Abstract
Phagocyte numbers and activities were compared in milk from 2 groups of uninfected mammary-gland quarters from 3 cows each: 6 quarters with a high (≥ 200 000/mL) somatic cell concentration (SCC), analyzed as 4 individual quarters and 1 pooled sample; and 12 quarters with a low SCC (< 200 000/mL), analyzed as 6 paired samples. The concentrations and ability of macrophages and polymorphonuclear (PMN) cells to phagocytize fluorescent microspheres were determined by flow cytometry after exposure of the cells to the microspheres. The macrophages and PMNs contained 2 major subpopulations, characterized by low phagocytic (LP) or high phagocytic (HP) ability. The quarters with high SCCs had significantly lower percentages of HP cells than did the quarters with low SCCs (P < 0.01). Whether mammary-gland quarters or cows were the unit of analysis, the HP/LP ratio was negatively related to the SCC (P < 0.04), which explained more than 50% of the SCC variability. Thus, poor bovine mammary-gland phagocytic ability may be associated with high SCC. Longitudinal studies are suggested to further explore and characterize these relationships.
Résumé
Le nombre et l’activité des phagocytes du lait provenant de deux groupes de quartiers non infectés de trois vaches ont été comparés : 6 quartiers avec un comptage de cellules somatiques (SCC) élevé (≥ 200 000/mL), analysés comme 4 quartiers individuels et 1 échantillon composite; et 12 quartiers avec un SCC bas (< 200 000/mL), analysés comme 6 échantillons pairés. Les concentrations et la capacité des macrophages et des cellules polymorphonucléaires (PMN) à phagocyter des microsphères fluorescentes ont été déterminées par cytométrie en flux après exposition des cellules aux microsphères. Les macrophages et les PMN comptaient deux sous-populations principales caractérisées par une faible capacité phagocytaire (LP) ou une capacité phagocytaire élevée (HP). Les quartiers avec des SCC élevés avaient un nombre significativement plus faible de cellules HP que les quartiers avec des SCC bas (P < 0,01). Indépendamment du fait que les quartiers ou les vaches soient considérés comme les unités d’analyse, le ratio HP/LP était corrélé négativement au SCC (P < 0,04) ce qui expliquait plus de 50 % de la variabilité des SCC. Ainsi, une pauvre capacité phagocytaire au niveau de la glande mammaire peut être associée avec des SCC élevés. Des études longitudinales sont de mises pour étudier et caractériser plus en détail ces relations.
(Traduit par Docteur Serge Messier)
Mastitis is a common and costly disease in dairy cattle (1). The most widely used method for detecting and measuring bovine mastitis is determination of the milk somatic cell concentration (SCC). Extensive study has demonstrated that the SCC can be used to estimate milk production losses associated with mastitis. Although a high SCC tends to indicate infection, it is not uncommon in uninfected cows (2,3). Current efforts to improve milk quality focus on decreasing the SCC of bulk milk.
In an effort to determine possible factors associated with a high SCC in milk, we previously investigated the phagocytic ability of milk phagocytes. At least 2 phagocyte subpopulations of different phagocytic ability (low versus high) were found in bovine milk, and the SCC was negatively correlated with phagocytic ability (4). However, milk was not cultured, and, hence, infection could have been a confounding factor (cows might have had a high SCC because they were fighting infection rather than because of differences in phagocytic ability). To establish whether a high SCC is associated with functional differences in phagocytic ability, we needed to study cows known to be free of infection.
The goals of this investigation were to determine if different phagocyte subpopulations exist in milk of cows free of bacterial infection and to compare the phagocytic ability of phagocyte populations present in the uninfected mammary-gland quarters of cows with either a high or a low SCC. Because cows with an SCC in milk below 200 000/mL display a lower risk of infectious mastitis than those with a higher SCC (5), that concentration was selected as the cut-off point for evaluating the phagocytic ability of bovine milk phagocytes. We used milk cell isolation and flow cytometry.
Six cows were selected according to the following criteria: nonperiparturient; having, within a 3-wk interval, 3 consecutive SCC measures in milk that were below (or above) 200 000/mL; and having, within the same period, at least 2 negative results of bacteriologic tests (lack of bacterial growth in cultured milk). The animals were housed, fed, and milked at North Carolina State University (NCSU) facilities according to protocols approved by the Institutional and Animal Care and Use Committee.
