Abstract
Actinobacillus seminis is a gram-negative bacterium of the Pasteurellaceae family that is involved in ovine epididymitis. Looking for a protein specific to this species, we determined the protein profile of subcellular fractions of A. seminis (American Type Culture Collection number 15768): proteins from the outer membrane (OMPs), inner membrane (IMPs), and cytoplasm (CPs). These profiles provide the first data, to our knowledge, regarding subcellular fractions of A. seminis. In the OMP fraction, we identified a protein with a molecular mass of 75 kDa that proved to be immunogenic and apparently specific for A. seminis. This conclusion was based on the reaction of hyperimmune serum of rabbits inoculated with whole cells of A. seminis that was tested against sonicated complete cells of reference strains and field isolates of Brucella ovis, Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni. No protein of these bacteria cross-reacted with the 75-kDa protein of A. seminis. Furthermore, when each type of hyperimmune serum was tested against the sonicated cells and each of the subcellular fractions of A. seminis, it did not recognize the A. seminis 75-kDa protein. We also isolated and identified this protein in microvesicles released to the culture supernatant. The results suggest that the 75-kDa protein could be used to establish a diagnostic test specific for ovine epididymitis caused by A. seminis.
Résumé
Actinobacillus seminis est une bactérie à Gram négatif de la famille des Pasteurellaceae associée à l’épididymite ovine. En cherchant pour une protéine spécifique à cette espèce, nous avons déterminé le profil de protéine de fractions sub-cellulaires d’A. seminis (ATCC 15768) : des protéines de la membrane externe (OMPs), de la membrane interne (IMPs) et du cytoplasme (CPs). Ces profils fournissent selon nous, les premiers résultats concernant les fractions sub-cellulaires d’A. seminis. Dans la fraction OMP, nous avons identifié une protéine avec un poids moléculaire de 75 kDa qui s’est avérée être immunogène et apparemment spécifique à A. seminis. Cette conclusion était basée sur la réaction de sérum hyperimmun de lapins inoculés avec des cellules entières d’A. seminis et qui a été testé avec des cellules entières soniquées de souches de référence et d’isolats cliniques de Brucella ovis, Mannheimia haemolytica, Pasteurella multocida et Histophilus somni. Aucune protéine des ces bactéries n’a donné de réaction croisée avec la protéine de 75 kDa d’A. seminis. De plus, lorsque des sérums hyperimmuns produits contre les autres bactéries mentionnées ci-dessus ont été testés avec les cellules soniquées et chacune des fractions sub-cellulaires d’A. seminis, aucun n’a reconnu la protéine de 75 Kda d’A. seminis. Nous avons également isolé et identifié cette protéine dans des micro-vésicules relâchées dans le surnageant de culture. Ces résultats suggèrent que la protéine de 75 kDa pourrait être utilisée pour mettre en place un test diagnostique spécifique pour l’épididymite ovine causée par A. seminis.
(Traduit par Docteur Serge Messier)
Introduction
Contagious ovine epididymitis is defined as infection of the testicle and epididymis in rams. This condition may occur in acute, subacute, and chronic phases and is clinically detected when the testicle degenerates and atrophies, resulting in subfertility or sterility (1). Ovine epididymitis damages young and adult animals, causing important losses to the sheep industry. This disease is a serious reproductive disorder, with marked pathological changes in the genital tract (2).
Among the etiologic agents of epididymitis are several bacterial species, such as Brucella ovis, Actinobacillus seminis, Mannheimia haemolytica (Pasteurella haemolytica), Pasteurella multocida, and Histophilus somni (Haemophilus somnus) (3). The route of entry is commonly a wound in the genital mucosa; however, for A. seminis the route has not been adequately investigated.
A pleomorphic gram-negative coccobacillus 1 × 1 to 1.4 μm, A. seminis is found in chains or palisades. It is nonmotile and non-sporulated. This facultative anaerobe has fermentative metabolism; it grows optimally in 10% CO2 at 37°C in media with blood or serum, producing small colonies after 24 h and large, white colonies (1 to 2 mm in diameter) after 48 h (4,5).
Reports of epididymitis caused by A. seminis are scarce. The first isolation was reported from Australia in 1960 by Baynes and Simmons (6), who described suppurative epididymitis caused by a gram-negative pleomorphic microorganism in a ram. In 1968 Baynes and Simmons (7) reported having isolated a microorganism from 3 rams with epididymitis. The same year, in New Zealand, A. seminis was isolated from a ram with epididymitis and polyarthritis (8). In Mexico, the first case of epididymitis produced by A. seminis, in animals imported from New Zealand, was reported in 1979 (9).
