi: 10.1038/327418a0. [DOI] [PubMed] [Google Scholar]
- 5.Campbell WC. Reply to Cherfas J. Ivermectin and faecal degradation. Parasitol Today. 1988;4:21–22. doi: 10.1016/0169-4758(88)90053-1. [DOI] [PubMed] [Google Scholar]
- 6.Wall R, Strong L. Ivermectin and cattle dung — a case for concern. Parasitol Today. 1988;4:107–108. doi: 10.1016/0169-4758(88)90038-5. [DOI] [PubMed] [Google Scholar]
- 7.Allen H. Antiparasitics: Products and Markets. Report SR 142. London, England: PJB Publications, 1995:145.
- 8.Wratten SD, Mead-Briggs M, Gettinby G, Ericsson G, Baggott DG. An evaluation of the potential effects of ivermectin on the decomposition of cattle dung pats. Vet Rec. 1993;133:365–371. doi: 10.1136/vr.133.15.365. [DOI] [PubMed] [Google Scholar]
- 9.Holter P, Strong L, Wall R, Wardhaugh K, Herd R. Effects of ivermectin on pastureland ecology. Vet Rec. 1994;135:211–212. doi: 10.1136/vr.135.9.211. [DOI] [PubMed] [Google Scholar]
- 10.Wratten SD, Mead-Briggs M, Gettinby G, Ericsson G, Baggott DG. Response to Holter et al. Effects of ivermectin on pastureland ecology. Vet Rec. 1994;135:212–213. [Google Scholar]
- 11.Wratten SD, Forbes AB. Environmental assessment of veterinary avermectins in temperate pastoral ecosystems. Ann Appl Biol. 1996;128:329–348. [Google Scholar]
- 12.McKellar QA. Ecotoxicology and residues of anthelmintic compounds. Vet Parasitol. 1997;72:413–35. doi: 10.1016/s0304-4017(97)00108-8. [DOI] [PubMed] [Google Scholar]
- 13.Herd R. Endectocidal drugs: ecological risks and counter-measures. Int J Parasitol. 1995;25:875–885. doi: 10.1016/0020-7519(95)00018-w. [DOI] [PubMed] [Google Scholar]
- 14.Forbes AB. Environmental assessments in parasitology: a balanced perspective. Int J Parasitol. 1996;26:567–569. doi: 10.1016/0020-7519(96)00019-7. [DOI] [PubMed] [Google Scholar]
- 15.Herd R. Ecotoxicity of the avermectins: a reply to Forbes. Int J Parasitol. 1996;26:571–572. doi: 10.1016/0020-7519(96)00038-0. [DOI] [PubMed] [Google Scholar]
- 16.Lysyk TJ, Colwell DD. Duration of efficacy of diazinon ear tags and ivermectin pour-on for control of horn fly (Diptera: Muscidae) J Econ Entomol. 1996;89:1513–1520. [Google Scholar]
- 17.Floate KD, Taylor WG, Spooner RW. Thin-layer chromatographic detection of ivermectin in cattle dung. J Chromatogr B Biomed Sci Appl. 1997;694:246–251. doi: 10.1016/s0378-4347(97)00153-9. [DOI] [PubMed] [Google Scholar]
- 18.Floate KD. Off-target effects of ivermectin on insects and on dung degradation in southern Alberta, Canada. Bull Entomol Res. 1998;88:25–35. [Google Scholar]
- 19.Floate KD. Does a repellent effect contribute to reduced levels of insect activity in dung from cattle treated with ivermectin? Bull Entomol Res. 1998;88:291–297. [Google Scholar]
- 20.Floate KD, Gill BD. Seasonal activity of dung beetles (Coleoptera: Scarabaeidae) associated with cattle dung in southern Alberta and their geographic distribution in Canada. Can Entomol. 1998;130:131–151. [Google Scholar]
- 21.Floate KD, Fox AS. Indirect effects of ivermectin residues across trophic levels: Musca domestica (Diptera: Muscidae) and Muscidifurax zaraptor (Hymenoptera: Pteromalidae) Bull Entomol Res. 1999;89:225–229. [Google Scholar]
- 22.Floate KD, Spooner RW, Colwell DD. Larvicidal activity of endectocides against pest flies in the dung of treated cattle. Med Vet Entomol. 2001;15:117–120. doi: 10.1046/j.1365-2915.2001.00269.x. [DOI] [PubMed] [Google Scholar]
- 23.Floate KD, Colwell DD, Fox AS. Reductions of non-pest insects in dung of cattle treated with endectocides: a comparison of 4 products. Bull Entomol Res. 2002;92:471–481. doi: 10.1079/ber2002201. [DOI] [PubMed] [Google Scholar]
- 24.Alberta Agriculture. The beef cow–calf manual. Agdex no. 420/10. Edmonton: Print Media Branch, Alberta Agriculture, 1992.
