Abstract
Avian influenza (H5N1) has recently been recognized as a new emerging infectious disease that may pose a threat to international public health. Most recent developments lead to the belief that H5N1 could become the cause of the next influenza pandemic. This review discusses the characteristics of H5N1 avian influenza virus as an emerging infectious disease with the potential for pandemic development. In addition, the current pandemic influenza alert status and guidelines for pandemic preparedness, treatment, and prevention are discussed.
Recent events such as the increase in cases of avian influenza in Southeast Asia and the cases of avian influenza in humans bring an important concern to public attention: infectious diseases cross species barriers and can lead to pandemics (1, 2). The same problem was brought to our attention during the outbreak of severe acute respiratory syndrome (SARS) in 2002–2003 (3, 4). Many infectious diseases are known to cross species barriers, and generally many of these infections occur because humans come into contact with an organism that is already capable of causing human infection. In other cases, a change in the host range of the microorganism occurs, making the infection of humans more effective. The majority of these infections are considered zoonotic, meaning that the usual host for the organism is a nonhuman vertebrate animal. However, most emerging infectious diseases are initially considered “spillovers” from the established animal reservoir. Many of these infections are also called “dead-end” infections because they die out eventually when effective human-to-human transmission cannot be established. However, these observations are often made retrospectively after the disappearance of a disease that temporarily caused human infections.
From a historical perspective, the assignment of “usual” hosts in some zoonoses is rather arbitrary, and the organism is found in several vertebrate species. In most cases, humans come into contact with the animal itself, animal excreta, or animal parts (e.g., feathers, meat), or an insect is the vector of transmission of the infective microorganism. Classic examples of these forms of disease transmission are Lyme disease (Borrelia burgdorferi), plague (Yersinia pestis), hantavirus, and rabies virus.
When cases of human monkeypox occurred in the USA in 2003–2004, the cases were linked to the close contact between humans and the infected animals (prairie dogs and other pet animals) (5). In Africa where the disease is endemic, outbreaks of monkeypox in humans are primarily associated with the hunting, skinning, preparing, and eating of infected rodents and monkeys (6). Person-to-person transmission in the African outbreaks has only occasionally been documented. When it occurred, transmission of the virus rarely extended beyond three or four transmission cycles. Ongoing research and vaccine development, as well as prohibitions on the import of exotic animals, should prevent another outbreak of monkeypox in the USA. Concerns have since been raised that monkeypox could potentially be used as a weapon for bioterrorists, and the outbreak in 2003 remains a constant reminder to health care providers to stay alert to the possibility of a new or re-emerging infectious disease. However, the likelihood that monkeypox will be a substantial bioweapon in the hands of terrorists remains low.
The outbreak of SARS coronavirus (SARS-CoV), on the other hand, represented a much greater threat to public health than monkeypox. Strong evidence exists that this new coronavirus was present in China in a population of animals, including the Himalayan palm civet (7). It appears that the virus broadened its host range by minimal molecular changes and then became capable of infecting humans. The concerning feature in SARS was that the virus, in addition to having a broadened host range, was capable of human-to-human transmission. SARS-CoV was isolated from sputum samples, nasal secretions, bronchial washings, serum samples, and fecal samples (8). Several cases were reported to have occurred in health care workers involved in high-risk aerosol- and droplet-generating procedures, such as airway manipulation, bronchoscopy, and intubation. Some of these cases occurred despite the use of personal protective equipment (8). Updated infection control guidelines are available from the Centers for Disease Control and Prevention (CDC) at http://www.cdc.gov/ncidod/sars/ic.htm.
Epidemiological evidence from SARS outbreaks in China, Taiwan, and Toronto suggested that transmission of the virus did not occur before the onset of symptoms or after resolution of symptoms. One study documented the shedding of SARS-CoV in stool for up to 64 hours after the resolution of symptoms, using reverse transcriptase–polymerase chain reaction for identification of viral RNA (8). However, later epidemiologic studies concluded that in general viral transmission in humans is somewhat inefficient, and newer seroprevalence studies suggest that SARS-CoV no longer circulates within the human population (9). Nevertheless, the possibility of clinically silent infections or of long-term virus carriers has not been fully excluded (4). According to several expert panels, the re-emergence of SARS-CoV with epidemic or pandemic potential is a low possibility at the present time.