In 1988, Healey et al (10) reported having separated proteins from strains of A. seminis and A. actinomycetemcomitans, both isolated from rams with epididymitis. The proteins from the 2 isolates had a similar molecular mass. However, other isolates identified as Actinobacillus spp. had different protein profiles, leading to the conclusion that diverse members of the Actinobacillus genus can probably be isolated from rams with epididymitis. The objective of our study was to identify a protein specific to A. seminis.
Materials and methods
Bacterial strains and growth conditions
The bacterial species used in this study are shown in Table I. All strains were grown on Brucella agar (BA; Becton Dickinson, Rutherford, New Jersey, USA) in 10% CO2 at 37°C.
Table I.
Bacterial strains and isolates used in this study
| Bacterium | Isolate | Origin |
|---|---|---|
| Actinobacillus seminis | 15768 | ATCC |
| Isolate | INIFAP–SAGARPA | |
| Brucella ovis | BP 2001 | BUAP |
| BP 2002 | ||
| Mannheimia haemolytica | GM31 | FESC, UNAM |
| EH 28 | ||
| CA22 | ||
| MA16 | ||
| SC17 | ||
| Pasteurella multocida | SM13 | FESC, UNAM |
| AC14 | ||
| SA15 | ||
| AE28 | ||
| SC17 | ||
| Histophilus somni | Isolate | INIFAP–SAGARPA |
ATCC — American Type Culture Collection; INIFAP–SAGARPA —Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias. Secretaría de Agricultura Ganadería, Desarrollo Rural, Pesca y Alimentación; BUAP — Benemérita Universidad Autónoma de Puebla; FESC, UNAM — Facultad de Estudios Superiores Cuautitlán, Universidad Nacional Autónoma de México
Subcellular fractions of A. seminis
All reagents were from Sigma Chemical Company, St. Louis, Missouri, USA. Bacterial subcellular fractions were obtained according to the method described by Morton et al (11) in 1996, with some modifications. American Type Culture Collection (ATCC) A. seminis 15768 was grown on BA for 24 h, suspended in 10 mM HEPES pH 7.2, and centrifuged at 1000 × g for 30 min. The pellet was washed twice and resuspended in 10 mM HEPES containing 0.1 M phenylmethylsulfonyl fluoride, 0.1 M p-hydroxymercury benzoate (PHMB), and 3% ethylene diamine tetraacetic acid. Cells was sonicated by 6 cycles of 1 min in a Vibra-Cell (Sonics & Materials; Newtown, Connecticut, USA), and cellular detritus was removed by centrifugation at 10 000 × g for 20 min. The supernatant was centrifuged at 150 000 × g for 1 h; the new supernatant contained the cytoplasmic proteins (CPs), and the pellet contained the total membranes. The membranes were resuspended in 10 mM HEPES containing 1% (w/v) sodium N-lauryl sarcosine and agitated for 30 min at 37°C (12). They were then centrifuged at 150 000 × g for 1 h. The pellet contained the outer membrane proteins (OMPs) and the supernatant the inner membrane proteins (IMPs). The OMPs were treated to remove lipids and detergent and were suspended in water according to the method of Wessel and Flügge (13). The purity of the OMPs was verified with the QCL-1000 Chromogenic Limulus Amebocyte Lysate (LAL) test for lipopolysaccharide (LPS) (BioWhittaker, East Rutherford, New Jersey, USA) (14), and LPS was stained with silver by the method of Tsai and Frasch (15). The IMPs and CPs were separately precipitated overnight with cold ethanol 1:4 (v/v) at 4°C. They were then centrifuged at 1000 × g for 20 min, and the sediment was dried by air flow and kept frozen until used. Protein concentration was determined by the method of Bradford (16).
Rabbit antiserum
Two New Zealand rabbits weighing 2 kg each were intramuscularly inoculated with A. seminis 15768 cells (1.5 × 108 colony-forming units [CFU]) in saline solution, initially with Freund’s complete adjuvant and 20 d later with Freund’s incomplete adjuvant. After 7 d, the rabbits were given intramuscular booster inoculations without adjuvant 3 times, at 1-wk intervals. A titer of 1:2050 for the antibody against A. seminis was obtained by dot enzyme-linked immunosorbent assay (ELISA-dot) with A. seminis sonicated cells used as antigen.