Milk collected from the cows revealed a low SCC (< 200 000/mL) in 3 and a high SCC (≥ 200 000/mL) in the other 3 when milk from the 4 quarters was examined as a pooled sample. Because milk from individual mammary-gland quarters may show different SCCs (and different health conditions) but the quarters share a single leukocyte pool, experiments were designed such that either the cow or the mammary-gland quarter could be regarded as the unit of analysis. Considering the cow as the unit of analysis, 2 groups of 3 animals each were investigated (1 group with a low and the other with a high SCC). Considering the mammary-gland quarter as the unit of analysis, we investigated 1 set of 12 low-SCC mammary quarters and 1 set of 6 high-SCC quarters. Low-SCC milk has lower leukocyte concentrations than high-SCC milk (6). Therefore, we expected to pool low-SCC samples, but not always high-SCC samples, in pairs. Accordingly, the low-SCC group was composed of 6 samples of milk pooled from 12 quarters of 3 cows, whereas the high-SCC group was composed of 4 individual samples and 1 pooled sample (2 quarters) from 3 cows.
The low-SCC samples were approximately 2 L each and consisted of composite milk collected from 2 quarters selected at random. In contrast, with the high-SCC cows 1 L of milk, obtained from a single mammary quarter selected at random, allowed enough cells to be measured; for 2 cows 2 samples were obtained in this way, but owing to milking difficulties a single sample of composite milk collected from 2 quarters was obtained from the 3rd cow. Hence, 11 mammary-quarter samples (pooled or not pooled) were investigated: 6 collected from low-SCC cows and 5 from high-SCC cows. By averaging the results for each animal, this design also allowed us to test the individual cow as a unit of analysis.
Milk samples were aseptically collected from each quarter of each cow on at least 2 occasions, with the use of procedures described by the National Mastitis Council (NMC) (7). Milk bacteriologic tests were conducted at the Mastitis Laboratory of the College of Veterinary Medicine of NCSU, also with the use of procedures described by the NMC (7). Somatic cell counts were conducted at DairyOne (Ithaca, New York, USA).
Milk was collected in sterile bottles containing 10 mL of gentamicin (Invitrogen, Carlsbad, California, USA) and transported on ice. Milk cells were isolated as described elsewhere (4). Briefly, milk was diluted in an equal volume of buffer containing phosphate-buffered saline, 10% acid citrate dextrose, and 20 mM ethylene diamine tetraacetic acid (EDTA) (PAE), then was centrifuged for 45 min at 350 × g at 15°C. The fat layer and supernatant were discarded, and the cell pellet was transferred to 50-mL vials and washed 3 times in PAE. The cells were then resuspended to approximately 1 × 106/mL in buffer containing 2% goat serum diluted in PAE containing 0.1% NaN3 and 10 mM EDTA (PAE-NaN3 buffer).
Aliquots containing approximately 1 mL (106) cells each were transferred to polypropylene tubes, to which 100 μL of 1 of the following was added: 1) PAE-NaN3 buffer (cells only), 2) an irrelevant mouse monoclonal antibody (against Distemper virus), 3) antibodies against bovine CD3 (backgating procedure that identifies lymphocytes), 4) antibodies against bovine CD11b (backgating procedure that identifies phagocytes), 5) fluorescein-coated latex microspheres in a 1:10 ratio of cells to microspheres 2.0 μm in diameter (Fluoresbrite plain YG; Polysciences, Warrington, Pennsylvania, USA), and 6) fluorescent microspheres only. All monoclonal antibodies were from VMRD (Pullman, Washington, USA). Tubes receiving fluorescent microspheres were kept at 37°C for 30 min; the contents were then washed 3 times and diluted with PAE-NaN3 buffer containing 2% paraformaldehyde, and the tubes were stored at 4°C until analysis. Tubes receiving monoclonal antibodies were kept on ice till the contents were washed 3 times; after incubation for 30 min at 4°C in a dark environment with fluoresceinated mouse antibody against goat antigens (Caltag Laboratories, Burlingame, California, USA), the contents were washed and similarly fixed.
