Abstract
Transient expression of foreign genes in plant tissues is a valuable tool for plant biotechnology. To shorten the time for gene functional analysis in fruits, we developed a transient methodology that could be applied to tomato (Solanum lycopersicum cv Micro Tom) fruits. It was found that injection of Agrobacterium cultures through the fruit stylar apex resulted in complete fruit infiltration. This infiltration method, named fruit agroinjection, rendered high levels of 35S Cauliflower mosaic virus-driven β-glucuronidase and yellow fluorescence protein transient expression in the fruit, with higher expression levels around the placenta and moderate levels in the pericarp. Usefulness of fruit agroinjection was assayed in three case studies: (1) the heat shock regulation of an Arabidopsis (Arabidopsis thaliana) promoter, (2) the production of recombinant IgA antibodies as an example of molecular farming, and (3) the virus-induced gene silencing of the carotene biosynthesis pathway. In all three instances, this technology was shown to be efficient as a tool for fast transgene expression in fruits.
The generation of stably transformed transgenic plants to assess gene function is a lengthy manipulative process. As an alternative, foreign gene expression in plants is often performed by transient transformation of cells or tissues. Recently, Agrobacterium-mediated transient gene expression (agroinfiltration) in plant leaves has become the favorite choice in many gene functional analyses (Kapila et al., 1997; Yang et al., 2000; Goodin et al., 2002). When Agrobacterium cell cultures are infiltrated into the intercellular spaces of leaf parenchyma, the transfer of T-DNA into the plant cell nucleus becomes a highly efficient event. The most popular host plant for agroinfiltration is Nicotiana benthamiana; however, the power of the technique has been also described for other species like Medicago sativum (D'Aoust et al., 2004), lettuce (Lactuca sativa), tomato (Solanum lycopersicum), and Arabidopsis (Arabidopsis thaliana; Wroblewski et al., 2005) among others. Efficiency of agroinfiltration varies from host to host, some seeming recalcitrant to the technique. The reasons for the differences in efficiency are not well described, but surely topological factors are partly to blame (compactness of the tissue, innervations pattern, etc.), and bacteria-host compatibility factors cannot be discarded (Wroblewski et al., 2005). Even in the species with limited transfer efficiency, agroinfiltration is often used as delivery system for replicons that either move systemically (viral RNA genomes) or amplify locally (deconstructed Tobacco mosaic viruses; Marillonnet et al., 2005).
Tomato fruit is a model for fleshy fruit development. Currently, several international efforts converge in the genomic characterization of tomato and related solanaceae species, including expressed sequence tags and genome sequencing projects (http://www.sgn.cornell.edu/). In addition, tomato fruits have been proposed as factories for the production of oral vaccines and other immunotherapeutic proteins (Sandhu et al., 2000; Jani et al., 2002; Ma et al., 2003; Walmsley et al., 2003). The lack of a high throughput transformation procedure and the length of time required to produce stable transgenic tomatoes make assessment of gene function and evaluation of xenoproteins in the tomato fruit a tedious and cumbersome process.
We have developed an agroinfiltration-based system (agroinjection), which allows transient expression of foreign genes directly in fruit tissues. We tested agroinjection as an assay tool for transgene studies in three scenarios: (1) the study of promoter activity assisted by reporter genes; (2) the analysis of xenoprotein production in fruits, as exemplified by IgA antibodies; and (3) the study of gene function by virus-induced gene silencing (VIGS).
RESULTS AND DISCUSSION
Infiltration of Tomato Fruit Tissues with Agrobacterium
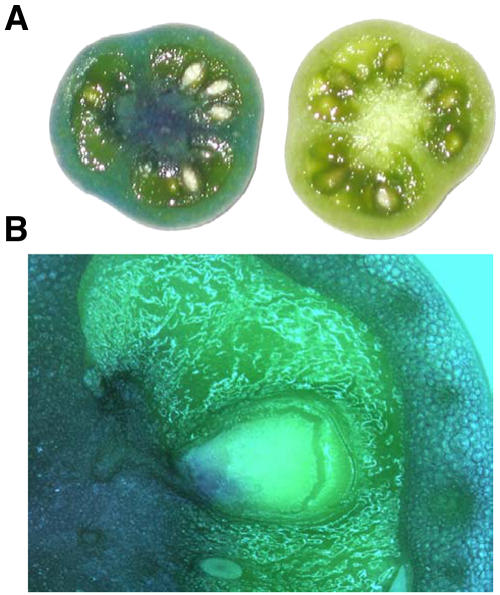
The versatility of agroinfiltration in N. benthamiana leaves prompted us to test the possibility of establishing a similar approach in tomato fruits. We first tested several methods for mechanically introducing bacteria in the fruit cell apoplast. Progression of the infiltration was monitored with Agrobacterium cultures stained with methylene blue. Needle-free syringe infiltration was found ineffective as well as vacuum-assisted infiltration of intact, detached fruits (data not shown). Sliced or half-cut fruits were effectively infiltrated, but the procedure inflicted severe tissue damage and was therefore discarded. Finally, we tested the injection of infiltration media into the fruit using a syringe with needle. A similar approach for fleshy fruits described earlier in the literature produced only partial fruit infiltration, limiting the possible applications of the technique (Spolaore et al., 2001). We found that when tomato fruits (cv Micro Tom) were injected through the stylar apex with 600 μL of infiltration medium containing methylene blue-stained bacteria, the infiltration solution reached the entire fruit surface (Fig. 1). Upon dissection, blue staining was observed in the central lamella, placenta, and pericarp, but not in the seed and locular tissues. Blue-stained bacteria accumulated preferentially in the placenta with less intense staining in the pericarp. Fruit infiltration was possible both in attached and detached fruits, however in the latter case a more extense infiltration was obtained as the peduncle remained attached to the fruit. This prevented media leakage during the process as the excess of infiltration solution could only find a way off the apoplast through the hydathods located at the tip of the sepals.