These initial considerations regarding infectious diseases with potential to cross species barriers lead to yet another disease of concern: avian influenza. In the last few years, a highly pathogenic subtype of avian influenza viruses, hpH5N1, has swept through poultry populations across Asia and now parts of Europe. These outbreaks are historically unprecedented in scale and are geographically spread (Figure). From December 2003 to mid July 2005, outbreaks of avian influenza (H5N1) in poultry occurred in nine countries(Cambodia, China, Indonesia, Japan, the Republic of Korea, the Lao People's Democratic Republic, Malaysia, Thailand, and Vietnam). Since late July 2005, outbreaks in domestic poultry as well as wild birds have been reported in the Russian Federation, Kazakhstan, and Mongolia. On October 13, 2005, tests conducted by the World Organization for Animal Health confirmed the presence of hpH5N1 avian influenza virus in samples taken from domestic birds in Turkey and Romania (10, 11). In addition, this virus has repeatedly jumped the species barrier and infected 125 humans as of mid November 2005, with cases occurring in Cambodia, Vietnam, Indonesia, and Thailand. Sixty-four of these cases were fatal (12). This trend of global spread of the disease, together with the increase in human cases of H5N1 avian influenza, raises strong public concern about the next steps the disease will take in evolving into a new influenza pandemic.
Figure.
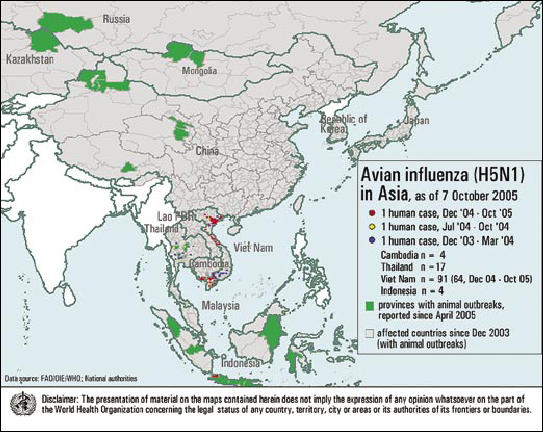
Avian influenza (H5N1) in Asia. Courtesy of the World Health Organization.
To improve understanding of the impact avian influenza may have on the public health infrastructure and the health care system, three aspects of influenza infection are discussed briefly in this article: What is the nature of the disease? What is the risk for a pandemic, and will it persist? What can be done to prevent or counteract disease outbreaks?
BIOLOGY AND NATURE OF AVIAN INFLUENZA
The family Orthomyxoviridae contains four genera, as defined by the Seventh Committee for the Taxonomy of Viruses: influenza A, B, and C viruses and thogavirus (sometimes referred to as influenza D virus). Influenza type A viruses can infect people, birds, pigs, horses, seals, whales, and other animals, but wild birds are the natural hosts for these viruses. Several subtypes of influenza A viruses exist and are classified based upon the proteins on the viral surface, specifically the hemagglutinin (HA or H) and the neuraminidase (NA or N) (13, 14). Sixteen different HA subtypes and 9 different NA subtypes have been described for influenza A, and many different combinations of HA and NA proteins are possible. However, only three major influenza A subtypes (i.e., H1N1, H1N2, and H3N2) are currently circulating in the human population. The HA and NA viral envelope proteins, as well as the M2 protein, are the medically most relevant proteins, since they are essential viral proteins that are targeted by host antibodies or antiviral drugs such as oseltamivir and rimantadine (2).
Influenza B viruses are normally found only in humans and are not further classified according to subtypes. On some occasions, influenza B viruses have caused human epidemics, but they have never been described as a cause of pandemics. Influenza C viruses cause only mild illness in humans and do not cause epidemics or pandemics.
