One strategy that has commonly been used to gain a more detailed understanding of ion chemistry in bulk solution is to investigate the structure and energetics of solvated ions in the gas phase. While solvation of singly charged metal ions has been studied extensively during the last two decades,1 significantly less information about doubly and triply charged metal ions is known. Solvated divalent metal ions can now be readily produced using electrospray ionization (ESI).2–5 This has led to a significant interest in obtaining thermochemical information about these ions with both experiment3–5 and theory.6–8 Kebarle and co-workers measured the Gibbs free energies of hydration for water molecules located in the second solvation shell of a variety of divalent metal ions using equilibrium experiments.3 More recently, Posey and co-workers combined ESI with laser photofragmentation mass spectrometry to study divalent transition metal–ligand complexes with methanol in the second solvation shell.4 Binding energies of inner solvent shell water molecules around Ni2+ and Ca2+ ions have been determined from blackbody infrared radiative dissociation (BIRD) experiments.5 Here, BIRD kinetics of the hexahydrated alkaline earth metal ions, Mg2+, Ca2+, Sr2+, and Ba2+, are presented. At low temperatures, binding energies obtained from these kinetic data are directly correlated with the radii of the metal ions (Mg2+ > Ca2+ > Sr2+ > Ba2+). In contrast, the binding energies at higher temperatures follow the trend Ca2+ > Mg2+ > Sr2+ > Ba2+. This is the first direct evidence for two distinct gas-phase structures for a hydrated divalent metal ion.
Experiments were performed using an external electrospray ionization source Fourier transform mass spectrometer that has been described previously.9 Hydrated alkaline earth M2+(H2O)n ions were generated from ~10−4 M aqueous solutions of the metal chloride salts using nanoelectrospray. Ions are loaded into the cell for 5 s during which time N2 gas (10−6 Torr) is introduced. Dissociation kinetics of the mass-selected ion are investigated at pressures <10−8 Torr. At these pressures, ion activation occurs by absorption of blackbody photons emitted by the heated vacuum chamber walls.9–11 Unimolecular dissociation rate constants in the zero-pressure limit are measured as a function of temperature (22–140 °C) from which Arrhenius activation parameters are obtained. Under these conditions, the ion population does not have a Boltzmann distribution of internal energies.10 Measured Arrhenius preexponentials and activation energies are smaller than those measured in the rapid energy exchange (REX) limit. To determine the threshold dissociation energies, master equation modeling of the kinetic data is necessary.12 The application of the master equation model to obtain threshold dissociation energies has been described in detail previously.11 The reverse activation barriers for these dissociation reactions should be negligible. Thus, the threshold dissociation energy should be equal to the binding energy.
For all metal ion hydrates, dissociation occurs exclusively by loss of one water molecule. The kinetics are first order at all temperatures. Figure 1 shows the Arrhenius plots for dissociation of M2+(H2O)6 and M2+(H2O)7. For the heptahydrated clusters, the dissociation rate constants follow the trend Ba2+ > Sr2+ > Ca2+ > Mg2+. This trend is consistent with the expected reactivity based on the ionic radii of the metals (water molecules attached to larger metal ions are expected to be less strongly bound). For the hexahydrated ions, this same ordering of rate constants is observed at low temperatures. In striking contrast, the order is Ba2+ > Sr2+ > Mg2+ > Ca2+ at higher temperatures; the slope of the Arrhenius plot for Mg2+(H2O)6 changes around 80°C! BIRD rate constants for the pentahydrated species have the same trend as that observed for the hexahydrated species at high temperature (Ba2+ > Sr2+ > Mg2+ > Ca2+).13 Thus, these dissociation kinetics do not follow the trend expected based on ionic radii. The change in the slope of the Arrhenius plot for Mg2+-(H2O)6 shows the presence of two distinct gas-phase structuresfor this ion. The first-order behavior (>80% precursor ion depletion) at all temperatures indicates the presence of one structure or several structures that interconvert.
Figure 1.