Counting of viable cells and measurement of phagocytosis-related fluorescence intensity were carried out after: a) identification of cell-side and forward light-scatter parameters of microspheres, lymphocytes, macrophages, and polymorphonuclear (PMN) cells; b) further validation of leukocyte identification based on side and forward scatter by use of monoclonal antibodies specific for lymphocytes (CD3+, CD11b−) or phagocytes (CD3−, CD11b+); and c) determination of autofluorescence (of nonviable cells, a negative control), as previously shown (6). In addition, lymphocyte-associated fluorescence (an additional negative control) and microsphere fluorescence background (microspheres not exposed to cells, a false-positive control) were also assessed. Viable cells per 500 μL were counted on the basis of light-scatter values, as previously described (6). Cells were analyzed with a flow cytometer (FACScalibur; Becton Dickinson, San Jose, California, USA). Medians, confidence intervals, and correlation coefficients were calculated and regression analysis was conducted with statistical software (Minitab, State College, Pennsylvania, USA).
Results of 3 consecutive SCC measurements for the 6 cows are shown in Table IA. All milk cultures were negative for bacteria.
Table I.
Somatic cell concentration (SCC)a and phagocytosis profile in bovine milk
| A. SCC determined within the 3 wk immediately before the study
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Measurement (thousands/mL)
|
||||||||
| Cow number | 1st | 2nd | 3rd | |||||
| 1 | 152 | 24 | 19 | |||||
| 2 | 47 | 67 | 14 | |||||
| 3 | 87 | 66 | 144 | |||||
| 4 | 283 | 838 | ND | |||||
| 5 | 346 | 639 | 2954 | |||||
| 6 | 326 | 350 | 841 | |||||
| B. Phagocytic ability and SCC | ||||||||
|---|---|---|---|---|---|---|---|---|
| Unit of analysis: pooled or nonpooled samples of mammary-quarter milkb | ||||||||
| Ratio of HP to LP cellsc |
Number (%) of viable leukocytesc |
|||||||
| Cow number, quarter | SCC | All cells | MOs | PMNs | LOs | MOs | PMNs | |
| Low SCC | ||||||||
| 1 | 1 LR, RF | 17 | 1.04 | 0.55 | 2.28 | 975 (45) | 899 (42) | 289 (13) |
| 2 | 1 LF, RR | 12 | 1.23 | 0.52 | 4.62 | 499 (53) | 316 (34) | 128 (14) |
| 3 | 2 LR, RF | 15 | 1.10 | 0.83 | 3.40 | 1243 (52) | 1021 (43) | 112 (5) |
| 4 | 2 LF, RR | 15 | 1.14 | 0.58 | 2.70 | 805 (50) | 674 (42) | 142 (9) |