The various subtypes of influenza A and influenza B viruses are further subclassified into different strains, which change seasonally. Humans can be infected by influenza viruses of all three types, but only influenza A viruses infect birds. Most of these viruses cause no significant illness in wild birds, which are typically the natural host for all subtypes of influenza A virus. However, domestic poultry, such as chickens and turkeys, develop a severe illness and can even die from influenza.
Avian influenza (H5N1) is a type A influenza virus. Many subtypes of avian influenza A have been described; some of the more recent isolates were H7 and H5 viruses. In addition, avian influenza viruses H5 and H7 can be further classified as “low pathogenicity” (lp, LPAI) and “high pathogenicity” (hp, HPAI) based on genetic features of the viral genome and the severity of illness they cause in poultry. HPAI is usually associated with high mortality (90%–100%) in poultry, but at this time it is not clear how the distinction of high and low pathogenicity relates to human disease.
Avian influenza viruses do not infect humans on a regular basis, and all influenza viruses usually show some receptor specificity for their hosts, i.e., birds and mammals. This receptor specificity of influenza viruses has been defined in terms of their recognition of sialic acid species and the type of glycosidic linkage between sialic acid and penultimate galactose in the host cell membranes (15). When avian influenza viruses cross species barriers and subsequently become infectious to humans, this is commonly believed to occur via an intermediate host, e.g., the pig. A change in viral envelope protein structure, together with the interaction between these viral surface proteins/ receptors and the cell surface receptors of mammalian cells, enables the virus to become infective for the new host. However, controversy exists within the scientific community about the existence and necessity of such an intermediate host in the transmission cycle of influenza viruses. In the past, several avian influenza viruses were able to directly infect humans. The human infections caused by the avian influenza hpH5N1 subtype in Hong Kong in 1997 also demonstrated a capability of direct human infection without passing through an intermediate host.
Influenza viruses can change in two ways. One type of change, “antigenic drift,” occurs through small changes in the virus that happen continually over time. Antigenic drift produces new virus strains that may not be recognized by antibodies to earlier influenza strains. The other type of change, “antigenic shift,” is an abrupt, major change in the influenza A viruses& hemagglutinin protein or hemagglutinin and neuraminidase protein combination that has not been seen in humans for many years.
PANDEMIC INFLUENZA
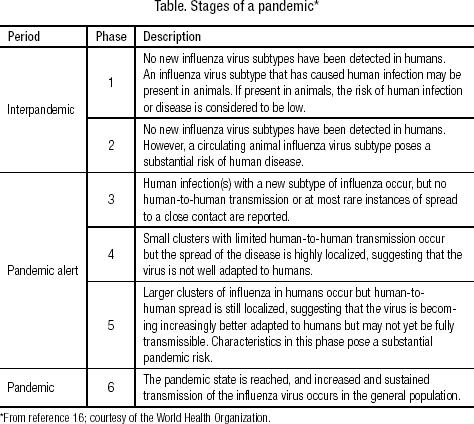
Pandemic influenza A viruses emerge as a result of antigenic shift. The appearance of a “new” influenza virus is the first step toward a pandemic. The stages in the development of pandemics are outlined in the Table (16). Three major influenza A pandemics were recorded during the 20th century: the 1918–1919 “Spanish flu” representing the H1N1 influenza virus, the 1957–1958 “Asian flu” (H2N2), and the 1968–1969 “Hong Kong flu” (H3N2) (17). The 1918–1919 pandemic caused the highest number of casualties: more than 500,000 people died in the USA, and more than 50 million people died worldwide. The “Asian flu” in 1957–1958 caused about 70,000 deaths in the USA, and the “Hong Kong flu” in 1968–1969 caused about 34,000 deaths. No prediction models exist to forecast the number of deaths for a future pandemic of avian influenza.
Table.