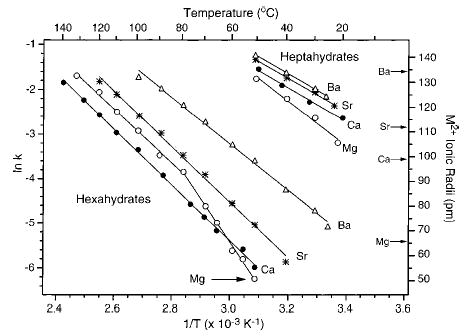
Arrhenius plots obtained from blackbody infrared radiative dissociation data of the hexa- and heptahydrated alkaline earth metal ions; Mg2+ (○), Ca2+ (•), Sr2+ (*), and Ba2+ (▵).
The measured zero-pressure limit Arrhenius parameters and the binding energies (Eo) obtained from master equation modeling of the kinetic data are given in Table 1. The low-temperature Eo’s consistently increase with decreasing cation size. At high temperatures, the binding energy of the sixth water molecule to Mg2+ (21.1 kcal/mol) is less than that at low temperatures (23.5 kcal/mol). It is also slightly less than that for the larger calcium cation (21.6 kcal/mol). For Mg2+(H2O)6, anomalously large radiative rate constants were used in the modeling in order to fit the kinetic data at low temperature. The reason for this is not clear, but this suggests that the error in Eo at low temperature may be larger than reported. The unexpected ordering of binding energies at high temperature is consistent with ΔH values recently reported by Kebarle and co-workers from equilibrium experiments at 170–225°C.3a Recent density functional theory (DFT) calculations by Pavlov et al.8 have been used to evaluate structures for hydrated divalent magnesium and calcium ions, M2+(H2O)m-(H2O)n, where m and n correspond to the number of water molecules in the first and second shell, respectively. The successive water binding energies (ΔEbinding) reported for these structures are also included in Table 1.
Table 1.
Measured Zero-Pressure Limit Arrhenius Parameters and EO Values Obtained from Master Equation Modeling for Loss of Water from M2+(H2O)6 Ionsa
| M | Ea (kcal/mol)b | log Ab | Eo (kcal/mol)c | ΔEbinding (kcal/mol)d |
|---|---|---|---|---|
| Mg | 19.3 ± 0.6 | 10.3 ± 0.4 | 23.5 ± 1.6 | 24.5,e 20.8,f |
| 12.3 ± 0.4 | 5.7 ± 0.2 | 21.1 ± 0.8 | 19.6,g 23.7h | |
| Ca | 12.4 ± 0.1 | 5.8 ± 0.1 | 21.6 ± 0.8 | 24.7,e 18.5,f 18.8,g 21.6h |
| Sr | 12.4 ± 0.3 | 6.1 ± 0.2 | 20.6 ± 1.0 | |
| Ba | 10.3 ± 0.3 | 5.4 ± 0.2 | 17.9 ± 0.6 |
Previously reported B3LYP calculations of successive binding energies are also given.
Reported errors are obtained from linear least squares analysis of the Arrhenius data.
Reported errors indicate both experimental error as well as the range of parameters used in the modeling.
Reference 8.
M2+(H2O)6.
M2+(H2O)5(H2O) where the water in the second shell is H-bonded to one water in the inner shell.
M2+(H2O)5(H2O) where the water in the second shell is H-bonded to two different waters in the first shell.
M2+(H2O)4(H2O)2.
To further investigate this unexpected reactivity of Mg2+(H2O)6, we also performed DFT calculations on all four hexahydrated divalent metals.14 Three different types of structures were studied in which the six water molecules are either all in the first shell or distributed between the first and second solvation shells around the metals. By the use of the nomenclature of Pavlov et al., the structures investigated were M2+(H2O)6, M2+(H2O)5(H2O),15 and M2+(H2O)4(H2O)2. Calculations indicate that the order of stability is M2+(H2O)6 > M2+(H2O)5(H2O) > M2+(H2O)4(H2O)2 for all of the metals. The energy differences between each of the higher energy structures and the M2+(H2O)6 conformation are reported in Table 2. Also included in the table are the values of Pavlov et al.8 for magnesium and calcium. At this level of theory, all three hexahydrated Mg2+ structures have energies within 5 kcal/mol. Several similar energy structures for hexahydrated barium ions were also found. However, no evidence of multiple gas-phase hexahydrated barium structures was observed over the temperature range of these experiments
Table 2.