| 5 | 3 LF, RR | 76 | 2.36 | 0.83 | 8.00 | 670 (65) | 308 (30) | 60 (6) |
| 6 | 3 LR, RF | 15 | 1.90 | 0.80 | 6.00 | 1114 (49) | 892 (39) | 257 (11) |
| Median | 15 | 1.18d | 0.69d | 4.01d | 890 (51) | 783 (40) | 135 (10)d | |
| High SCC | ||||||||
| 7 | 4 RF | 472 | 0.37 | 0.25 | 0.50 | 1729 (53) | 1354 (42) | 175 (5) |
| 8 | 4 LR | 2031 | 0.55 | 0.32 | 1.21 | 5153 (62) | 2830 (34) | 275 (3) |
| 9 | 5 RF | 263 | 0.45 | 0.38 | 1.00 | 5235 (65) | 2605 (32) | 222 (3) |
| 10 | 5 LF | 1434 | 0.18 | 0.06 | 0.17 | 3119 (63) | 1691 (34) | 177 (4) |
| 11 | 6 LR, RF | 504 | 0.88 | 0.40 | 2.96 | 2472 (48) | 2285 (44) | 429 (8) |
| Median | 504d | 0.45 | 0.32 | 1.00 | 3119 (62)d | 2285 (34)d | 222 (4) | |
| Unit of analysis: cowe | ||||||||
| Low SCC | ||||||||
| 1 | 14 | 1.13 | 0.53 | 3.45 | 737 (49) | 607 (38) | 208 (13) | |
| 2 | 15 | 1.12 | 0.70 | 3.05 | 1024 (51) | 847 (42) | 127 (7) | |
| 3 | 46 | 2.13 | 0.81 | 7.00 | 892 (57) | 600 (34) | 158 (8) | |
| Median | 15 | 1.13d | 0.70d | 3.45d | 892 (51) | 607 (38) | 158 (8) | |
| High SCC | ||||||||
| 4 | 1251 | 0.46 | 0.28 | 0.86 | 3441 (57) | 2092 (38) | 225 (4) | |
| 5 | 848 | 0.31 | 0.22 | 0.58 | 4177 (64) | 2148 (33) | 199 (3) | |
| 6 | 504 | 0.88 | 0.40 | 2.96 | 2472 (48) | 2285 (44) | 429 (8) | |
| Median | 848d | 0.46 | 0.28 | 0.86 | 3441 (57)d | 2148 (38)d | 225 (4) | |
ND — not done; HP — high phagocytic ability; LP — low phagocytic ability; MOs — macrophages; PMNs — polymorphonuclear cells; LOs — lymphocytes; LR — left rear; RF — right front; LF — left front; RR — right rear
Average from 4 (A) or 1 to 2 (B) mammary quarters
Composite (LR, RF) or single (RF) mammary quarters
Based on values for light-scatter parameters
Significantly higher than in the other SCC group (P < 0.05)
Individual cow values are the average of 2 separate measurements, each derived from pooled or nonpooled quarter samples, except for high-SCC cow 6, for which there was a single measurement from 2 quarter samples
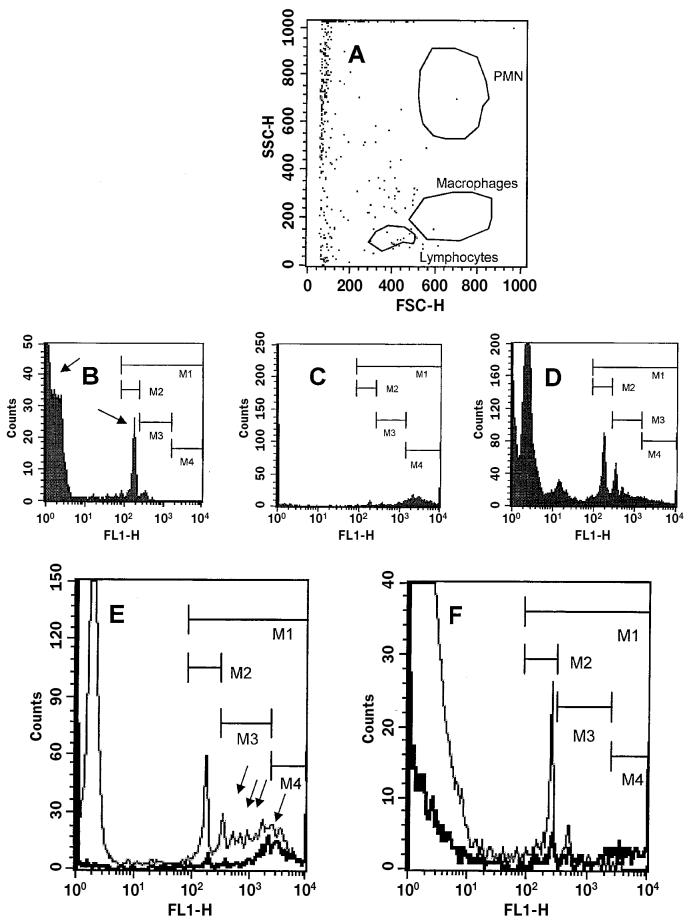
The side and forward light-scatter values of the fluorescent microspheres minimally overlapped with those of leukocytes and were close to the origin of the forward-scatter scale, which indicated uniformity in size. In contrast, dispersion along the side-scatter scale revealed major variability in bead density (Figure 1A).
Figure 1.
