Abstract
Nonsynaptic release of ATP from electrically stimulated dorsal root gangion (DRG) axons inhibits Schwann cell (SC) proliferation and arrests SC development at the premyelinating stage, but the specific types of purinergic receptor(s) and intracellular signaling pathways involved in this form of neuron–glia communication are not known. Recent research shows that adenosine is a neuron–glial transmitter between axons and myelinating glia of the CNS. The present study investigates the possibility that adenosine might have a similar function in communicating between axons and premyelinating SCs. Using a combination of pharmacological and molecular approaches, we found that mouse SCs in culture express functional adenosine receptors and ATP receptors, a far more complex array of purinergic receptors than thought previously. Adenosine, but not ATP, activates ERK/MAPK through stimulation of cAMP-linked A2A adenosine receptors. Both ATP and adenosine inhibit proliferation of SCs induced by platelet-derived growth factor (PDGF), via mechanisms that are partly independent. In contrast to ATP, adenosine failed to inhibit the differentiation of SCs to the O4+ stage. This indicates that, in addition to ATP, adenosine is an activity-dependent signaling molecule between axons and premyelinating Schwann cells, but that electrical activity, acting through adenosine, has opposite effects on the differentiation of myelinating glia in the PNS and CNS.
Keywords: Purinergic receptor, Schwann-cell development, cell proliferation, ERK/MAPK, adenosine, ATP, neuron-glia interactions, myelination
INTRODUCTION
It is becoming increasingly clear that neuronal activity can have a significant influence on development and myelination of glial cells (Fields and Stevens-Graham, 2002; Fields and Stevens, 2000), making it important to determine the molecular mechanisms for this communication. Extracellular ATP has been identified as an important, activity-dependent axonal signal that inhibits the proliferation and differentiation of SCs, the myelinating glia in the PNS (Stevens and Fields, 2000). ATP released from electrically stimulated sensory axons induces calcium transients, CREB phosphorylation and gene expression in associated SCs (Stevens and Fields, 2000), but the specific types of purinergic receptor(s), and intracellular signaling pathways involved in this form of neuron–glia communication are not known. Moreover, ATP can be degraded to adenosine by extracellular ectonucleotidases (Zimmermann et al., 1998), and adenosine has been shown recently to mediate activity-dependent communication between axons and oligodendrocyte progenitor cells (Stevens et al., 2002). Adenosine receptors in premyelinating SCs have not been identified, and the effects of adenosine on SC proliferation and development have not been studied previously.
Evidence indicates that glia in the CNS express a complex array of purinergic receptors (Fields and Stevens, 2000). Astrocytes and microglia express several subtypes of adenosine and ATP receptors, often in the same cell (King et al., 1996; Neary et al., 1998). A similar picture is beginning to emerge for cells of the oligodendrocyte lineage (Stevens et al., 2002), however it is not known which types of ATP receptors are expressed in developing SCs, and whether developing, premyelinating SCs express functional adenosine receptors.
There are many types of ATP receptors, which can be categorized broadly into two families: the ligand-gated ionotropic (P2X) receptors; and the G-protein-coupled metabotropic (P2Y) ATP receptors (Ralevic and Burnstock, 1998). Hydrolysis of extracellular ATP by ectonucleotidases can generate adenosine (Zimmermann, 2000), which, subsequently, can activate four subtypes of metabotropic adenosine (P1) receptors that differ, in part, by intracellular signaling through changes in either intracellular calcium or cAMP (Klotz, 2000; Ralevic and Burnstock, 1998).
The ERK/MAPK cascade, which is involved in regulating cell proliferation and differentiation, can be activated by either P1 or P2 purinergic receptors through subsequent changes in either intracellular cAMP or calcium, but this varies in different types of cells. The functional consequences of ERK activation on cell proliferation are highly dependent on the cell type and biological context with respect to developmental stage and other factors that regulate cell development and proliferation. ERK signaling has been shown to mediate mitogenic signaling by ATP (P2) purinergic receptors in human and rodent astrocytes. Activation of P2 receptors stimulates astrocyte proliferation (Franke et al., 1999; Neary et al., 1999; Neary et al., 1998), both in culture (Abbracchio et al., 1994; Neary et al., 1994; Rathbone et al., 1992) and in vivo (Franke et al., 1999; Franke et al., 2001). This P2 receptor-mediated mitogenic signaling is independent of calcium and dependent on MEK, an upstream activator of ERK/MAPK (Lenz et al., 2001; Neary et al., 1999). Interestingly, in contrast to astrocytes, extracel-lular ATP inhibits Schwann cell proliferation (Stevens and Fields, 2000). Although the mechanisms controlling the anti-proliferative response to ATP in SCs are unknown, differences in specific purinergic receptor subtypes and ERK/MAPK signaling might underlie the disparate functional effects in response to extracellular ATP in Schwann cells and astrocytes.
OBJECTIVE
The present study uses a co-culture system of mouse DRG neurons and SCs in dishes equipped with stimulating electrodes to determine which purinergic receptors are present on premyelinating SCs and test whether adenosine contributes to activity-dependent communication between axons and premyelinating SCs. Differences in the intracellular signaling pathways activated by ATP and adenosine in premyelinated SCs will be explored, with particular emphasis on the ERK/MAPK signaling pathway and the effects on SC proliferation and differentiation.
MATERIALS AND METHODS
Cell culture
DRG neurons were obtained from embryonic day 13.5 mice and plated at a density of 0.35×106 cells ml−1 into the side compartments of multicompartment chambers. DRG cultures were maintained in medium containing 5% horse serum supplemented with 50 ng ml−1 nerve growth factor (NGF) according to previously published methods (Fields et al., 1990). SCs, obtained from the sciatic nerve of postnatal mice (P2) were cultured and purified using the Brockes method as described previously (Stevens et al., 1998), with the following modifications. SCs were cultured on poly-L-Lysine coated dishes in medium containing 5% horse serum. The following day, cultures were treated with 10−5 M cytosine arabinoside to prevent proliferation of non-neuronal cells. Contaminating fibroblasts were eliminated by complement-mediated lysis during passaging with antibody to Thy1.1 one week later, and purified SCs were used in experiments within 1 week. Purified SCs were maintained in medium containing 5% horse serum, without exogenous growth factors and mitogens until passaged and replated onto collagen-coated dishes/coverslips, or co-cultured with DRG neurons. Premyelinated SCs in coculture and monoculture were cultured for 48–72 hours in DRG growth medium without NGF and incubated overnight in serum-free medium before all experiments.
A2A-receptor-knockout mice
Generation of mice with a targeted disruption of the gene for A2A receptor have been described in detail previously (Chen et al., 1999). The mice used in this study were derived from heterozygous breeding pairs and bred on a c57BL/6 background.
Chemicals
ATP, adenosine, 2-(methylthio)-adenosine diphosphate (2Me-SADP), 2-(methylthio)-adenosine triphosphate (2MeSATP), αβ-methylene adenosine 5'-triphosphate (αβ-meATP), 2'-3'-o-(4-benzoyybenzoyl)-adenosine 5'-triphosphate (BzATP), uridine-5' triphosphate (UTP), apyrase were purchased from Sigma. NECA, CGS21680, CCPA, IBMECA, ZM241385, MRS2179 were purchased from Tocris Cookson Inc.
Electrical stimulation
Neurons were dissociated from the DRG of fetal mice and cultured for 3 weeks in multicompartment chambers equipped for electrical stimulation (Fields and Nelson, 1992). Axons grew into the central compartment by passing beneath high-electrical resistance barriers separating two side compartments. This allowed electrical stimulation of DRG neurons (10 Hz) from a custom-made, multi-channel stimulator. The stimulus was monitored continually by an oscilliscope and by light emitting diodes in series with stimulating electrodes in each dish. Up to 24 cultures could be stimulated simultaneously inside the incubator. Electrophysiological recording in DRG neurons labeled with DiI show that only those neurons with axons traversing the barrier are stimulated to fire action potentials (Li et al., 1996).