- 25.Environment Canada. Narrative Descriptions of Terrestrial Ecozones and Ecoregions of Canada. Prairies Ecozone. Available at http://www.ec.gc.ca/soer-ree/English/Framework/Nardesc/praire_e.cfm Last accessed 18 February 2005.
- 26.Rew R, Vercruysse J. Use of macrocyclic lactones to control cattle parasites in the USA and Canada. In: Vercruysse J, Rew RS, eds. Macrocyclic Lactones in Antiparasitic Therapy. Wallingford, England: CABI Publishing, 2002:248–261.
- 27.Stafford K, Coles GC. Nematode control practices and anthelmintic resistance in dairy calves in the south west of England. Vet Rec. 1999;144:659–661. doi: 10.1136/vr.144.24.659. [DOI] [PubMed] [Google Scholar]
- 28.Health Canada. Drug Product Database. Available at http://www.hc-sc.gc.ca/hpb/drugs-dpd/index.html Last accessed 18 February 2005.
- 29.Steel JW. Assessment of the effects of the macrocyclic lactone class of chemicals on dung beetles and dung degradation in Australia. In: NRA Special Review of Macrocyclic Lactones. Canberra, Australia: National Registration Authority for Agricultural and Veterinary Chemicals, 1998:15–79.
- 30.Sommer C, Steffansen B. Changes with time after treatment in the concentrations of ivermectin in fresh cow dung and in cow pats aged in the field. Vet Parasitol. 1993;48:67–73. doi: 10.1016/0304-4017(93)90145-d. [DOI] [PubMed] [Google Scholar]
- 31.Cook DF, Dadour IR, Ali DN. Effect of diet on the excretion profile of ivermectin in cattle faeces. Int J Parasitol. 1996;26:291–295. doi: 10.1016/0020-7519(95)00132-8. [DOI] [PubMed] [Google Scholar]
- 32.Lifschitz A, Virkel G, Sallovitz J, et al. Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle. Vet Parasitol. 2000;87:327–338. doi: 10.1016/s0304-4017(99)00175-2. [DOI] [PubMed] [Google Scholar]
- 33.Lifschitz A, Virkel G, Imperiale F, et al. Moxidectin in cattle: correlation between plasma and target tissues disposition. J Vet Pharmacol Ther. 1999;22:266–273. doi: 10.1046/j.1365-2885.1999.00222.x. [DOI] [PubMed] [Google Scholar]
- 34.Alvinerie M, Sutra JF, Galtier P, et al. Persistence of ivermectin in plasma and faeces following administration of a sustained-release bolus to cattle. Res Vet Sci. 1998;66:57–61. doi: 10.1053/rvsc.1998.0240. [DOI] [PubMed] [Google Scholar]
- 35.Laffont CM, Alvinerie M, Bousquet-Mélou A, Toutain P-L. Licking behaviour and environmental contamination arising from pour-on ivermectin for cattle. Int J Parasitol. 2001;31:1687–1692. doi: 10.1016/s0020-7519(01)00285-5. [DOI] [PubMed] [Google Scholar]
- 36.Barber S, Alvinerie M. Comment on “A comparison of persistent anthelmintic efficacy of topical formulations of doramectin, eprinomectin, ivermectin and moxidectin against naturally acquired nematode infections of beef calves” and problems associated with mechanical transfer (licking) of endectocides in cattle. Vet Parasitol. 2003;112:255–257. doi: 10.1016/s0304-4017(02)00436-3. [DOI] [PubMed] [Google Scholar]
- 37.Pfizer. Environmental assessment: doramectin 1% injectable solution for treatment of parasitic infections in cattle. Rep. Projpc/uk67994/injc/08envir/nadaez.doc. New York: Pfizer, 1996.