Assessment by flow cytometry of mammary-gland cell capacity to phagocytize fluorescent microspheres in cows with a low or high somatic cell concentration (SCC) in milk. A, forward (FSC-H) and side (SSC-H) light-scatter values for microspheres (dots) and leukocytes (polygons). B, fluorescence histogram of nonviable and viable cells, showing that nonviable cells (arrow pointing left) display high counts but very low fluorescence (2 orders of magnitude lower than cells showing a distinct peak in the center of the plot); the arrow pointing right indicates the threshold above which < 5% lymphocytes are observed, the cut-off point above which phagocytosis-related fluorescence is measured. C, fluorescence intensity of microspheres not exposed to cells. D, fluorescence intensity of all cells after incubation with fluorescent microspheres; < 2% of cells are under the region denoted as M4 and thus indicate false-positive fluorescence (the upper limit of phagocytosis-specific fluorescence). E, F, representative examples of fluorescence intensity profiles of ungated cells of cows with low (E) and high (F) SCCs after incubation with fluorescent microspheres, showing only 1 spike under M2 (cell subset of low phagocytic [LP] ability) and at least 4 spikes (17.0% of all cells) under M3 (cell subset of high phagocytic [HP] ability) for the low-SCC cow (E), and only 1 peak (0.2% of all cells) under M3 for the high-SCC cow (F). The background fluorescence of microspheres alone is overlaid (darker line), displaying lower counts than those for the low-SCC cow but higher counts than those for the high-SCC cow (lighter line). The data for the low-SCC cow show more HP cells (5.7% under M4, above background, E) than the data for the high-SCC cow (0.01% under M4, below background, F). PMN — polymorphonuclear cells; FL1-H — fluorescence height or intensity (relative units).
The fluorescence intensity associated with lymphocytes (Figure 1B) led to objective identification of the cut-off point above which fluorescence specifically associated with phagocytosis could be measured: the point at which 5% or fewer lymphocytes were found (under M1). Some microspheres not exposed to cells (Figure 1C) were able to attach, even after washing, and displayed very intense fluorescence (under M4) that was considered false-positive fluorescence, or the upper limit of phagocytosis-specific fluorescence. When preparations containing phagocytes exposed to fluorescent microspheres were analyzed (Figure 1D), several fluorescence peaks were observed. After exclusion of fluorescence values below the lymphocyte-related, negative cut-off point (below M1) and above the microsphere-related, false-positive cut-off point (above M4), phagocytosis-specific fluorescence was expressed as 2 major peaks (within M2 and M3), which indicates that bovine phagocytes can have 2 subpopulations of different phagocytic ability: low (under M2) and high (under M3). Representative examples of phagocyte subpopulations in cows with low and high SCCs are shown in Figures 1E and 1F, respectively. For low-SCC cows, there were several fluorescence peaks (under M3), characterizing HP cells. For high-SCC cows, there was a single peak, representing LP cells, and no peak (or only marginally noticeable peaks) representing HP cells.
Cells from all mammary-quarter samples were analyzed with the same instrument settings (voltage and detectors) and the same threshold (channel 90). Four types of cells were evaluated: all leukocytes (observations conducted without identification of individual cell types, or “ungated” measurements), lymphocytes only, macrophages only, and PMNs only.
The median HP/LP ratios were 2 to 4 times greater for the low-SCC cows than for the high-SCC cows (P < 0.004), whether ungated or gated measurements were analyzed (Table IB).
Significantly higher total numbers of viable mononuclear cells (both lymphocytes and macrophages), but not of PMNs, were observed per sample (approximately 500 μL) from high-SCC cows compared with low-SCC cows (P < 0.01, Mann–Whitney test). The difference in median number of viable macrophages was greater (2.91 times: 2285 for the high-SCC cows versus 783 for the low- SCC cows, P < 0.05) than the difference in HP/LP ratio (2.16 times greater for the low-SCC than the high-SCC cows), suggesting that the increased numbers of macrophages at least partially compensated for the functional deficit in PMN HP/LP ratio in the high-SCC cows (although the macrophage HP/LP ratio was lower for high-SCC cows than for low-SCC cows). Furthermore, the difference in the total number of viable leukocytes (2.5 to 3 times greater in high-SCC cows than in low-SCC cows, depending on whether mammary quarters or cows were regarded as the unit of analysis) seemed to be much less than the difference in SCC (at least 34 times greater for high-SCC cows than for low-SCC cows). This suggests that cell viability is much poorer in high-SCC cows than in low-SCC cows.
On the basis of mammary-quarter analysis, low-SCC cows may be differentiated from high-SCC cows by nonoverlapping HP/LP ratio intervals. When all phagocytes were considered, the lower limit of the HP/LP interval for low-SCC cows (1.04) was higher (with 95% confidence) than the upper limit for high-SCC cows (0.88). Expressed as the log HP/LP ratio, animals with values below 0.039 may be expected, with 95% confidence, to have poor phagocytic ability per cell and to have a high SCC. Cow-based analysis displayed similar results (Figures 2A to 2C).