Stages of a pandemic*
It should also be mentioned that subtypes of avian influenza A other than H5N1 have sporadically caused human disease, and additional subtypes have caused disease in domestic birds (18). An outbreak of H7N3 avian influenza occurred in February 2004 in poultry in the Fraser Valley region of British Columbia, Canada. Culling operations and other efforts controlled the outbreak of the virus. During this period, two cases of laboratory-confirmed human infection with H7 influenza virus were reported: one person was involved in culling operations; the other person was a poultry worker. Both patients developed conjunctivitis and flulike symptoms, which resolved upon treatment with oseltamivir. In October 2003, a human case of H7N2 avian influenza was reported from New York. Poultry infections with H7N2 were also reported from Maryland, New Jersey, and Delaware in 2004. An outbreak of H5N2 avian influenza occurred in poultry in Texas in February 2004. No human cases were reported during this outbreak. Although it appears that the risk of human infection by these other subtypes of avian influenza viruses is low, it must be considered that human infection is generally a possibility.
PREVENTING AND COUNTERACTING DISEASE OUTBREAKS
The CDC has published interim guidelines to help limit the possibility of human infections during outbreaks of avian influenza in domestic birds and poultry in the USA. Human influenza is thought to transmit primarily via large respiratory droplets; treatment for epidemic human influenza A rests on annual vaccination campaigns, antiviral treatment, and standard contact precautions plus droplet precautions, which are recommended for the care of patients infected with human influenza. However, given the uncertainty about the exact modes by which avian influenza may first transmit between humans, additional precautions for health care workers involved in the care of patients with documented or suspected avian influenza may be prudent. The rationale for the use of additional precautions for avian influenza as compared with human influenza includes the following (19):
It is possible that avian influenza H5N1 (HPAI) carries a higher risk of serious disease and increased mortality compared with known forms of human influenza A.
It is a current concern that avian H5N1 viruses are becoming more infectious for humans, thus facilitating infection in a greater number of people and resulting in more clusters.
It could also be possible that the avian H5N1 viruses might eventually become capable of effective human-to-human transmission.
These concerns, which are widely shared among public health officials, are based on risk assessments conducted by the World Health Organization (WHO) and CDC. Based on these risk analyses, the CDC issued interim recommendations for the treatment of patients with suspected avian influenza. All patients coming to a hospital or emergency room with fever and respiratory symptoms should be managed according to the recommendations for respiratory hygiene and cough etiquette. Information about their most recent travel history will help in establishing the possibility of an avian influenza case. The CDC recommends that all patients with such symptoms and a history of travel to a country where avian influenza activity has been reported should be managed using isolation precautions similar to those recommended during the SARS epidemic. These measures include standard precautions (i.e., careful hand hygiene and awareness of the infectious potential of patients and material they came into contact with), contact precautions (i.e., gowns, gloves, etc.), and eye protection (i.e., goggles and face shields) (19). In addition, patients with suspected avian influenza should be placed in an airborne isolation room with negative air pressure and 6 to 12 air changes per hour. The exhaust air should be vented directly to the outside, or if recirculation is necessary, a high-efficiency particulate air (HEPA) filter should be used. Personnel directly involved in the care of such patients should wear a fit-tested respirator (e.g., N-95 air filter). Additional information regarding these recommendations is available at the websites of the CDC and the WHO (16, 19). Other measures that may control an outbreak include vaccination of health care workers and good surveillance and monitoring of health care workers.
SUMMARY AND CONCLUSIONS
Experts at the WHO believe that the world has now come closer to another influenza pandemic than at any other time since 1968 (16). The WHO expert panel considers the current pandemic alert to be phase 3 (Table) (16). Evidence shows that avian influenza hpH5N1 is now epidemic in parts of Asia and has recently been identified in some European countries. Several human cases of avian influenza have occurred in Asian countries. Therefore, the initial requirements for the development toward a pandemic have been established except for larger clusters of human disease and, most important, efficient human-to-human transmission. However, each human infection represents an opportunity for avian influenza to gain the ability to effectively infect humans, with possible consecutive human-to-human transmission. In addition, some ominous changes have been observed in the disease in animals, and outbreaks have occurred despite aggressive control measures including the culling of more than 140 million poultry. Wild migratory birds, historically the reservoir of avian influenza A viruses, are now dying in large numbers from hpH5N1 influenza virus, while domestic ducks do not show significant signs of the disease and play a more silent role in maintaining the transmission of the virus by shedding large quantities of it.