Serum antibody against B. ovis was donated by Facultad de Estudios Superiores Cuautitlán, Universidad Nacional Autónoma de México, and antibody against M. haemolytica, P. multocida, and H. somni was donated by Centro Nacional de Investigaciones Disciplinarias en Microbiología Animal, Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias, Secretaría de Agricultura Ganadería, Desarrollo Rural, Pesca y Alimentación, México. All antibodies were produced in rabbits with the use of sonicated cells. The titers (1:2000 for B. ovis and 1:1000 for the other bacteria) were determined by ELISA-dot with use of the sonicated cells as antigen.
Two New Zealand rabbits weighing 2 kg each were intramuscularly inoculated with the 75-kDa protein of A. seminis 15768, obtained by elution with 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). A titer of 1:4000 was obtained by ELISA-dot using the eluted protein as antigen.
Protein preparation, purification, and testing
Whole cells and each subcellular fraction of A. seminis were separated by 10% SDS-PAGE (17); each well contained 10 μg of protein. Proteins were electrotransferred (18) to 0.45-μm nitrocellulose membranes (Gibco Laboratories, Carlsbad, California, USA) for 1 h at 400 mA by means of a Mini Trans-Blot Electrophoretic Transfer Cell (BioRad Laboratories, Hercules, California, USA). To visualize the A. seminis proteins recognized by the rabbit antiserum, a peroxidase-labeled goat IgG conjugate against rabbit antigen, diluted 1:1000 (Sigma), was used.
To determine which proteins of A. seminis are species-specific, whole cells of A. seminis were separated by 10% SDS-PAGE, transferred, and exposed to rabbit hyperimmune serum against the other bacteria, with use of the same enzyme conjugate.
To purify the 75-kDa protein from A. seminis 15768, the OMPs were separated by 12% SDS-PAGE in a Hoefer chamber 16 × 0.2 × 18 cm and stained with Coomassie blue (Hoefer Scientific Instruments, San Francisco, California, USA). The 75-kDa OMP was cut from the gel and electroeluted in a MiniProtean III chamber (BioRad) for 3 h at 60 mA. The eluted material was precipitated with 4 volumes of ethanol at 4°C, centrifuged at 10 000 × g for 20 min, and then resuspended in deionized water. The protein concentration was measured and purity checked with the use of 12% SDS-PAGE and Western-blot testing with the rabbit antiserum to the 75-kDa protein.
To determine if the 75-kDa protein was species-specific for A. seminis, field isolates were obtained from epididymal tissues of rams infected with A. seminis, B. ovis, M. haemolytica, P. multocida, and H. somni. Whole cells were separated by 12% SDS-PAGE (17), and then the proteins were electrotransferred to 0.45-μm nitrocellulose membranes for 1 h at 400 mA and exposed to the rabbit antiserum to the A. seminis 75-kDa protein, with use of the same enzyme conjugate.
Isolation of A. seminis membrane microvesicles
After centrifugation of cultured A. seminis 15768 at 10 000 × g for 20 min at 4°C, the supernatant was filtered sequentially through membranes of pore diameters 0.45 μm and then 0.22 μm (Millipore, Billerica, Massachusetts, USA) to remove residual cells. Vesicles were recovered by ultracentrifugation (150 000 × g for 3 h at 5°C) and kept at −20°C until used (19).
Electron microscopy
For negative staining, cultured A. seminis 15768 was harvested by centrifugation at 10 000 × g for 20 min at 4°C. Whole cells or vesicles (15-μL) were placed onto carbon and copper grids coated with polyvinyl formal (Formvar), stained with 25% (v/v) phosphotungstic acid, and observed with an electron transmission microscope (JEM-100S; JEOL, Tokyo, Japan) at a magnification of × 40 000 (20).
For scanning electron microscopy, cultured A. seminis 15768 with vesicles were dehydrated in graded alcohols, dried, coated with gold, and observed with a scanning electron microscope (JSM-5410LV; JEOL) at a magnification of × 20 000 (20).
Immunoblotting of membrane microvesicles
To detect the 75-kDa protein in membrane microvesicles of A. seminis, after electrophoresis the vesicle proteins were transferred to a nitrocellulose membrane for 1 h at 400 mA and then exposed to the rabbit antiserum to the A. seminis 75-kDa protein, with use of the same enzyme conjugate.