Calcium imaging
Intracellular Ca2+ was monitored in SCs cultured 48–72 hours on Poly-L-Lysine+ collagen-coated glass coverslips. Confocal microscopy (BioRad MRC 1024) with the calcium-sensitive indicator fluo-3 (Molecular Probes) was used to measure changes in fluorescence intensity (ΔF/Fo) caused by calcium transients in SCs in response to purinergic agonists. Solutions were applied locally through a multi-barrel pipette using electronically controlled valves (Harvard Apparatus). Measurements were carried out at room temperature in HEPES-buffered balanced salt solution, pH 7.2. A Nikon 40×, 0.7 n.a. long-working-distance lens was used for confocal imaging of cells grown on plastic dishes. A 40× Nikon, 1.3 n.a. lens was used on cells cultured on 0.17 nm-thick glass coverslips. Either scanning argon ion or krypton-argon lasers emitting at 488 nM were used for excitation and imaged with a pin-hole setting of 3.2–4.5 mm. The optical sectioning by confocal microscopy allowed us to distinguish calcium responses in SCs from responses in neurons and axons.
Immunocytochemistry
SC/DRG co-cultures were stimulated either electrically (10 Hz, 30 minutes) or SCs in monoculture stimulated pharmacologically (30 minutes) and fixed immediately with 1–4% paraform-aldehyde. Cells were permeabilized with 0.2% Triton X-100 and nonspecific peroxidases were blocked with 3% hydrogen peroxide, followed by 5% normal goat serum. Cultures were incubated with antibody against CREB phosphorylated at Ser133 (1:800, Cell Signaling), phosphorylated ERK1/2 MAPK (1:500, Cell Signaling) in 3% BSA/PBS overnight at 4°C. Cultures were then incubated with biotinylated goat anti-rabbit antibody (Vector Labs) and localized with the ABC method. The relative intensity of the stain was quantified by using image densitometry on a video microscope (Image-1, Universal Imaging). The expression of A2A receptors in mouse SCs was detected using a monoclonal antibody against the receptor (Santa Cruz) at 1:200 dilution and localized with Alexa 488-donkey anti-goat IgG (Molecular Probes) at 1:1500 dilution.
Experimental design and data analysis
The relative intensity of nuclear staining after immunocytochemistry was compared by imaging densitometry from multiple culture dishes representing controls and all relevant experimental treatments. Only DRG neurons that extend axons under the high-electrical resistance barriers into the central compartment are electrically stimulated (Li et al., 1996), therefore, imaging densitometry was performed in 10–15 random fields along the central barrier region of each side compartment (Fig. 3A). This quantification method takes into account the heterogeneity of MAPK staining in our cocultures because not all SCs are associated with electrically stimulated axons. Images were acquired from at least 15 randomly chosen fields in each culture using a Nuvicon video camera and digitized on an 8-bit scale for storage and optical densitometry using Image-1 software (Universal Imaging). Statistical analysis was based on the mean staining intensity of SC cytoplasm and nuclei in each dish, determined from measurements of all SCs in each field (thus, n=number of cultures). All values were normalized to the mean nuclear-staining intensity of control cultures in each experiment to allow pooling of replicate experiments [arbitrary O.D. units = (nuclear staining density/average staining density of control nuclei) × 100 – 100]. This yields a scale of 0–55 O.D. units from gray (unstimulated control) to pure black (Fields et al., 1997). The results are presented as mean ± S.E.M. and statistical comparisons evaluated by either ANOVA or two-sample t-test using the Minitab statistical analysis software (State College, PA).
Fig. 3. Action potentials phosphorylate ERK1/2 MAPK in SCs.
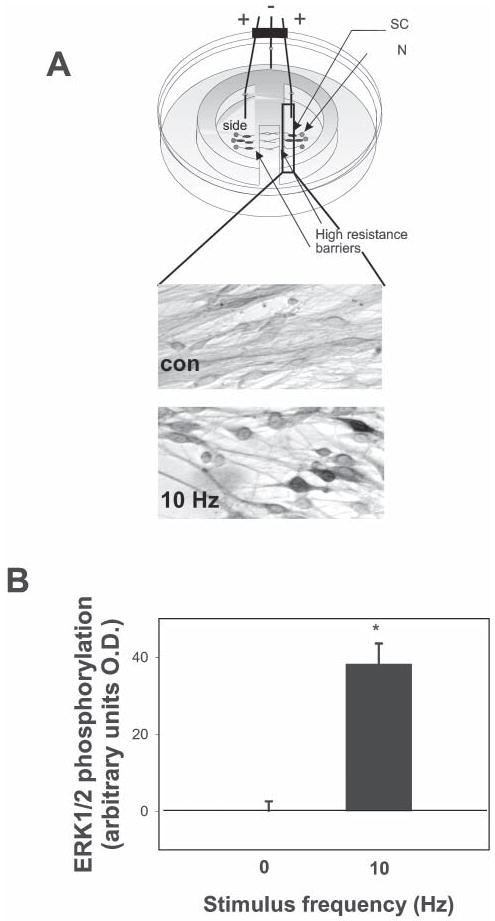
(A) SCs plated on DRG axons were stimulated at 10 Hz for 30 minutes in cultures equipped with platinum electrodes. Co-cultures were then incubated with a polyclonal antibody against phosphorylated MAPK (ERK1/2), and the mean staining intensity in both nuclei and cytoplasm quantified by image densitometry. Representative fields in control (Con) and stimulated dishes (10 Hz) are shown. Note the darkly stained, spindle-shaped SCs on axons that have been stimulated to fire action potentials. (B) The concentration of phospho-ERK1/2 was significantly increased in SCs cultured on axons stimulated at 10 Hz compared with unstimulated controls (*P<<0.0001, n=21).
RT-PCR
RNA was isolated using TRIzol (Invitrogen) from purifed SC monocultures and RT-PCR performed using 1 μg RNA in a Retroscript kit (Ambion). RT product (5 μl) was amplified using SuperTaq (Ambion) in 30 cycles of 94°C for 1 minute and 60°C for 1 minute, with 72°C for 7 minutes after the final cycle. Primers for mouse: A2a, 5'-CTCACGCAGAGTTCCATCTTC-3' and 5'-GAAGCAGTTGATGATGTGCAG-3' (500 bp); A2b, 5'-CAGACCCCCACCAACTACTTT-3' and 5'-TGTCAGAGGACAGCAGCTTTT-3' (396 bp). Products were resolved by electrophoresis on a 2% agarose gel.
Proliferation and TUNEL assay
After 48 hours in culture SCs were serum-starved for 18–24 hours before treatment with growth factors (either 10 ng ml−1 PDGF) either with or without selective purinergic agonists for 24 hours. Cultures were pulsed with BrdU (Boehringer Mannheim) for 6 hours, fixed and stained according to manufacturer instructions. Cultures were then counterstained with Hoechst nuclear stain (Molecular Probes) at a dilution of 1:2000 for 10 minutes. The proliferation rate was calculated as the ratio of BrdU: Hoechst-positive SC nuclei in each microscope field. All SC nuclei stained with BrdU and Hoechst were counted in each microscope field. Randomly chosen fields (10–15) were sampled to obtain a mean for each culture well; n=number of culture wells.
For the apoptosis assay, SCs were fixed with 4% paraform-aldehyde for 1 hour following treatment. Cells were stained for TUNEL according to manufacturer’s protocol (Roche), and the % of TUNEL-positive cells determined from the ratio of TUNEL: Hoechst-positive cells in each microscope field.
Differentiation assay
Differentiation was induced in 1-week-old DRG/SC cocultures by adding ascorbic acid (50 μg ml−1) in medium containing 5–10% horse serum either with or without purinergic agonists. Morphology changes were observed 3–4 days later and SCs stained with antibodies against the O4 antigen. Live cultures were incubated with monoclonal O4 antibody (1:10 for 1 hour) and antigens detected using a fluorescein-conjugated goat-anti-mouse IgM antibody (Jackson Immunoresearch). The 04 antibody was generously provided by Dr. Vittorio Gallo.
cAMP assay
Intracellular cAMP levels were measured using a nonacetylation competitive enzymeimmunoassay (Biotrak, Amersham Pharmacia). SCs (15 000–20 000 well−1) were cultured for 48–72 hours in collagen-coated, 96-well plates and serum (5% horse serum) removed from the culture medium 18–24 hours before assay. SCs were treated with adenosine receptor agonists (NECA, adenosine and CGS21690) (100 μM) for 30 minutes at 35°C. SCs were subsequently lysed for 10 minutes, and the cAMP assay performed according to the Biotrak kit protocol. The optical density for each sample and standard was calculated in replicate, and the mean concentration of intracellular cAMP determined from the standard curve (12.5–3200 fmol well−1).