Figure 2.
Relationship between the HP/LP ratio and the SCC for all cells and subsets in low-SCC and high-SCC cows and overall. Boxplots display the median, lower, and upper values (central, lower, and upper horizontal lines) of individual cows (3 per group). A, all cells (ungated observations). B, macrophages. C, PMNs. D, line (and 95% confidence interval [CI]) of the HP/LP ratio (all phagocytes) regressed on the SCC for 6 cows.
When mammary-gland quarters were the unit of analysis, a negative association was found between the log HP/LP ratio and the log SCC (P ≤ 0.01, Table IIA). When the cow was the unit of analysis, the macrophage HP/LP ratio explained 72% of the SCC variablity (P < 0.03, Table IIB). However, all phagocytes explained 63% of the SCC variability (P < 0.04, Figure 2D). Therefore, measurement of all cells (ungated observations) is not worse than measurement of specific phagocytes (macrophages or PMNs).
Table II.
Relationships between the somatic cell concentration (SCC) and the HP/LP ratio
| A. Unit of analysis: pooled or nonpooled samples of mammary-quarter mik (n = 11) | ||||
|---|---|---|---|---|
| Response | HP/LP predictor | Regression equation | R2 (adjusted) | P-value |
| SCC | All cells | log SCC = 4.194 – 1.982 log HP/LP | 50.1 | 0.009 |
| SCC | MOs | log SCC = 1.94 – 3.53 log HP/LP | 54.5 | 0.009 |
| SCC | PMNs | log SCC = 5.42 – 1.25 log HP/LP | 44.5 | 0.01 |
| B. Unit of analysis: cow (n = 6) | ||||
| Response | HP/LP predictor | Regression equation | R2 (adjusted) | P-value |
| SCC | All cells | SCC = 333 – 629 log HP/LP | 63.1 | 0.036 |
| SCC | MOs | SCC = − 282 – 891 log HP/LP | 72.1 | 0.02 |
| SCC | PMNs | SCC = 815 – 471 log HP/LP | 65.8 | 0.031 |
HP — high phagocytic ability; LP — low phagocytic ability; MOs — macrophages; PMNs — polymorphonuclear cells
In agreement with previous reports (8), multiple fluorescence peaks were displayed (in low-SCC cows) by HP cells. Cells from high-SCC cows seemed to phagocytize only 1 bead at most. It cannot be ruled out that the LP peak represents passive bead attachment. Only HP cells can be regarded as truly functioning phagocytes (those displayed under M3 in Figure 1D). Because bovine phagocytes display a relatively intense autofluorescence that may prevent the identification of negative and positive cells (9), the lack of phagocytic ability typical of lymphocytes was used to exclude from the analysis the fluorescence associated with these cells. Because microsphere fabrication conditions may result in particle heterogeneity (10), as observed in this study in relation to particle density (Figure 1A), the very bright fluorescence exhibited by beads alone was excluded from the analysis as false-positive fluorescence. Measurement of ungated cells resulted in a faster procedure and probably an even more valid one since separate cell gating (one for each cell type) is prone to induce additional sources of error. Hence, we conclude that measurement of ungated phagocytes (after evaluation of microsphere fluorescence range and identification of lymphocyte nonspecific fluorescence) provides a rapid and reliable procedure for estimating the phagocytic ability of bovine mammary-gland cells.
The results indicated that decreased phagocytic ability was related to higher numbers of cells (expressed as a higher SCC). Such an association suggests a compensatory mechanism. Although not studied in adult cattle before, differences in phagocyte counts and phagocytic ability have been reported for young cattle, whose phagocytes have poorer phagocytic ability but compensate for this deficit with larger numbers (11). The mechanism is physiologic in young animals, but decreased phagocytic ability per cell that is not compensated has been reported in human chronic granulomatous disease, in which both recurrent infection and sterile inflammation may occur (12).