Results
Protein profiles of A. seminis subcellular fractions
Figure 1A shows the protein patterns of the whole cells, OMPs, CPs, and IMPs separated by SDS-PAGE (lanes 2, 3, 4, and 5, respectively). A pattern corresponding to smooth LPS was found in A. seminis in a gel with urea (not shown). The amebocyte LAL test showed that the OMP fraction had a low level of contamination with LPS (0.17 endotoxin units/mL), but contamination was not found in the IMP and CP fractions, which demonstrated their purity. Figure 1B shows the corresponding Western-blot results, which were different for each fraction, showing high antigenic reactivity by the 3 subcellular fractions when tested with the antiserum against whole cells of A. seminis. A 75-kDa polypeptide was observed in the OMP and CP fractions (lanes 3 and 4, respectively). These antigenic protein profiles provide the first data, to our knowledge, regarding various subcellular fractions of A. seminis.
Figure 1.
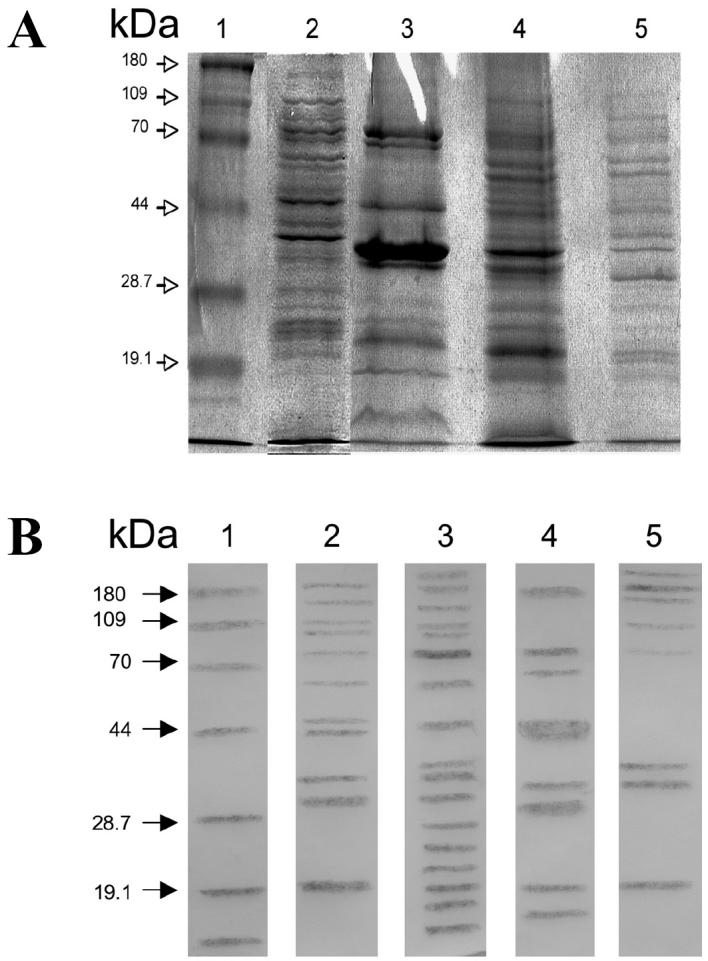
A, proteins of Actinobacillus seminis separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Lane 2, whole-cell extracts; lane 3, outer membrane proteins (OMPs); lane 4, cytoplasmic membrane proteins (CPs); lane 5, inner membrane proteins (IMPs). B, corresponding results of Western blot testing with rabbit antibody against A. seminis. In all the figures, lane 1 consists of molecular mass markers.
Specific protein of A. seminis
As Figure 2A shows, Western-blot tests of whole-cell extracts with rabbit hyperimmune serum against A. seminis revealed a protein of approximately 75 kDa in A. seminis (lane 2) but not in B. ovis, M. haemolytica, P. multocida, or H. somni. On the other hand, antibodies against the other bacteria did not recognize the 75-kDa protein in whole-cell extracts (Figure 2B). These results suggest that the 75-kDa protein could be specific to A. seminis.
Figure 2.