RESULTS
Premyelinating mouse SCs express functional adenosine receptors
Because extracellular ATP can be hydrolyzed to adenosine by ectonucleotidases (Zimmermann and Braun, 1996; Zimmermann et al., 1998), we considered adenosine as a candidate activity-dependent axonal signal in communication with SCs. Timelapse, confocal, calcium imaging was used to determine whether premyelinating SCs in monoculture responded to adenosine receptor agonists. Consistent with published reports, adenosine (300 μM), selective agonists at A1 adenosine receptors (100 μM CCPA) and A3 adenosine receptors (100 μM IBMECA), and the general adenosine receptor agonist NECA (100 μM) failed to elicit calcium responses in SCs (data not shown). However, the A2A family of adenosine receptors signal through cAMP and are not coupled to PLC/IP3 (Fredholm et al., 2000; Schulte and Fredholm, 2003); thus, A2B receptors would not be detected in the calcium-imaging experiments described above. The A2B subclass of adenosine receptors are also positively coupled to cAMP, and can mediate PLC/IP3-dependent increases in intracellular calcium (Cai2+) in some cell types (Feoktistov and Biaggioni, 1995; Yakel et al., 1993). Using RT-PCR with specific primers for mouse A2A and A2B receptors, we detected adenosine receptor mRNA transcripts for both A2 receptor subtypes in mouse SCs in culture (Fig. 1A). The presence of A2A receptor protein in SCs was also detected immunocytochemically with an A2A receptor-specific monoclonal antibody (Fig. 6A).
Fig. 1. Pre-myelinating SCs express functional adenosine receptors.
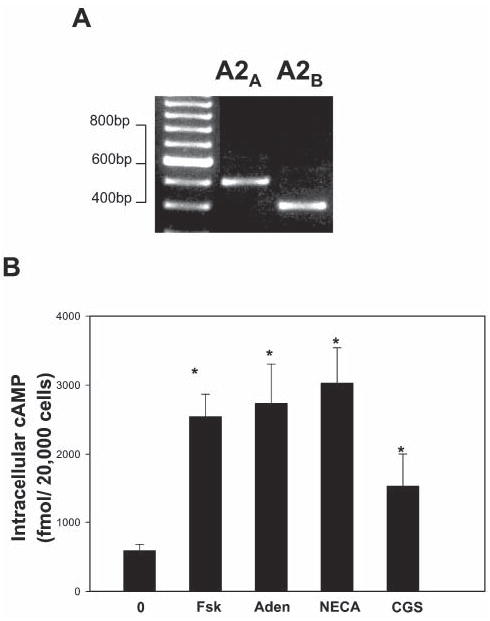
(A) mRNA encoding cAMP-dependent A2A and A2B receptors was detected by RT-PCR using specific adenosine receptor primers in SCs after 2 days in monoculture. (B) An enzyme immunoassay was used to monitor changes in intracellular cAMP in SCs in response to adenosine receptor agonists. Increases in cAMP were observed in SCs following a 30-minute treatment with 100 μM adenosine (Aden), the general adenosine receptor agonist NECA and the selective A2A receptor agonist CGS21680 (CGS). Forskolin (Fsk) (20 μM) was used as a positive control (P<0.003, ANOVA, n=30). *Significantly different from control.
Fig. 6. Adenosine-dependent activation of ERK1/2 in SCs is mediated by activation of A2A receptors.
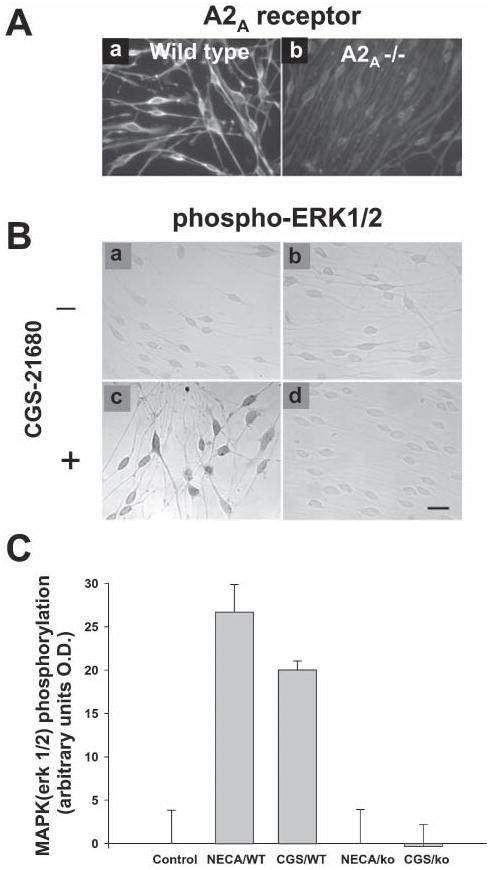
(A) Consistent with RT-PCR evidence (Fig. 1A), premyelinating SCs in culture express A2A receptor protein, determined by immunostaining with an antibody that is specific for the A2A receptor (a). This receptor is absent from SCs cultured from A2A−/− mice (Chen et al., 1999) (b). (Ba-d) SCs from wild-type and A2A−/− mice were treated for 30 minutes with the A2 receptor agonist CGS21680 (1 μM) and phospho-ERK1/2 levels were determined immunocytochemically. No response to the A2 adenosine receptor agonist CGS-21680 was observed in SCs cultured from A2A−/− mice (d) (scale bar; 20 μm). (C) Quantitative analysis shows that neither the nonspecific adenosine receptor agonist NECA (1 μM) (NECA/KO) nor the A2A-receptor selective agonist CGS21680 (1 μM) (CGS/KO) increased phospho-ERK1/2 levels in A2A−/− SCs compared with wild-type controls (NECA/WT and CGS/WT respectively) (P<0.001, ANOVA, n=30).
Consistent with these findings, activation of adenosine receptors in SCs resulted in significant increase in intracellular cAMP concentrations (Fig. 1B). Purified SCs were treated for 30 minutes with adenosine, the general P2 receptor agonist NECA and the A2A receptor-selective agonist CGS21680 (each at 100 μM), and cAMP accumulation was measured by competitive enzyme immunoassay. As shown in Fig. 1B, each agonist significantly increased cAMP levels in SCs compared with unstimulated controls. Together, these data are the first to show that SCs in vitro express functional adenosine receptors, and to implicate adenosine as a possible activity-dependent signal between neurons and premyelinating SCs.
Adenosine inhibits growth factor-induced SC proliferation
We previously reported that activity-dependent ATP release from axons significantly inhibits axon-stimulated SC proliferation (Stevens and Fields, 2000). Because of the finding that premyelinating SCs express cAMP-linked adenosine receptors, we investigated the effects of adenosine on proliferation of SCs to determine whether the inhibitory effects of ATP are due to breakdown to adenosine. Confirming our previous results (Stevens and Fields, 2000), direct application of ATP to SC monocultures inhibited growth factor-stimulated SC proliferation. Consistent with our previous interpretation, 2MeSATP, a non-degradable ATP receptor agonist, also inhibited SC proliferation. This indicates that SC proliferation is inhibited in part by activation of ATP (P2) receptors (Fig. 2A) and not necessarily by hydrolysis to adenosine.
Fig. 2. Agonists of ATP (P2) and adenosine (P1) receptors inhibited growth factor-mediated SC proliferation.