Because a pandemic threat cannot be predicted, a sensitive early warning system is necessary to detect changes in the behavior of the virus. Improving pandemic surveillance additionally requires more research in secure laboratory settings to investigate viral reassortment, biologic behavior, and transmissibility. In addition, vaccines and antiviral drugs are the most important medical interventions for reducing morbidity and mortality during a pandemic. But neither will be available in adequate supply in any country at the beginning of a pandemic. Antiviral drugs will assume an especially critical role in the management of patients because of the initial short supply of vaccines. In addition, nonpharmaceutical measures will have to be considered in all countries to reduce morbidity, mortality, and social disruption.
The above descriptions of two diseases, SARS and avian influenza, illustrate the epidemiologic significance of the transmission of infectious agents from animals to humans. This transmissibility depends on several factors, such as the density of the infected animal hosts (e.g., poultry), the frequency with which humans come into contact with infected animals, the susceptibility of the human host to the virus, and the biology of the virus itself, including the efficacy of human-to-human transmission. In Asia, many of these prerequisites for pandemic development have been met: large numbers of poultry remain infected with hpH5N1 influenza A virus, close contacts between susceptible humans and infected animals occur frequently, and increasing numbers of human infections have recently been reported. Even if human-to-human transmission has not been conclusively identified at this point, we can anticipate additional human cases, and the risk of a more efficient human-to-human transmission of the virus remains a possibility. In conclusion, the possibility that hpH5N1 avian influenza could become the next pandemic subtype of influenza A virus is real. Given the constantly changing nature of influenza viruses, the exact timing and severity of the next pandemic cannot be predicted.
Furthermore, three other influenza A viruses have caused pandemics in the past 100 years and continue to circulate in the human population, causing yearly, smaller epidemics. With the focus on the possibility of a pandemic caused by H5N1 avian influenza, we should not forget the burden of illness caused by these other influenza A viruses already known to cause significant human infections, which have considerable annual morbidity and mortality rates. As we consider a future in which we must be concerned with increasing numbers of emerging infectious diseases, it is imperative that we augment existing public health and research capabilities. The global and domestic surveillance of infectious diseases by organizations like the WHO or the CDC has been remarkable and has provided us with successful tools to prepare for and contain disease outbreaks. However, as the current developments of the hpH5N1 avian influenza epidemic illustrate, these disease surveillance and control efforts must be increased to safeguard public health as well as to improve animal welfare. Although the disease will continue to break the species barrier, the ultimate goal of all disease surveillance, research, and development is the avoidance of a major pandemic of H5N1 avian influenza.
Comment
Data presented in this article reflect the status quo as of November 14, 2005, the date it was submitted with final revisions to Baylor University Medical Center Proceedings and accepted for publication. Changes in epidemiology and the course of the disease may occur between the time of submission and publication. Interested readers are encouraged to review more recent publications from the WHO and the CDC when assessing the risk of an avian influenza outbreak/pandemic.
References
- 1.Department of Communicable Disease Surveillance and Response, Global Influenza Program, World Health Organization. WHO intercountry-consultation: influenza A/H5N1 in humans in Asia, Manila, Philippines, 6–7 May 2005 [publication no. WHO/CDS/CSR/GIP/2005.7]. Geneva: WHO, 2005. Available at http://www.who.int/entity/csr/resources/publications/influenza/WHO_CDS_CSR_GIP_2005_7/en/; accessed October 14, 2005.