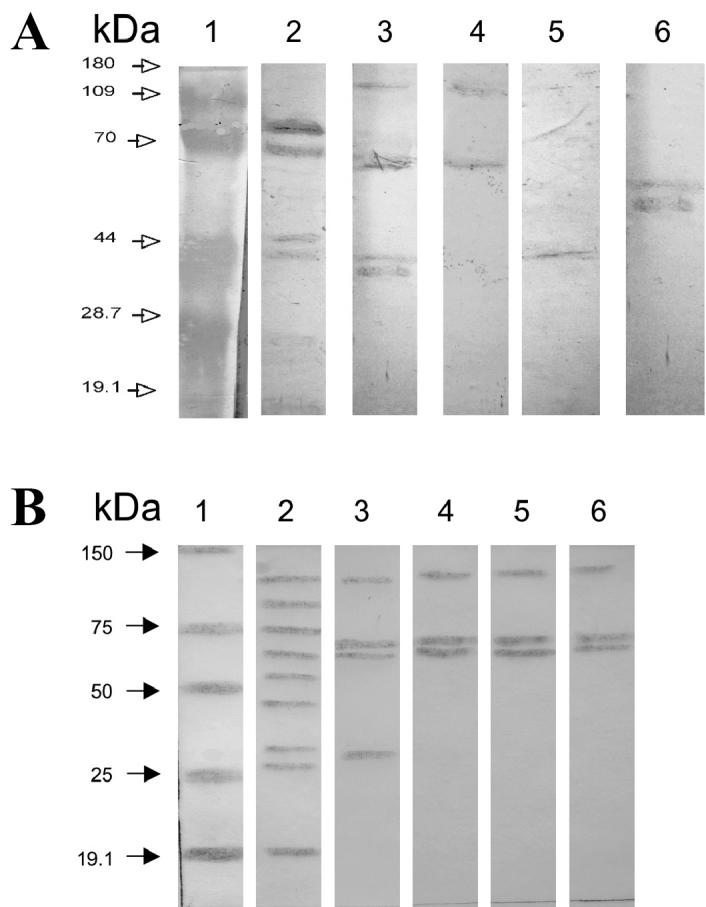
Demonstration of the 75-kDa protein that is apparently specific for Actinobacillus seminis (A, lane 2) by Western blot testing of extracts from whole cells of bacteria associated with contagious epididymitis. A, extracts of each bacterium were exposed to antibody against A. seminis: lane 2, A. seminis; lane 3, Brucella ovis; lane 4, Histophilus somni; lane 5, Mannheimia haemolytica; lane 6, Pasteurella multocida. B, extract from A. seminis was exposed to antibody against each bacterium: lane 2, antibody against A. seminis; lane 3, antibody against B. ovis; lane 4, antibody against H. somni; lane 5, antibody against M. haemolytica; lane 6, antibody against P. multocida.
When the 75-kDa protein was purified from cellular extracts by electroelution (Figure 3A, lane 3) and then exposed to hyperimmune serum against A. seminis and the other microorganisms, Western-blot testing revealed that the eluted protein was recognized only by the hyperimmune serum against A. seminis (Figure 3B, lane 2). Also, Western-blot testing with serum against whole-cell and field isolates of the same bacteria (Table I), obtained from epididymal tissues of rams, revealed that the 75-kDa protein was recognized by serum against isolates of A. seminis but not by serum against the other bacterial species (data not shown). These results confirm the specificity of the 75-kDa protein of A. seminis.
Figure 3.
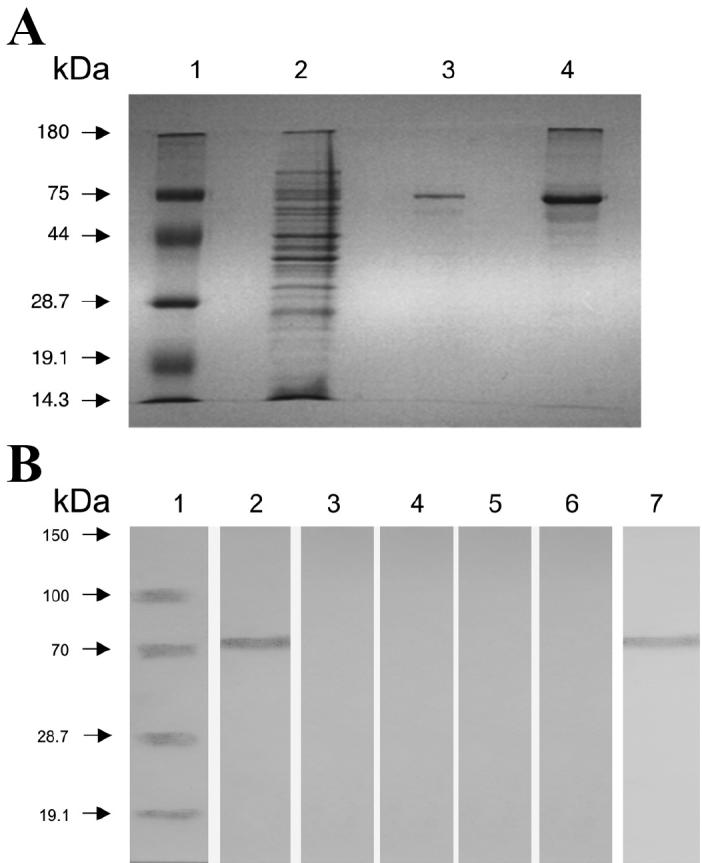
A, separation by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of whole cells (lane 2), the 75-kDa protein (lane 3), and microvesicles (lane 4) of Actinobacillus seminis. B, Western-blot results, showing recognition of the 75-kDa protein in A. seminis microvesicles only by A. seminis whole cells (lane 2) and not by antibodies against field isolates of Brucella ovis (lane 3), Histophilus somni (lane 4), Mannheimia haemolytica (lane 5), Pasteurella multocida (lane 6), and A. seminis (lane 7).