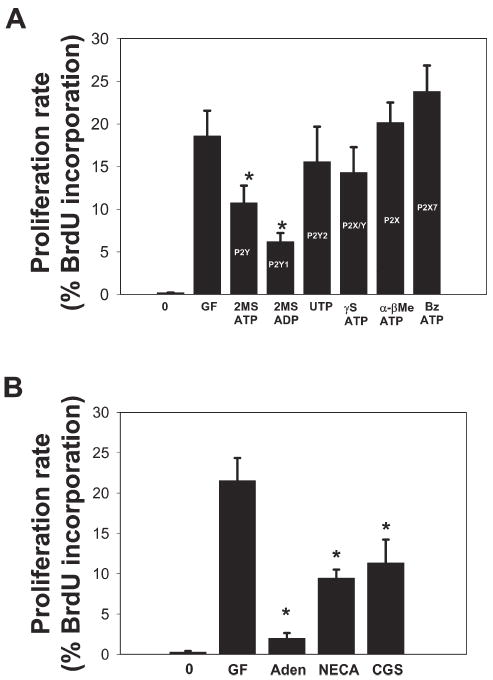
(A) Treatment of SC cultures for 24 hours with either the non-hydrolysable ATP (P2) receptor agonist 2MSATP or the selective P2Y1 receptor agonist 2MSADP, significantly inhibited PDGF-stimulated SC proliferation (control, 18.6 ± 2.1; 2MSADP, 6.2 ± 1.0; P<0.004, t-test, n=11). By contrast, agonists selective for P2X receptors (100 μM α-βMeATP and Bz ATP) and P2Y2/4 receptors (100 μM UTP) did not inhibit PDGF-induced SC proliferation. The P2 receptor subtype stimulated primarily by these agonists is indicated on each bar of the histogram. (B) Adenosine (Aden, 100 μM for 24 hours) inhibited PDGF (GF)-stimulated SC proliferation ~10fold. A similar inhibition was observed with the nonselective P2 receptor agonist NECA (100 μM) and the A2A receptor-selective agonist, CGS21680 (CGS, 100 nM) (P<0.001, ANOVA, n=30). *Significantly different from control.
To further identify the specific subtype of ATP receptor that inhibits SC proliferation, a series of ATP receptor-selective agonists were applied and SC proliferation quantified (Fig. 2A). This series of experiments showed that the inhibitory effect of ATP on SC proliferation was mimicked by the selective P2Y1 receptor agonist, 2MeSADP (control, 18.6 ± 2.1; 2MeSADP, 6.2 ± 1.0; P<0.004, t-test, n=11). By contrast, agonists selective for P2X receptors (αβmeATP and BzATP, 100 μM), and P2Y2/4 receptors (UTP, 100 μM) failed to inhibit SC proliferation (Fig. 2A).
To determine the effects of adenosine on SC proliferation, selective adenosine receptor agonists were applied to SC monocultures that had been serum starved for 24 hours. PDGF (10 ng ml−1) was applied 15 minutes after the addition of purinergic agonists to stimulate entry in to the cell cycle, and SC proliferation rates were determined 24 hours later using a BrdU-incorporation assay. As shown in Fig. 2B, pretreatment with adenosine receptor agonists significantly inhibited growth factor-stimulated SC proliferation. Adenosine (100 μM) inhibited SC proliferation ~10fold (control, 21.6 ± 2.8%; adenosine 2 ± 0.65%; t-test, n=6), as did the general adenosine receptor agonist NECA (100 μM) and the A2A-selective agonist CGS21680 (100 nM) (Fig. 2B). We found no evidence of cell death following treatment with adenosine agonists at concentrations that inhibited SC proliferation because the total number of SCs following the 24-hour period did not fall below unstimulated, control cultures. The absence of apoptosis was further confirmed using a TUNEL assay.
These findings demonstrate that purinergic signaling molecules can override the potent mitogenic effects of growth factors in developing SCs. Taken together, our results indicate that adenosine inhibits growth factor-induced SC proliferation through activation A2 adenosine receptors, and ATP does so through activation of P2Y1 receptors. Considering that A2 adenosine receptors act through cAMP and P2Y receptors act through intracellular calcium, it appears likely that these two axon-derived signaling molecules inhibit SC proliferation through partly independent intracellular signaling mechanisms.
Action potential-dependent activation of ERK1/2 MAPK is not mediated by ATP
Previously, we have reported evidence that the ERK/MAPK pathway is activated in premyelinated SCs by neural impulse activity (Stevens and Fields, 2000). To determine whether this is mediated by activation of either ATP receptors or adenosine receptors (or both), DRG neurons were stimulated electrically (10 Hz, 30 min) and phosphorylated ERK1/2 MAPK determined immunocytochemically in SCs cocultured with DRGs in a multicompartment chamber equipped with stimulating electrodes (Stevens and Fields, 2000; Stevens et al., 1998) (Fig. 3A). Electrical stimulation of DRG neurons (10 Hz, 30 minutes) significantly increased the concentrations of phospho-ERK1/2 MAPK in premyelinated SCs associated with electrically stimulated DRG axons compared with unstimulated controls (P<0.0001; n=21, t-test) (Fig. 3B).
Surprisingly, both ATP and the non-hydrolysable γSATP, failed to phosphorylate ERK/MAPK in SCs in monoculture over a wide range of concentrations. This is despite functional expression of both P2X and P2Y receptors in premyelinating SCs and the robust calcium responses generated in SCs stimulated with these agonists (Fig. 4A). As shown in Fig. 4B, the agonists UTP (100 μM), which is selective for P2Y2, P2Y4 and P2Y6 receptors, and αβMeATP, which is selective for P2X receptors, were also ineffective in phosphorylating ERK/MAPK in SCs. In fact, treatment of SC with the potent P2 receptor agonist, 2MeSATP, significantly inhibited ERK1/2 levels in SCs compared with unstimulated controls (P<0.001, t-test, n=15) (Fig. 4B). These findings indicate that ATP does not mediate activity-dependent ERK signaling under these conditions, and that another signaling molecule mediates this activity-dependent communication between axons and SCs.
Fig. 4. SCs express functional P2 receptors, but extracellular ATP does not mediate action potential-dependent activation of ERK1/2 in SCs.
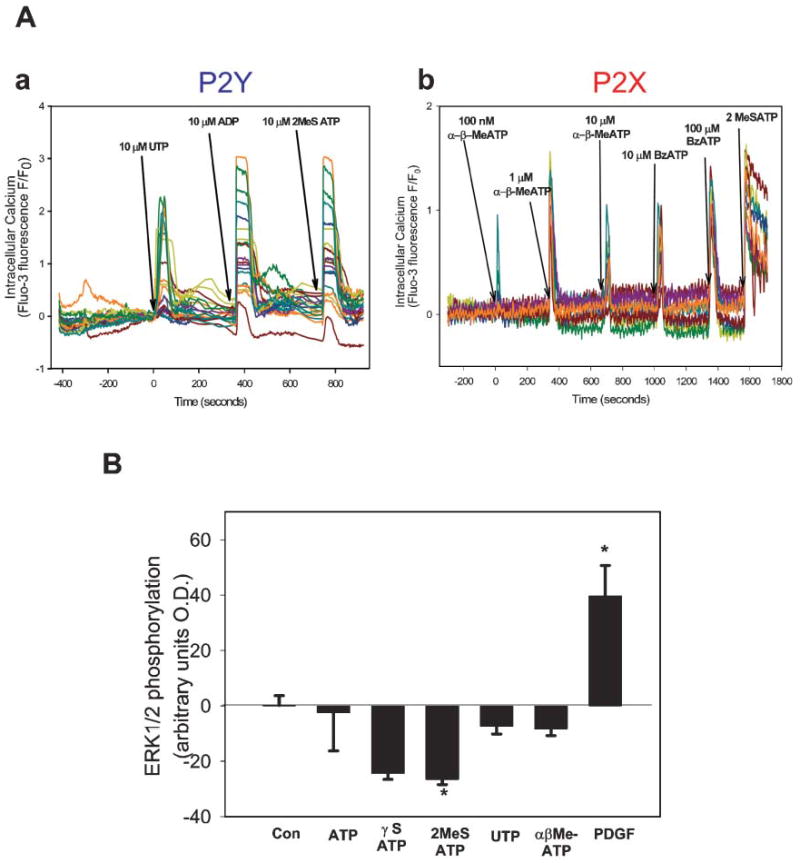
(A) Time-lapse confocal microscopy was used to monitor changes in the intracellular calcium concentration in SCs in monoculture, in response to the application of specific purinergic receptor agonists. One representative experiment is shown in each graph; each colored trace represents the calcium response in a different SC in the microscope field. (Aa) The presence of P2Y receptor subtypes in cultured SCs is revealed by robust responses to P2Y1 receptor agonists 2MeSADP (10 μM) and 2MeSATP (10 μM), and the P2Y2/4 agonist UTP (10 μM). (Ab) Calcium increases were also observed in response to the P2X agonists αβMeATP (a general P2X receptor agonist) at 100 nM–10 μM and BzATP (selective for P2X7 receptors) at 10 μM and 100 μM. All the SCs in this experiment also responded to the P2 receptor agonist 2MeSATP (100 μM), indicating the presence of both P1 and P2 receptors in many of the same cells. (B) Phosphorylation of ERK/MAPK was measured by immunocytochemistry in SCs in culture 30 minutes after treatment with P2 receptor agonists. ATP and a nonhydrolysable ATP agonist, γSATP failed to activate ERK/MAPK in SCs over a wide range of concentrations. Agonists selective for P2Y2,4,6 receptors (UTP, 10 μM) and P2X receptor subtypes (αβMeATP, 10 μM) were also ineffective. Treatment of SCs with the potent P2 receptor agonist 2MeSATP significantly inhibited phospho-ERK1/2 levels in SCs compared with unstimulated controls (P<0.001, ANOVA, n=31). *Significantly different from control.