B3LYP Energy Differences between M2+(H2O)5(H2O) and M2+(H2O)4(H2O)2 and the Most Stable M2+(H2O)6 Structurea
| structure | ΔE(elec. + ZPE)b (kcal/mol) | ΔEtotalc (kcal/mol) |
|---|---|---|
| Mg2+(H2O)5(H2O)d | 2.3 | 4.9 |
| Mg2+(H2O)4(H2O)2 | 4.8 | 4.4 |
| Ca2+(H2O)5(H2O)d | 4.2 | 5.9 |
| Ca2+(H2O)4(H2O)2 | 9.6 | 8.7 |
| Sr2+(H2O)5(H2O)d | 3.2 | |
| Sr2+(H2O)4(H2O)2 | 7.5 | |
| Ba2+(H2O)5(H2O)d | 1.6 | |
| Ba2+(H2O)4(H2O)2 | 3.9 |
Previously reported values for Mg2+ and Ca2+ are also shown.
This work;
Values from Pavlov and co-workers (ref 8).
Values reported for structures in which the second shell water molecule is hydrogen-bonded to two inner-shell water molecules.
Although the experimental evidence for two distinct structures for Mg2+(H2O)6 is clear, the exact nature of these isomers is not. One possibility is that the isomers have different number of water molecules in the first and second shell. The results of DFT calculations show that all three hexahydrated Mg2+ structures investigated have 0 K energies within 5 kcal/mol (Table 2). The entropies of Mg2+(H2O)5(H2O) and Mg 2+(H2O)4(H2O)2 under standard conditions are higher than that of Mg2+(H2O)6. The opposite is true for the Ca2+, Sr2+, and Ba2+ hexahydrates. The anomalous entropy for Mg2+(H2O)6 is presumably due to the crowding of the six water molecules around the much smaller metal ion. This would suggest that the higher temperature structure corresponds to a two-shell structure. Another possible explanation is that one of the isomers is a salt-bridge structure (Scheme 1). Such a salt-bridge structure was found to be stable at the semiempirical PM3 level of theory, but not with the density functional levels of theory applied. The proton-transfer process necessary for the formation of a salt-bridge is expected to occur more readily for hydrated Mg than for the larger metal hydrates.3b
Scheme 1.
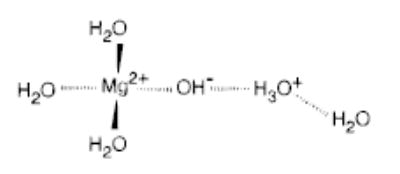
In conclusion, evidence for two distinct isomeric structures of Mg2+(H2O)6 is presented. This is the first experimental evidence for two isomeric structures of hydrated metal dications. Binding energies of water to Mg2+(H2O)7 and Mg2+(H2O)6 (low temperature) follow the expected trend in cation size. These values for Mg2+(H2O)6 (high temperature) do not. Calculations suggest that the most stable structure for M2+(H2O)6 is one in which all of the water molecules are in the inner shell. However, further experiments and higher level calculations are necessary to definitively elucidate these structures.
Acknowledgments
The authors thank Dr. Maria Pavlov (University of Stockholm) for providing the coordinates for Mg2+(H2O)n and Ca2+-(H2O)n. We also acknowledge the financial support provided by the National Science Foundation (CHE-9726183) and the National Institutes of Health (IR29GM50336-01A2, and fellowship support for R.A.J.).