Membrane microvesicles of A. seminis
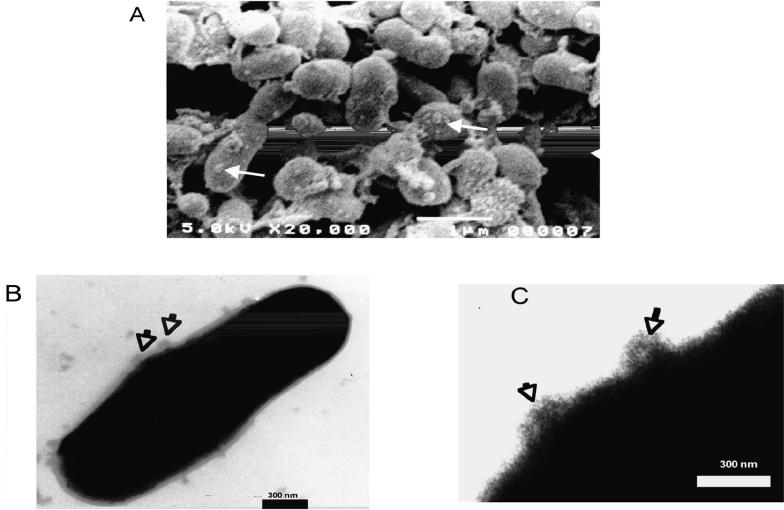
Membrane vesicles associated with cells from A. seminis cultures in the early stationary phase were observed by scanning electron microscopy (Figure 4A) and negative-staining transmission electron microscopy (Figures 4B and 4C). The vesicle diameter varied from 20 to 100 nm, as was described for A. acinomycetemcomitans (21) and A. pleuropneumoniae (19). After concentration by centrifugation and analysis by SDS-PAGE, the vesicles were found to be rich in the 75-kDa protein and other proteins (Figure 3A, lane 4). The 75-kDa protein was recognized in the microvesicles by antibodies against A. seminis whole cells (Figure 5, lane 2) and field isolate but not by antibodies against the other bacteria (Figure 5, lanes 3, 4, 5, and 6).
Figure 4.
Vesicles (arrows) on the surface of Actinobacillus seminis cells, shown by (A) scanning electron microscopy (× 20 000; bar = 1 μm) and (B, C) transmission electron microscopy after staining with phosphotungstic acid (× 40 000; bars = 300 nm).
Figure 5.
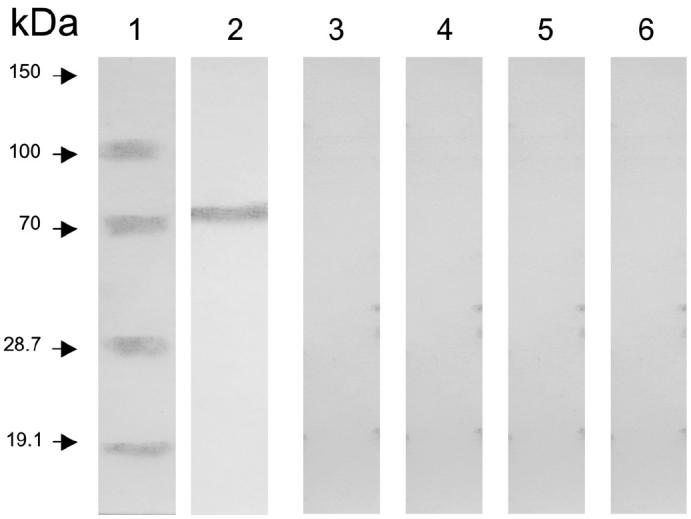
Immunoblot analysis of Actinobacillus seminis microvesicles, showing recognition of the 75-kDa protein by antibody against A. seminis (lane 2) but not by antibody to Brucella ovis (lane 3), Histophilus somni (lane 4), Mannheimia haemolytica (lane 5), or Pasteurella multocida (lane 6).