Activity-dependent activation of A2 adenosine receptors phosphorylate ERK1/2 MAPK in SCs
To determine whether adenosine receptor activation phosphorylates ERK1/2 in SCs, agonists selective for A1 (CCPA), A2 (CGS21680) and A3 (IBMECA) receptors were applied to purified SC monocultures, and levels of phosphorylated ERK1/2 MAPK measured immunocytochemically, as described previously. The non-specific adenosine receptor agonist, NECA increased phospho-ERK1/2 levels in SCs, with maximal increases occurring at lower doses (1 μM) (Fig. 5). A similar increase in phosphorylated ERK/MAPK was induced by treatment with the more selective agonist CGS21680 (10–30 μM), which is specific for the A2A receptor (P<0.003, n=9) (Fig. 5A). By contrast, A1 and A3 receptor-selective agonists (10 μM CPA and 10 μM IBMECA, respectively), had no significant effects (P<0.005, n=19) (Fig. 5A).
Fig. 5. Activation of adenosine receptors phosphorylates ERK/MAPK in SCs.
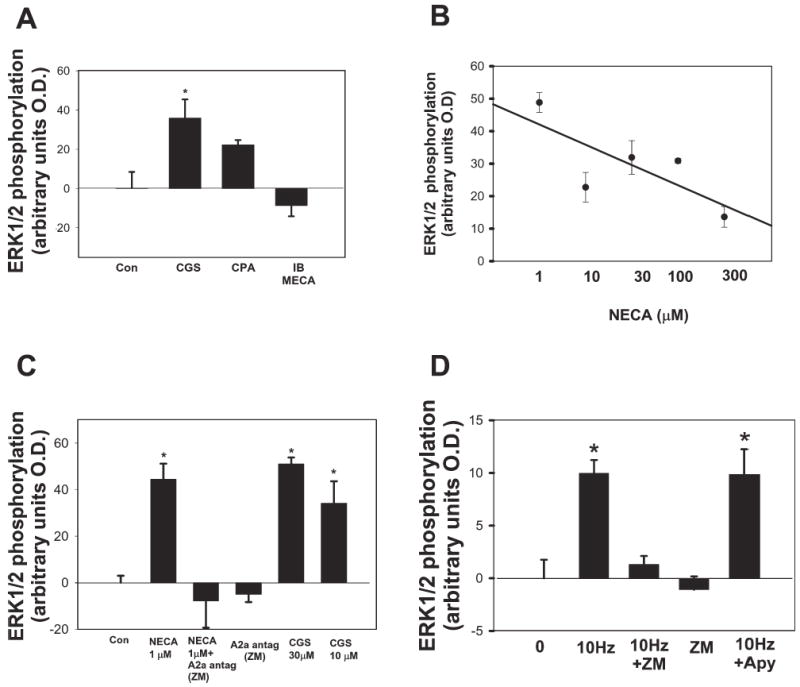
(A) The selective A2 receptor agonist CGS21680 (CGS, 10 μM for 30 minutes) significantly increased phospho-ERK1/2 in SCs (P<0.02, t-test, n=8). By contrast, selective A1 (10 μM CPA) and A3 (10 μM IBMECA) receptor agonists had no significant effect (P<0.005, ANOVA, n=19). (B) The nonselective adenosine receptor agonist, NECA, phosphorylated ERK/MAPK in SCs, with maximal activation at lower doses (1 μM). Higher concentrations of NECA (300 μM) had less affect on ERK/MAPK, possibly because of co-activation of other subtypes of purinergic receptors at higher concentrations of this general P2 receptor agonist. (C) Antagonists of specific purinergic receptor subtypes provide complementary results to the effects on ERK/MAPK phosphorylation when specific agonists applied to SCs and further identify the A2A receptor subtype that mediates the response of ERK/MAPK to adenosine. Pre-incubation of SCs in monoculture with the A2A receptor antagonist ZM241385 (ZM, 1 μM) completely blocked the NECA-stimulated (1 μM) increase in phospho-ERK1/2 in SCs. There was no significant effect on phospho-ERK1/2 levels in SCs following treatment with ZM241385 alone (P<0.0001, ANOVA, n=16). (D) Action potentials in DRG axons mediate phosphorylation of ERK/MAPK in SCs, primarily by activating P2 receptors. This is shown by pre-incubating DRG/SC co-cultures with the A2 receptor antagonist ZM241385 (ZM), and measuring phospho-ERK/MAPK levels in SCs after stimulating axons at 10 Hz for 30 minutes. Electrical stimulation in the presence of ZM241385 significantly inhibited the activity-dependent increase in phospho-ERK1/2 levels following electrical stimulation of DRG neurons (P<0.0001, 10 Hz vs 10 Hz + ZM; n=15, t-test). Treatment of cocultures with ZM241385 alone did not inhibit basal phospho-ERK/MAPK levels. In contrast to ZM241385, electrical stimulation of axons in the presence of apyrase (Apy) (27 U ml−1), which degrades extracellular ATP, failed to block the activity-dependent phosphorylation of ERK/MAPK in SCs (P<0.0001, n=33, ANOVA). This indicates that P2 receptor activation is not necessary for activation of the ERK/MAPK pathway in SCs in response to action potentials. *Significantly different from control.
Because NECA can activate both A2A and A2B receptors, the A2A-selective antagonist, ZM241385 was used to discriminate between these two receptor subtypes. Pre-incubation with 1 μM ZM241385 blocked the NECA-stimulated increase in phospho-ERK1/2 in SCs, but there were no significant effects on phospho-ERK1/2 levels in SCs following treatment with ZM241385 alone (Fig. 5C). In addition, the adenosine receptor-mediated phosphorylation of ERK1/2 was prevented in SCs cultured from A2A-receptor deficient (A2A−/−) mice (Chen et al., 1999) (Fig. 6). Neither NECA (1 μM) nor the A2A-selective agonist CGS21680 (1 μM) increased phospho-ERK1/2 levels in A2A−/− SCs compared to wild-type controls (Fig. 6B). We were unable to test directly the involvement of A2B receptors because selective A2B agonists and antagonists are not available; however, the findings from studies using A2A−/− mice indicate strongly that adenosine-stimulated phosphorylation of ERK1/2 in SCs is mediated by the A2A receptor.
In addition to ATP and adenosine, several soluble, signaling molecules could be released from electrically-stimulated DRG axons to activate ERK/MAPK in SCs in coculture. However, treatment of cocultures of DRG neurons and SCs with the selective A2A receptor antagonist ZM241385, significantly inhibited phosphorylation of ERK/MAPK in SCs in response to electrical stimulation of axons (P<0.0001; n=15, t-test) (Fig. 5D). In contrast to the inhibition of ERK/MAPK phosphorylation seen when A2A receptor activation is inhibited with ZM241385, electrical stimulation in the presence of apyrase (27 U ml−1) failed to inhibit activity-dependent phosphorylation of ERK/MAPK in SCs (Fig. 5D). Apyrase is an enzyme that rapidly degrades extracellular ATP (Guthrie et al., 1999). Therefore, the increase in ERK/MAPK phosphorylation in SCs on electrically stimulated axons was not dependent on activation of ATP receptors. Indeed, there was an increase in basal ERK/MAPK levels in SCs treated with apyrase in unstimulated cocultures (27 ± 2.41 optical unit increase above control; P<0.001, t-test, n=28), which is consistent with the inhibitory effect of ATP (P2) receptor activation on ERK/MAPK phosphorylation observed in monoculture (Fig. 4B). Together, the results indicate that extracellular adenosine is a primary signaling molecule that activates ERK/MAPK in SCs in response to electrical activity in DRG axons, and demonstrate that this signaling occurs through activation of the A2A subtype of adenosine receptors.