- 2.The World Health Organization Global Influenza Program Surveillance Network Evolution of H5N1 avian influenza viruses in Asia. Emerg Infect Dis. 2005;11(10):1515–1521. doi: 10.3201/eid1110.050644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, Ling AE, Humphrey CD, Shieh WJ, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang JY, Cox N, Hughes JM, LeDuc JW, Bellini WJ, Anderson LJ, SARS Working Group A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 4.Berger A, Drosten CH, Doerr HW, Stuermer M, Preiser W. Severe acute respiratory syndrome (SARS)—paradigm of an emerging viral infection. J Clin Virol. 2004;29(1):13–22. doi: 10.1016/j.jcv.2003.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis. 2004;15(4):280–287. doi: 10.1053/j.spid.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reed KD, Melski JW, Graham MB, Regnery RL, Sotir MJ, Wegner MV, Kazmierczak JJ, Stratman EJ, Li Y, Fairley JA, Swain GR, Olson VA, Sargent EK, Kehl SC, Frace MA, Kline R, Foldy SL, Davis JP, Damon IK. The detection of monkeypox in humans in the Western hemisphere. N Engl J Med. 2004;350(4):342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 7.Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, Luo SW, Li PH, Zhang LJ, Guan YJ, Butt KM, Wong KL, Chan KW, Lim W, Shortridge KF, Yuen KY, Peiris JS, Poon LL. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302(5643):276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 8.Christian MD, Poutanen SM, Loutfy MR, Muller MP, Low DE. Severe acute respiratory syndrome. Clin Infect Dis. 2004;38(10):1420–1427. doi: 10.1086/420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massad E, Burattini MN, Lopez LF, Coutinho FAB. Forecasting versus projection models in epidemiology: the case of the SARS epidemics. Med Hypotheses. 2005;65(1):17–22. doi: 10.1016/j.mehy.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith J. Deadly Asian bird flu reaches fringes of Europe. Reuters.com, October 13, 2005. Available at http://today.reuters.com/news/newsArticle.aspx?type=topNewsstory=2005-10-13T184411Z_01_ROB340139_ RTRUKOC_0_US-BIRDFLU.xml; accessed October 14, 2005.
- 11.Department of Communicable Disease Surveillance and Response, Global Influenza Program, World Health Organization. Avian influenza—new areas with infection in birds—update 34. Geneva: WHO, October 13, 2005. Available at http://www.who.int/csr/don/2005_10_13/en/print.html; accessed October 14, 2005.
- 12.World Health Organization, Epidemic and Pandemic Alert and Response. Cumulative number of confirmed human cases of avian influenza A/ (H5N1)reported to WHO. Geneva: WHO, November 9, 2005. Available at http://www.who.int/csr/disease/avian_influenza/country/cases_table_2005_11_9/en/index.html; accessed November 13, 2005.
- 13.Lamb RA, Krug RM. Orthomyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fundamental Virology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 725–769. [Google Scholar]
- 14.Fouchier RAM, Munster V, Wallensten A, Bestebroer TM, Herfst S, Smith D, Rimmelzwaan GF, Olsen B, Osterhaus ADME. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol. 2005;79(5):2814–2822. doi: 10.1128/JVI.79.5.2814-2822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gambaryan A, Yamnikova S, Lvov D, Tuzikov A, Chinarev A, Pazynina G, Webster R, Matrosovich M, Bovin N. Receptor specificity of influenza viruses from birds and mammals: new data on involvement of the inner fragments of the carbohydrate chain. Virology. 2005;334(2):276–283. doi: 10.1016/j.virol.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Department of Communicable Disease Surveillance and Response, Global Influenza Program, World Health Organization. WHO global influenza preparedness plans: the role of WHO and recommendations for national measures before and during pandemics [publication no. WHO/CDS/CSR/ GIP/2005.5]. Geneva: WHO, 2005. Available at http://www.who.int/csr/resources/publications/influenza/WHO_CDS_CSR_GIP_2005_5.pdf; accessed October 14, 2005.
- 17.Centers for Disease Control and Prevention (CDC). Avian influenza (bird flu): information about influenza pandemics. Atlanta, GA: CDC, October 17, 2005. Available at http://www.cdc.gov/flu/avian/gen-info/pandemics.htm; accessed October 17, 2005.
- 18.Centers for Disease Control and Prevention (CDC). Avian influenza (bird flu): Outbreaks in North America. Atlanta, GA: CDC, October 17, 2005. Available at http://www.cdc.gov/flu/avian/outbreaks/us.htm; accessed October 17, 2005.
- 19.Centers for Disease Control and Prevention (CDC). Interim recommendations for infection control in health-care facilities caring for patients with known or suspected avian influenza. Atlanta, GA: CDC, May 21, 2004. Available at http://www.cdc.gov/flu/avian/professional/infect-control.htm; accessed October 14, 2005.