Discussion
Infectious epididymitis in rams is a major disease in countries devoted to the industrialized breeding of sheep. Brucella ovis was reported as the main etiologic agent in Australia in 1960; however, A. seminis and H. somni are also commonly isolated from lesions and produce clinical signs and reproductive problems in rams (3). Analysis of the protein profiles and outer membrane antigens of A. seminis and A. actinomycetemcomitans, which are considered to be transient ovine genital flora, suggests that these opportunistic pathogens constitute a diverse group of bacteria (10). However, A. seminis is one of the most common isolates and produces clinical signs, lesions, and reproductive problems in rams that may be similar to those of B. ovis. Transmission of A. seminis is assumed to be venereal, through direct contact between mucous membranes of all natural orifices when crowding occurs during the premating period owing to increased homosexual activity. Ewes are considered to be possible intermediate carriers, promoting ewe-to-lamb transmission (6).
Identification and characterization of microorganisms has historically been based on morphologic, biochemical, serologic, toxigenic, and genetic characteristics. More recently, examination of CP patterns by means of PAGE has been used to distinguish taxonomically among strains within a species and among organisms within a genus or family. Currently the diagnostic technique used to identify A. seminis is bacteriologic and includes Gram’s and modified Ziehl–Nielsen staining. The latter differentiates A. seminis and B. ovis. This technique is performed until the animal shows the characteristic signs of the infection. There is no specific biochemical test to distinguish A. seminis from the other bacterial species involved in epididymitis.
The OMPs of many gram-negative bacteria have been studied. Some have been identified as porins, Fe-receptors, invasins, and factors in resistance to serum opsonization and killing in Escherichia coli and in the genera Neisseria, Yersinia, Actinobacillus, and others (22). Healey et al (10) added n-octyl-β-D-glucopyranoside to A. seminis isolates and used SDS-PAGE and spectrophotometric scanning of gels to analyze protein extracts from A. seminis (ATCC 15768) and A. actinomycetemcomitans. Bacterial isolates were grouped according to protein profile. Group 1 consisted of A. seminis, A. actinomycetemcomitans, and 7 field isolates of Actinobacillus spp., all of which had common protein bands of approximate molecular masses of 94, 64, 60, 52, 44, and 26 kDa. Group 2 had a unique protein profile, with relatively few protein bands in common.
To the best of our knowledge, this is the 1st report of data on the proteins in subcellular fractions of A. seminis. The polypeptide with a molecular mass of 75 kDa identified in the fractions and had an apparently specific immunogenic reaction: there was no cross-reaction of the hyperimmune serum from rabbits inoculated with whole cells of A. seminis against sonicated complete cells of B. ovis, M. haemolytica, P. multocida, or H. somni. Furthermore, when each type of hyperimmune serum of rabbits inoculated with whole cells of B. ovis, M. haemolytica, P. multocida, and H. somni was exposed to sonicated cells and each of the subcellular fractions of A. seminis, there was no recognition of the A. seminis 75-kDa protein by the other species.
Gram-negative bacteria form microvesicles, or blebs, as a result of outer membrane budding. These structures range in diameter from 20 to 500 nm and are released into the culture medium. They contain mainly outer membrane components and associated toxins and enzymes (19,21). Their release could therefore be a mechanism of tissue damage in epididymitis caused by A. seminis. They also provide an excellent source for the purification and characterization of biologically active molecules. However, it is still necessary to conduct tests with serum from animals naturally infected with microorganisms other than A. seminis that cause epididymitis. In addition, it is important to demonstrate a lack of cross-reaction with other members of the Actinobacillus group and to prove the specificity of the 75-kDa protein for A. seminis. Validation tests are necessary before the 75-kDa protein can be used in a diagnostic test for ovine epididymitis caused by A. seminis.