ATP, but not adenosine, arrests SC differentiation
Treatment with ATP has been shown to arrest SC differentiation and prevent the formation of myelin in DRG–SC cocultures (Stevens and Fields, 2000). Because the present results show that SCs also express functional adenosine receptors, it is necessary to determine whether this developmental arrest is mediated by ATP (P2) receptors, adenosine (A2) receptors, or both. Consistent with our published findings (Stevens and Fields, 2000), treatment of SC/DRG cocultures for 4 days with 300 μM ATP (in ascorbic acid-containing medium) prevented the normal developmental shift from spindle-shaped to rounded, flattened morphology (not shown) and prevented the expression of the O4 antigen, compared with controls (Fig. 7). Inhibition of O4 expression was also seen following treatment with the non-degradable P2 receptor agonist, 2MeSATP (100 μM), which indicates the involvement of ATP (P2) receptors, rather than dependence on breakdown of ATP to adenosine. By contrast, treatment with 300 μM adenosine failed to inhibit expression of O4 in SCs (P<0.001; ANOVA, n=16), despite its potent anti-proliferative effect (Fig. 7A). There were no differences in the total number of SCs and no evidence of apoptosis in these cultures, as determined by cell counts by TUNEL assay following any of these treatments (not shown).
Fig. 7. ATP, but not adenosine, arrests maturation and differentiation of SCs.
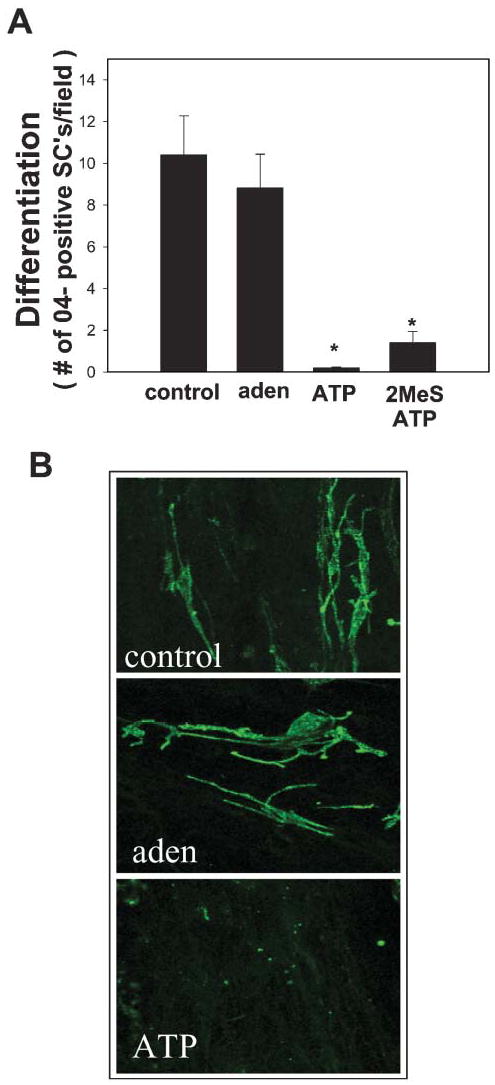
(A) Treating SCs with either ATP (300 μM) or the non-hydrolysable analog 2MeSATP (100 μM) in coculture with DRG neurons for 4 days prevented expression of the 04 antigen, a marker of SC lineage progression from an immature stage (P<0.001, n=16, ANOVA). Treatment with 300 μM adenosine (aden) had no inhibitory effect. (B) The morphology and differentiation of SCs in adenosine-treated cultures (middle) was similar to control cells (top), but SCs retained the spindle-shaped immature morphology and failed to express the O4 antigen in cocultures treated with ATP (bottom). *Significantly different from control.
CONCLUSIONS
Premyelinated mouse SCs express functional adenosine receptors of the A2 subtype in cell culture.
Electrical stimulation of DRG neurons activates the ERK/MAPK pathway in associated premyelinating SCs by activating A2A adenosine receptors on SCs, not by ATP receptor activation.
Activation of A2A adenosine receptors and metabotropic ATP receptors inhibits PDGF-induced proliferation of SCs via different intracellular signaling pathways.
Activation of ATP (P2), but not adenosine (P1) receptors, arrests SC differentiation at the premyelinating stage.
DISCUSSION
The present work identifies adenosine as a novel, axonally derived signal that mediates activity-dependent communication between neurons and premyelinating SCs. The results show that cultured mouse SCs express functional receptors for adenosine and ATP. Although adenosine and ATP receptors are both activated in SCs in response to neural impulses, and both receptors inhibit growth factor-induced proliferation of SCs, we observed opposing effects of ATP and adenosine on MAPK signaling and differentiation of SCs. These differential functional responses are mediated by distinct subtypes of purinergic receptors (A2A and P2Y) that probably act through different intracellular signaling pathways in response to neural impulse activity.
Premyelinating SCs express functional adenosine receptors
All four subtypes of adenosine receptor have been shown to be expressed in cultured and acutely isolated oligodendrocyte progenitor cells (Stevens et al., 2002) and in astrocytes (Pilitsis and Kimelberg, 1998), but the adenosine receptors in PNS myelinating glia have not been previously characterized. Calcium-linked A1 adenosine receptors have been observed in perisynaptic SCs that surround the neuromuscular junction (Robitaille, 1995), but these specialized PNS glia are structurally and functionally distinct from the SCs that are associated with extrasynaptic myelinated and non-myelinated axons.
The present study provides several lines of evidence that premyelinating SCs in culture express functional A2 adenosine receptors. RT-PCR and immunocytochemical analysis revealed that SCs specifically express A2A adenosine receptor mRNA and protein. Importantly, activation of A2 adenosine receptors in SCs resulted in significant cAMP accumulation, and phosphorylation of ERK1/2 MAPK, indicating that these receptors are functional. Because this class of adenosine receptors is positively coupled to cAMP, and not necessarily to Cai2+, it is possible that A2 adenosine receptors have been overlooked in other studies in which adenosine failed to elicit calcium responses in SCs (Lyons et al., 1994; Mayer et al., 1998; Mayer et al., 1997).
Adenosine is an activity-dependent signal activating ERK/MAPK in SCs
The ERK/MAPK pathway plays a central role in regulating growth factor-dependent proliferation and differentiation of most mammalian cells, including developing SCs (Maurel and Salzer, 2000; Meintanis et al., 2001). Growth factors such as PDGF and neuregulins signal through the Ras/Raf/MAPK pathway to regulate cell-cycle progression in SCs (Kim et al., 1997a; Kim et al., 2001b; Maurel and Salzer, 2000), but the effect of adenosine on ERK/MAPK signaling in SCs has not been explored previously.
Our results implicate adenosine as an important activitydependent signal that activates ERK signaling in premyelinating SCs in response to electrical activity in axons. First, activity-dependent phosphorylation of ERK/MAPK was inhibited significantly by the selective A2 receptor antagonist ZM241385. Consistent with these data, the selective A2A receptor agonist, CGS21680 caused a dose-dependent phosphorylation of ERK, as did NECA at nM concentrations. Importantly, NECA and CGS failed to increase phosphorylated ERK1/2 levels in SCs cultured from A2A−/− mice, which specifically implicates the A2A subtype of adenosine receptor. That ATP failed to activate ERK under the conditions of our study, further excludes involvement of ATP (P2) receptors in activity-dependent activation of ERK/MAPK.
Extracellular ATP is hydrolyzed to ADP, AMP and, ultimately, to adenosine, which can modulate cell function via its own receptors. The rate and degree of ATP hydrolysis, is tightly regulated by several complex families of ectonucleotidases that act together to terminate nucleotide signaling at their respective receptors (Zimmermann et al., 1998). We reported previously that ATP is released nonsynaptically from DRG neurons in an activity-dependent manner (Stevens and Fields, 2000). The present findings indicate that sufficient ATP is hydrolyzed to adenosine during the 30-minute-stimulation period in DRG/SC co-cultures to exert functional effects in SCs. However, we have not ruled out the alternative possibility, that adenosine is released directly from DRG neurons in an activity-dependent manner. Future studies are necessary to determine whether ectonucleotidases are involved in regulating activity dependent axon–SC communication. Interestingly, extracellular ATP failed to activate ERK1/2 in SCs grown without axons, which indicates that ectonucleotidases controlling the synthesis of adenosine from ATP might be more active in SCs cocultured with DRG axons. Consistent with this notion, agents that increase intracellular cAMP levels (and, thus, mimic axonal signals) have been shown recently to upregulate significantly an ATPase in SCs in culture (Bermingham et al., 2001).