- 38.Merck. Ivomec Eprinex (eprinomectin) pour on for beef and dairy cattle: environmental assessment. Rep. NADA 141-079EA. Rahway, New Jersey: Merck, 1996.
- 39.Fort Dodge Animal Health. Environmental assessment: Cydectin moxidectin 0.5% pour on cattle. Doc. Z154314. Overland Park, Kansas: Fort Dodge Animal Health, 1997.
- 40.Halley BA, VandenHeuvel WJA, Wislocki PG. Environmental effects of the usage of avermectins in livestock. Vet Parasitol. 1993;48:109–125. doi: 10.1016/0304-4017(93)90149-h. [DOI] [PubMed] [Google Scholar]
- 41.Bloom RA, Matheson JC. Environmental assessment of avermectins by the US Food and Drug Administration. Vet Parasitol. 1993;48:281–294. doi: 10.1016/0304-4017(93)90163-h. [DOI] [PubMed] [Google Scholar]
- 42.Blume RR. A checklist, distributional record, and annotated bibliography of the insects associated with bovine droppings of pastures in American north of Mexico. Southwest Entomol 1985; Suppl 9.
- 43.Merritt RW, Anderson JR. The effects of different pasture and rangeland ecosystems on the annual dynamics of insects in cattle droppings. Hilgardia. 1977;45:31–71. [Google Scholar]
- 44.Fender WM. Native earthworms of the Pacific northwest: an ecological overview. In: Hendrix PF, ed. Earthworm Ecology and Biogeography in North America. Boca Raton, Florida: Lewis Publishers, 1995:53–66.
- 45.Mohr CO. Cattle droppings as ecological units. Ecol Monogr. 1943;13:275–298. [Google Scholar]
- 46.Holter P. Food utilization of dung-eating Aphodius larvae. Oikos. 1974;25:71–79. [Google Scholar]
- 47.Tineralla PP, Fauske GM. Occurrence of Onthophagus nuchicornis (Coleoptera: Scarabaeidae) in North Dakota. Entomol News. 1999;110:22–26. [Google Scholar]
- 48.Macqueen A, Beirne BP. Dung burial activity and fly control potential of Onthophagus nuchicornis (Coleoptera: Scarabaeidae) in British Columbia. Can Entomol. 1975;107:1215–1220. [Google Scholar]
- 49.Macqueen A, Beirne BP. Effects of cattle dung and dung beetle activity on growth of beardless wheatgrass in British Columbia. Can J Plant Sci. 1975;55:961–967. [Google Scholar]
- 50.Schmidt CD. Activity of an avermectin against selected insects in aging manure. Environ Entomol. 1983;12:455–457. [Google Scholar]
- 51.Farkas R, Gyurcsó A, Börzsönyi L. Fly larvicidal activity in the faeces of cattle and pigs treated with endectocide products. Med Vet Entomol. 2003;17:301–306. doi: 10.1046/j.1365-2915.2003.00443.x. [DOI] [PubMed] [Google Scholar]
- 52.Wardhaugh KG, Holter P, Whitby WA, Shelley K. Effects of drug residues in the faeces of cattle treated with injectable formulations of ivermectin and moxidectin on larvae of the bush fly, Musca vetustissima, and the house fly, Musca domestica. Aust Vet J. 1996;74:370–374. doi: 10.1111/j.1751-0813.1996.tb15448.x. [DOI] [PubMed] [Google Scholar]
- 53.Anderson JR, Merritt RW, Loomis EC. The insect-free cattle dropping and its relationship to increased dung fouling of rangeland pastures. J Econ Entomol. 1984;77:133–141. [Google Scholar]