Acknowledgments
The first author is a recipient of a scholarship (#93884) from the Consejo Nacional de Ciencia y Tecnología (CONACYT), México. This work was partially supported by the Cátedra de Inmunodignóstico de Enfermedades Bacterianas y Micóticas de la Coordinación de Investigación, FES Cuautitlán, UNAM-México, and by CONACYT Project # G38590-B. The authors thank Eliseo Hernández, Sofía González, and Rodolfo Robles, of the Electron Microscopy Unit of FES Cuautitlán, UNAM, for their assistance and Adriana Palomino for her collaboration in the revision of manuscript.
References
- 1.Appuhamy S, Coote JG, Low JC, Parton R. PCR methods for rapid identification and characterization of Actinobacillus seminis strains. J Clin Microbiol. 1998;36:814–817. doi: 10.1128/jcm.36.3.814-817.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Healey MC, Hwang HH, Kleinschuster SJ, Gharpure HM, Johnston AV. Production and preliminary characterization of monoclonal antibodies to Actinobacillus sp. isolated from epididymitis lesions in a ram. Am J Vet Res. 1985;46:1297–1302. [PubMed] [Google Scholar]
- 3.Genetzky R. Epididymitis in rams. Food Anim. 1995;17:447–454. [Google Scholar]
- 4.Burgess GW. Ovine contagious epididymitis. Anim Rev Vet Microbiol. 1982;7:551–575. doi: 10.1016/0378-1135(82)90049-9. [DOI] [PubMed] [Google Scholar]
- 5.Koneman W. Diagnóstico Microbiológico. 5th ed., Buenos Aires, Argentina: Ed Panamericana 1999;394:395.
- 6.Baynes I, Simmons G. Ovine epididymitis caused by Actinobacillus seminis. Aust Vet J. 1960;36:454–459. [Google Scholar]
- 7.Baynes I, Simmons G. Clinical and pathological studies of border Leicester rams naturally infected with Actinobacillus seminis. Aust Vet J. 1968;44:339–343. doi: 10.1111/j.1751-0813.1968.tb14399.x. [DOI] [PubMed] [Google Scholar]
- 8.Ekdahl MO, Money DFL, Martin CA. Some aspects of epididymitis of rams in New Zealand. N Z Vet J. 1968;16:81–82. doi: 10.1080/00480169.1968.33750. [DOI] [PubMed] [Google Scholar]
- 9.Pérez DP, Trigo TF, De la Higuera JJ, Flores CR. Diagnóstico y descripción de un brote de epididimitis ovina en México causada por Brucella ovis. Vet Mex. 1979;10:221–226. [Google Scholar]
- 10.Healey MC, Hwang HH, Kleinschuster SJ, Johnston AV, Symons KS. Comparison and partial characterization of the protein profiles and outer membrane antigens of Actinobacillus species isolated from ram lambs with epididymitis. Am J Vet Res. 1988;49:1824–1831. [PubMed] [Google Scholar]
- 11.Morton RJ, Simons KR, Confer AW. Major outer proteins of Pasteurella haemolytica serovars 1–15: comparison of separation techniques and surface-exposed proteins on selected serovars. Vet Microbiol. 1996;51:319–330. doi: 10.1016/0378-1135(96)00010-7. [DOI] [PubMed] [Google Scholar]
- 12.Filip C, Fletcher G, Wulff JL, Earthart CF. Solubilization of cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wessel D, Flügge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 14.Young NS, Levin J, Prendergast RA. An invertebrate coagulation system activated by endotoxin: evidence for enzymatic mediation. J Clin Invest. 1972;51:1790–1797. doi: 10.1172/JCI106980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai C, Frasch CE. A sensitive silver stain for detection of lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 16.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Towbin H, Stachelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Negrete E, García R, Reyes M, Godínez D, de la Garza M. Membrane vesicles released by Actinobacillus pleuropneumoniae contain proteases and Apx toxins. FEMS Microbiol Lett. 2000;191:109–113. doi: 10.1111/j.1574-6968.2000.tb09326.x. [DOI] [PubMed] [Google Scholar]
- 20.Kadurugamuwa TJ, Beveridge TJ. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol. 1995;177:3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer DH, Fives-Taylor PM. Evidence that extracellular components function in adherence of Actinobacillus actinomycetemcomitans to epithelial cells. Infect Immun. 1993;61:4933–4936. doi: 10.1128/iai.61.11.4933-4936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corbeil LB. Molecular aspects of some virulence factors of Haemophilus sommus. Can J Vet Res. 1999;54:57–62. [PubMed] [Google Scholar]