Adenosine is a candidate axon-derived signal elevating intracellular cAMP in SCs during development
Cyclic AMP has been implicated as an important second messenger that regulates SC proliferation and differentiation, and it is thought to mimic axon signals in vitro (Morgan et al., 1991). The identity of the axon signal(s) that increase cAMP in developing SCs remain a mystery, but our findings identify adenosine as a possible candidate. A2 adenosine receptors are coupled positively to adenylate cyclase via Gs proteins to increase intracellular cAMP levels (Klinger et al., 2002; Schulte and Fredholm, 2000; Schulte and Fredholm, 2003; Seidel et al., 1999). The present study has demonstrated that activation of A2 receptors results in significant accumulation of cAMP in SCs in culture, suggesting that adenosine may activate ERK/MAPK via the cAMP-dependent PKA pathway, as has been demonstrated in several cell types (Klinger et al., 2002; Schulte and Fredholm, 2000; Schulte and Fredholm, 2003; Seidel et al., 1999).
ERK/MAPK appears to integrate signals from multiple intracellular signaling cascades to regulate genes involved in cell cycle and differentiation (Bhalla and Iyengar, 2001; Bhalla et al., 2002). Activation of ERK/MAPK underlies the mitogenic effects of many growth factors, but ERK can also mediate cell-cycle arrest (Pumiglia and Decker, 1997). In SCs, the ERK/MAPK cascade represents a point of cross-talk between growth factors and cAMP signaling pathways, whereby cAMP can either enhance or inhibit growth-factor mediated ERK signaling (and cell-cycle progression) (Kim et al., 1997a; Kim et al., 1997b; Kim et al., 2001a). This might help explain our present findings that acute activation of A2 adenosine receptors strongly activated ERK/MAPK in SCs, but inhibited growth factor-induced stimulation of SC proliferation. Future studies are necessary to determine whether signaling from A2A receptors to ERK via cAMP regulates cell cycle progression in SCs.
Differential roles of ATP and adenosine during SC development and plasticity
The onset of high-frequency, neural-impulse activity and possible release of ATP and adenosine from sensory axons corresponds to a period in development in which SCs stop proliferating and differentiate into mature myelinating or nonmyelinating phenotypes (Fields and Stevens, 2000; Fitzgerald, 1987). The present study indicates that electrical activity in axons could overcome the mitogenic signals and regulate glial development by releasing ATP and adenosine. Although ATP and adenosine both inhibit growth factor-stimulation of SC proliferation, they are likely to act through different mechanisms. ATP, unlike adenosine, can inhibit ERK/MAPK. The signaling pathways and mechanisms by which ATP negatively regulates mitogenic signaling in SCs are not characterized, but our results suggest the involvement of metabotropic P2Y receptors. It is likely that activation of A2 receptors by adenosine inhibits growth factor-stimulated SC proliferation through a cAMP-dependent pathway. Although the specific mechanisms by which these two signaling pathways inhibit PDGF-induced proliferation are not known, our findings indicate possible interactions in intracellular signaling systems that are upstream of ERK/MAPK.
ATP and adenosine have opposing effects on the differentiation of and myelination by CNS and PNS glia. ATP appears to be the dominant purinergic signal that inhibits SC differentiation and myelination (Stevens and Fields, 2000), whereas adenosine had no inhibitory effect. By contrast, adenosine is the primary activity-dependent signal promoting differentiation of premyelinating progenitor cells into myelinating oligodendrocytes in the CNS (Stevens et al., 2002). Differences in the number and types of purinergic receptors, and interactions between purinergic receptors and other signaling systems in the extracellular environment may underlie diverse biological outcomes in CNS and PNS glia. Possible competitive interactions between ATP and adenosine-linked signaling pathways could also play an important role during the premyelinating period, when purinergic receptor expression, ectonucleotidase activity and ATP release may be regulated developmentally. Collectively, our findings indicate that extracellular ATP and adenosine regulate MAPK signaling in SCs to meet functional requirements during development, regeneration and nervous system plasticity.
Acknowledgments
We thank Brian Weinberg and Aimee Kim for assistance with SC cultures, Aimee Kim for MAPK immunocytochemistry and quantification, Deborah Jones for assistance with calcium imaging experiments, Michail Sitkovsky for help with homozygous A2A−/− breeding pairs, Daniel Abebe for assistance with breeding and maintenance of mouse colonies, Ken Jacobson for advice on the pharmacology of purinergic receptors, and Philip Lee for assistance with RT-PCR and helpful discussions. A2A−/− mice were provided by Drs. J.-F. Chen and M.A. Schwarzschild at Massachusetts General Hospital and Boston University School of Medicine.
References
- Abbracchio MP, Saffrey MJ, Hopker V, Burnstock G. Modulation of astroglial cell proliferation by analogues of adenosine and ATP in primary cultures of rat striatum. Neuroscience. 1994;59:67–76. doi: 10.1016/0306-4522(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Bermingham JR, Jr, Shumas S, Whisenhunt T, Rosenfeld MG, Scherer SS. Modification of representational difference analysis applied to the isolation of forskolin-regulated genes from Schwann cells. Journal of Neuroscience Research. 2001;63:516–524. doi: 10.1002/jnr.1046. [DOI] [PubMed] [Google Scholar]
- Bhalla U.S. and Iyengar R. (2001) Functional modules in biological signalling networks. Novartis Foundation Symposia 239, 4–13; discussion 13–5, 45–51. [DOI] [PubMed]
- Bhalla US, Ram PT, Iyengar R. MAP kinase phosphatase as a locus of flexibility in a mitogen-activated protein kinase signaling network. Science. 2002;297:1018–1023. doi: 10.1126/science.1068873. [DOI] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, et al. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. Journal of Neuroscience. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feoktistov I, Biaggioni I. Adenosine A2b receptors evoke interleukin-8 secretion in human mast cells. An enprofylline-sensitive mechanism with implications for asthma. Journal of Clinical Investigation. 1995;96:1979–1986. doi: 10.1172/JCI118245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Eshete F, Stevens B, Itoh K. Action potential-dependent regulation of gene expression: temporal specificity in Ca2+, cAMP-responsive element binding proteins, and mitogen-activated protein kinase signaling. Journal of Neuroscience. 1997;17:7252–7266. doi: 10.1523/JNEUROSCI.17-19-07252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Neale EA, Nelson PG. Effects of patterned electrical activity on neurite outgrowth from mouse sensory neurons. Journal of Neuroscience. 1990;10:2950–2964. doi: 10.1523/JNEUROSCI.10-09-02950.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Nelson PG. Activity-dependent development of the vertebrate nervous system. International Review of Neurobiology. 1992;34:133–214. doi: 10.1016/s0074-7742(08)60098-7. [DOI] [PubMed] [Google Scholar]
- Fields RD, Stevens B. ATP: an extracellular signaling molecule between neurons and glia. Trends in Neurosciences. 2000;23:625–633. doi: 10.1016/s0166-2236(00)01674-x. [DOI] [PubMed] [Google Scholar]
- Fields RD, Stevens-Graham B. New views of neuronglia communication. Science. 2002;298:483–690. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. Spontaneous and evoked activity of fetal primary afferents in vivo. Nature. 1987;326:603–605. doi: 10.1038/326603a0. [DOI] [PubMed] [Google Scholar]
- Franke H, Krugel U, Illes P. P2 receptor-mediated proliferative effects on astrocytes in vivo. Glia. 1999;28:190–200. [PubMed] [Google Scholar]
- Franke H, Krugel U, Schmidt R, Grosche J, Reichenbach A, Illes P. P2 receptor-types involved in astrogliosis in vivo. British Journal of Pharmacology. 2001;134:1180–1189. doi: 10.1038/sj.bjp.0704353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn Schmiedeberg’s Archives of Pharmacology. 2000;362:364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. Journal of Neuroscience. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HA, DeClue JE, Ratner N. cAMP-dependent protein kinase A is required for Schwann cell growth: interactions between the cAMP and neuregulin/tyrosine kinase pathways. Journal of Neuroscience Research. 1997a;49:236–247. [PubMed] [Google Scholar]