Whether considering mammary quarters or cows as the unit of analysis, this study has confirmed the presence of milk phagocytes of different phagocytic ability (4), in uninfected cows of a different herd and environment. Evaluation of the phagocytic ability of bovine milk phagocytes may elucidate whether high SCC is caused by abnormal phagocytosis or other factors. Because high SCC is undesirable even in the absence of infection (2,3), monitoring of phagocyte subpopulations may optimize dairy health and management- related decision-making. To that extent, longitudinal studies are recommended.
Acknowledgments
This study was funded by the New York State Department of Agriculture and Markets and the Department of Population Health & Pathobiology, College of Veterinary Medicine, NCSU. The cooperation and assistance of the staff and management of the Dairy Educational Unit of NCSU was appreciated.
References
- 1.Mayer E. The role of the veterinary profession and its adaptation to the challenges of ameliorating bovine productivity in the southern hemisphere on the dawn of the third millenium. In: Proceedings of the XVI World Buiatrics Congress, Salvador, Bahia, Brazil, 1990:2–24.
- 2.Labohm R, Gotz E, Luhofer G, Hess RG, Bostedt H. Factors influencing the somatic milk-cell-count in dairy cows. 1. Influence of bacteriological findings, stage and number of lactation. Milchwissenschaft-Milk Sci Int. 1998;53:63–66. [Google Scholar]
- 3.Middleton JR, Hardin D, Steevens B, Randle R, Tyler JW. Use of somatic cell counts and California mastitis test results from individual quarter milk samples to detect subclinical intra-mammary infection in dairy cattle from a herd with a high bulk tank somatic cell count. J Am Vet Med Assoc. 2004;224:419–423. doi: 10.2460/javma.2004.224.419. [DOI] [PubMed] [Google Scholar]
- 4.Rivas AL, Tadevosyan R, Quimby FW, Coksaygan T, Lein DH. Identification of subpopulations of bovine mammary-gland phagocytes and evaluation of sensitivity and specificity of morphologic and functional indicators of bovine mastitis. Can J Vet Res. 2002;66:165–172. [PMC free article] [PubMed] [Google Scholar]
- 5.Green MJ, Burton PR, Green LE, et al. The use of Markov chain Monte Carlo for analysis of correlated binary data: patterns of somatic cells in milk and the risk of clinical mastitis in dairy cows. Prev Vet Med. 2004;64:157–174. doi: 10.1016/j.prevetmed.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Rivas AL, Quimby FW, Blue J, Coksaygan O. Longitudinal evaluation of bovine mammary gland health status by somatic cell counting, flow cytometry, and cytology. J Vet Diagn Invest. 2001;13:399–407. doi: 10.1177/104063870101300506. [DOI] [PubMed] [Google Scholar]
- 7.National Mastitis Council Writing Committee. Laboratory Handbook on Bovine Mastitis. Revised ed. Madison, Wisconsin: National Mastitis Council, 1999.
- 8.Steinkamp JA, Valdez YE, Lehnert BE. Flow cytometric, phase-resolved fluorescence measurement of propidium iodide uptake in macrophages containing phagocytized fluorescent microspheres. Cytometry. 2000;39:45–55. doi: 10.1002/(sici)1097-0320(20000101)39:1<45::aid-cyto7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Kampen AH, Tollersrud T, Larsen S, Roth JA, Frank DE, Lund A. Repeatibility of flow cytometric and classical measurement of phagocytosis and respiratory burst in bovine polymorphonuclear leukocytes. Vet Immunol Immunopathol. 2004;97:105–114. doi: 10.1016/j.vetimm.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Berkland C, Kim K, Pack DW. PLG microsphere size controls drug release rate through several competing factors. Pharm Res. 2003;20:1055–1062. doi: 10.1023/a:1024466407849. [DOI] [PubMed] [Google Scholar]
- 11.Menge C, Neufeld B, Hirt W, et al. Compensation of preliminary blood phagocyte immaturity in the newborn calf. Vet Immunol Immunopathol. 1998;62:309–321. doi: 10.1016/s0165-2427(98)00109-3. [DOI] [PubMed] [Google Scholar]
- 12.Brown JR, Goldblatt D, Buddle J, Morton L, Thrasher AJ. Diminished production of anti-inflammatory mediators during neutrophil apoptosis and macrophage phagocytosis in chronic granulomatous disease (CGD) J Leukoc Biol. 2003;73:591–599. doi: 10.1189/jlb.1202599. [DOI] [PubMed] [Google Scholar]