References
- 1.a Castleman AW, Jr, Bowen KH., Jr J Phys Chem. 1996;100:12911–12944. and references therein. [Google Scholar]; b Keesee RG, Castleman AW., Jr J Phys Chem Ref Data. 1986;15:1011–1071. [Google Scholar]; c Dzidic I, Kebarle P. J Phys Chem. 1970;74:1466–1474. [Google Scholar]; d El-Shall MS, Schriver KE, Whetten RL, Meot-Ner (Mautner) M. J Phys Chem. 1989;93:7969–7973. [Google Scholar]; e Dalleska NF, Honma K, Sunderlin LS, Armentrout PB. J Am Chem Soc. 1994;116:3519–3528. [Google Scholar]; f Berg C, Achatz U, Beyer M, Joos S, Albert G, Schindler T, Niedner-Schatteburg G, Bondybey VE. Int J Mass Spectrom Ion Processes. 1997;167/168:723–734. [Google Scholar]
- 2.Schmelzeisen-Redeker G, Bütfering L, Röllgen FW. Int J Mass Spectrom Ion Processes. 1989;90:139–150. [Google Scholar]
- 3.a Peschke M, Blades AT, Kebarle P. J Phys Chem A. 1998;102:9978–9985. [Google Scholar]; b Blades AT, Jayaweera P, Ikonomou MG, Kebarle P. J Chem Phys. 1990;92:5900–5906. [Google Scholar]
- 4.Spence TG, Burns TD, Guckenberger VGB, Posey LA. J Phys Chem A. 1997;101:1081–1092. [Google Scholar]
- 5.Rodriguez-Cruz SE, Jockusch RA, Williams ER. J Am Chem Soc. 1998;120:5842–5843. doi: 10.1021/ja980716i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a Katz AK, Glusker JP, Beebe SA, Bock CW. J Am Chem Soc. 1996;118:5752–5763. [Google Scholar]; b Markham GD, Glusker JP, Bock CL, Trachtman M, Bock CW. J Phys Chem. 1996;100:3488–3497. [Google Scholar]
- 7.Glendening ED, Feller D. J Phys Chem. 1996;100:4790–4797. [Google Scholar]
- 8.Pavlov M, Siegbahn PEM, Sandström M. J Phys Chem A. 1998;102:219–228. [Google Scholar]
- 9.Price WD, Schnier PD, Williams ER. Anal Chem. 1996;68:859–866. doi: 10.1021/ac951038a. [DOI] [PubMed] [Google Scholar]
- 10.a Price WD, Schnier PD, Jockusch RA, Strittmatter EF, Williams ER. J Am Chem Soc. 1996;118:10640–10644. doi: 10.1021/ja961812r. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Dunbar RC, McMahon TB, Tholmann D, Tonner DS, Salahub DR, Wei D. J Am Chem Soc. 1995;117:12819–12825. [Google Scholar]
- 11.a Price WD, Schnier PD, Williams ER. J Phys Chem B. 1997;101:664–673. doi: 10.1021/jp9628702. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jockusch RA, Williams ER. J Phys Chem A. 1998;102:4543–4550. doi: 10.1021/jp980264w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.For this modeling, vibrational frequencies and transition dipole moments for the Mg2+, Ca2+, and Sr2+ systems were obtained at the RHF/STO-3G level and at the B3LYP/LANL2DZ level for Ba2+(H2O)6 Microcanonical radiative rate constants are calculated using these values and varied over a 9-fold range to account for errors in the calculated values. For Mg2+-(H2O)6, a 16-fold range was required to fit the data at low temperature. Dissociation rate constants are obtained by using RRKM theory and adjusted to provide REX limit A-factors between 1014 and 1017.5 s−1 to take into account a range of transition state entropies.
- 13.Manuscript under preparation.
- 14.Relative energies for the hexahydrated metal ions were calculated using the hybrid density functional method B3LYP. Geometry optimizations were done using the double-ζ basis set LACVP**, which incorporates an effective core potential for Ca, Sr, and Ba (Hay, P. J.; Wadt, W. R. J. Chem. Phys. 1985, 82, 299–310) and uses 6-31G** for Mg, O, and H. Single-point energy calculations were done at the optimized geometries replacing the basis sets on Mg, O, and H with the larger 6-311G++(2d,2p) basis set. RHF frequency analyses confirmed that each structure is an energy minimum. Zero-point vibrational energies were calculated by scaling the RHF frequencies by 0.91. All of the calculations were performed using Jaguar v. 3.0 software (Schrodinger Inc., Portland, OR, 1997).
- 15.Two different M2+(H2O)5(H2O) structures were studied; one in which the water in the second shell is hydrogen-bonded to one water in the inner shell, the other in which the outer water is hydrogen-bonded to two inner-shell water molecules. The latter structure is 4–6 kcal/mol more stable for all four hexahydrated metals.