- Kim HA, Ling B, Ratner N. Nf1-deficient mouse Schwann cells are angiogenic and invasive and can be induced to hyper-proliferate: reversion of some phenotypes by an inhibitor of farnesyl protein transferase. Molecular and Cellular Biology. 1997b;17:862–872. doi: 10.1128/mcb.17.2.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HA, Ratner N, Roberts TM, Stiles CD. Schwann cell proliferative responses to cAMP and Nf1 are mediated by cyclin D1. Journal of Neuroscience. 2001a;21:1110–1116. doi: 10.1523/JNEUROSCI.21-04-01110.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Yoon MY, Choi SL, Kang I, Kim SS, Kim YS, et al. Effects of stimulation of AMP-activated protein kinase on insulin-like growth factor 1- and epidermal growth factor-dependent extracellular signal-regulated kinase pathway. Journal of Biological Chemistry. 2001b;276:19102–19110. doi: 10.1074/jbc.M011579200. [DOI] [PubMed] [Google Scholar]
- King BF, Neary JT, Zhu Q, Wang S, Norenberg MD, Burnstock G. P2 purinoceptors in rat cortical astrocytes: expression, calcium-imaging and signalling studies. Neuroscience. 1996;74:1187–1196. doi: 10.1016/0306-4522(96)00209-6. [DOI] [PubMed] [Google Scholar]
- Klinger M, Kuhn M, Just H, Stefan E, Palmer T, Freissmuth M, et al. Removal of the carboxy terminus of the A2A-adenosine receptor blunts constitutive activity: differential effect on cAMP accumulation and MAP kinase stimulation. Naunyn Schmiedeberg’s Archives of Pharmacology. 2002;366:287–298. doi: 10.1007/s00210-002-0617-z. [DOI] [PubMed] [Google Scholar]
- Klotz KN. Adenosine receptors and their ligands. Naunyn Schmiedeberg’s Archives of Pharmacology. 2000;362:382–391. doi: 10.1007/s002100000315. [DOI] [PubMed] [Google Scholar]
- Lenz G, Goncalves D, Luo, Z., Avruch J, Rodnight R, Neary JT. Extracellular ATP stimulates an inhibitory pathway towards growth factor-induced cRaf-1 and MEKK activation in astrocyte cultures. Journal of Neurochemistry. 2001;77:1001–1009. doi: 10.1046/j.1471-4159.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- Li M, Jia M, Fields RD, Nelson PG. Modulation of calcium currents by electrical activity. Journal of Neurophysiology. 1996;76:2595–2607. doi: 10.1152/jn.1996.76.4.2595. [DOI] [PubMed] [Google Scholar]
- Lyons SA, Morell P, McCarthy KD. Schwann cells exhibit P2Y purinergic receptors that regulate intracellular calcium and are up-regulated by cyclic AMP analogues. Journal of Neurochemistry. 1994;63:552–560. doi: 10.1046/j.1471-4159.1994.63020552.x. [DOI] [PubMed] [Google Scholar]
- Maurel P, Salzer JL. Axonal regulation of Schwann cell proliferation and survival and the initial events of myelination requires PI 3-kinase activity. Journal of Neuroscience. 2000;20:4635–4645. doi: 10.1523/JNEUROSCI.20-12-04635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C, Quasthoff S, Grafe P. Differences in the sensitivity to purinergic stimulation of myelinating and non-myelinating Schwann cells in peripheral human and rat nerve. Glia. 1998;23:374–382. doi: 10.1002/(sici)1098-1136(199808)23:4<374::aid-glia9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Mayer C, Wachtler J, Kamleiter M, Grafe P. Intracellular calcium transients mediated by P2 receptors in the paranodal Schwann cell region of myelinated rat spinal root axons. Neuroscience Letters. 1997;224:49–52. doi: 10.1016/s0304-3940(97)13457-7. [DOI] [PubMed] [Google Scholar]
- Meintanis S, Thomaidou D, Jessen KR, Mirsky R, Matsas R. The neuronglia signal beta-neuregulin promotes Schwann cell motility via the MAPK pathway. Glia. 2001;34:39–51. [PubMed] [Google Scholar]
- Morgan L, Jessen KR, Mirsky R. The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP-, N-CAM-, NGF-receptor-) depends on growth inhibition. Journal of Cell Biology. 1991;112:457–467. doi: 10.1083/jcb.112.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary JT, Baker L, Jorgensen SL, Norenberg MD. Extra-cellular ATP induces stellation and increases glial fibrillary acidic protein content and DNA synthesis in primary astrocyte cultures. Acta Neuropathologica. 1994;87:8–13. doi: 10.1007/BF00386249. [DOI] [PubMed] [Google Scholar]
- Neary JT, Kang Y, Bu Y, Yu E, Akong K, Peters CM. Mitogenic signaling by ATP/P2Y purinergic receptors in astrocytes: involvement of a calcium-independent protein kinase C, extracellular signal-regulated protein kinase pathway distinct from the phosphatidylinositol-specific phospholipase C/calcium pathway. Journal of Neuroscience. 1999;19:4211–4220. doi: 10.1523/JNEUROSCI.19-11-04211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary JT, McCarthy M, Kang Y, Zuniga S. Mitogenic signaling from P1 and P2 purinergic receptors to mitogen-activated protein kinase in human fetal astrocyte cultures. Neuroscience Letters. 1998;242:159–162. doi: 10.1016/s0304-3940(98)00067-6. [DOI] [PubMed] [Google Scholar]
- Pilitsis JG, Kimelberg HK. Adenosine receptor mediated stimulation of intracellular calcium in acutely isolated astrocytes. Brain Research. 1998;798:294–303. doi: 10.1016/s0006-8993(98)00430-2. [DOI] [PubMed] [Google Scholar]
- Pumiglia KM, Decker SJ. Cell cycle arrest mediated by the MEK/mitogen-activated protein kinase pathway. Proceedings of the National Academy of Sciences of the USA. 1997;94:448–452. doi: 10.1073/pnas.94.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacological Reviews. 1998;50:413–492. [PubMed] [Google Scholar]
- Rathbone MP, Middlemiss PJ, Kim JK, Gysbers JW, DeForge SP, Smith RW, et al. Adenosine and its nucleotides stimulate proliferation of chick astrocytes and human astrocytoma cells. Neuroscience Research. 1992;13:1–17. doi: 10.1016/0168-0102(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Robitaille R. Purinergic receptors and their activation by endogenous purines at perisynaptic glial cells of the frog neuromuscular junction. Journal of Neuroscience. 1995;15:7121–7131. doi: 10.1523/JNEUROSCI.15-11-07121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte G, Fredholm BB. Human adenosine A(1), A(2A), A(2B), and A(3) receptors expressed in Chinese hamster ovary cells all mediate the phosphorylation of extracellular-regulated kinase 1/2. Molecular Pharmacology. 2000;58:477–482. [PubMed] [Google Scholar]
- Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cellular Signalling. 2003;15:813–827. doi: 10.1016/s0898-6568(03)00058-5. [DOI] [PubMed] [Google Scholar]
- Seidel MG, Klinger M, Freissmuth M, Holler C. Activation of mitogen-activated protein kinase by the A(2A)-adenosine receptor via a rap1-dependent and via a p21(ras)-dependent pathway. Journal of Biological Chemistry. 1999;274:25833–25841. doi: 10.1074/jbc.274.36.25833. [DOI] [PubMed] [Google Scholar]
- Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science. 2000;287:2267–2271. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- Stevens B, Porta S, Haak LL, Gallo V, Fields RD. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36:855–868. doi: 10.1016/s0896-6273(02)01067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Tanner S, Fields RD. Control of myelination by specific patterns of neural impulses. Journal of Neuroscience. 1998;18:9303–9311. doi: 10.1523/JNEUROSCI.18-22-09303.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakel JL, Warren RA, Reppert SM, North RA. Functional expression of adenosine A2b receptor in Xenopus oocytes. Molecular Pharmacology. 1993;43:277–280. [PubMed] [Google Scholar]
- Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedeberg’s Archives of Pharmacology. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- Zimmermann H, Braun N. Extracellular metabolism of nucleotides in the nervous system. Journal of Autonomic Pharmacology. 1996;16:397–400. doi: 10.1111/j.1474-8673.1996.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann H, Braun N, Kegel B, Heine P. New insights into molecular structure and function of ectonucleotidases in the nervous system. Neurochemistry International. 1998;32:421–425. doi: 10.1016/s0197-0186(97)00126-5. [DOI] [PubMed] [Google Scholar]