Figure 1.
Extent of agroinfiltration of tomato fruits using agroinjection. A, Fruit slices from tomatoes agroinjected with methylene blue-stained bacteria (left) and with an unstained culture (right). B, Close up from a tomato agroinjected with stained bacteria. Blue color reveals the tissues reached by the infiltrated culture.
Agroinjection as a Transient Expression System
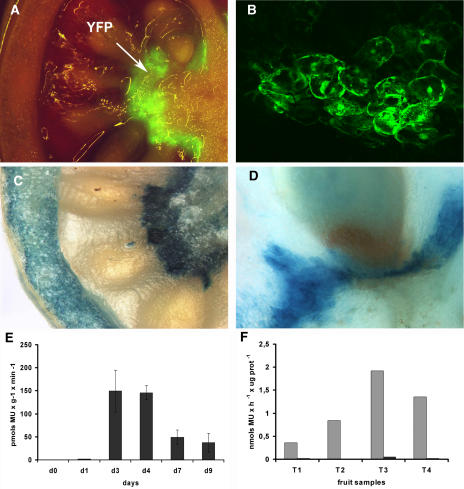
Once infiltration in most fruit tissues was confirmed, we proceeded to test fruit agroinjection as a transient expression system of foreign genes using yellow fluorescence protein (YFP, a yellow version of green fluorescence protein) and β-glucuronidase (GUS) as reporter genes. Tomato fruits at mature green stage (22–25 d after anthesis) were agroinjected with a double-reporter plasmid pBIN-YFP/GUS containing YFP and GUS genes directed by the 35S promoter (Fig. 2). High levels of glucuronidase activity were detected in agroinjected fruits 4 d after agroinfiltration (Fig. 2E). GUS activity decreased thereafter until ripening. At ripen stages (9 d post injection [dpi]), measurement of GUS activity using standard techniques was unreliable probably due to endogenous activity (data not shown). Under UV light, high levels of yellow fluorescence were clearly visible around the placenta tissue of 4-dpi fruits (Fig. 2A). Confocal microscopy confirmed nucleocytoplasmic localization of plant-expressed YFP (Fig. 2B). Occasionally, strong YFP fluorescence was also observable at the inner side of the pericarp (data not shown). Histochemical GUS staining of 4-dpi fruits revealed a pattern similar to that found for YFP (Fig. 2, C and D); however, moderate levels of GUS staining were also found in the pericarp, probably due to the higher sensibility of the method (Fig. 2C). Interestingly, despite our efforts, agroinfiltration of tomato leaves with pBIN-YFP/GUS constructs rendered no YFP fluorescence and negligible levels of GUS activity (data not shown). Since the 35S promoter is known to be active in tomato leaves of stably transformed plants, this observation seems to indicate that tissue susceptibility to Agrobacterium infection plays and important role in the efficiency of agroinfiltration methodology.
Figure 2.
Plant promoter-driven expression of reporter genes in tomato fruits. A, pBIN-YFP/GUS-agroinfiltrated tomato at 4 dpi showing YFP fluorescence in the placental tissue under UV light. B, Confocal microscope image of a similar sample showing YFP fluorescence in cells from the placenta-locule transition zone. C, Histochemical GUS staining of a pBIN-YFP/GUS-agroinfiltrated tomato. D, Close up of the seed-placenta joining region decorated with GUS staining. E, Time course of glucuronidase activity in agroinfiltrated tomatoes. Bars show the average activity (pmol methylumbelliferone × g fresh weight−1 × min−1) of four tomatoes per time point ± sd. F, Heat shock induction of GUS activity directed by Arabidopsis HSP70 promoter. pHSP70∷GUS-agroinjected tomatoes at 4 dpi were separated in two halves and incubated for 6 h at 42°C and 25°C, respectively. Graph compares GUS-specific activity (pmol methylumbelliferone × μg protein−1 × h−1) of heat-shocked pieces (gray bars) with the negligible specific activity of control pieces (black bars).
The capacity for modulating transgene expression using agroinjection was tested with a construct containing the Arabidopsis heat shock-regulated promoter HSP70B fused to GUS (Aparicio et al., 2005). Four pHSP70B:GUS-agroinjected tomatoes were incubated 3 dpi in the plant, and then harvested and cut in two halves, one of the pieces incubated 6 h at 42°C and the other left at 25°C for the same period of time. As shown in Figure 2F, the pHSP70B:GUS-agroinjected tomato fruits showed the capacity to activate HSP70B promoter in response to heat shock in all the samples tested. Together, these observations indicate that reporter genes can be efficiently expressed in fruit tissues via agroinjection, retaining the capacity to modulate transcription in response to environmental (temperature) factors.
We found that the spatial expression patterns observed with agroinjection seem at least partially governed by constraints imposed by the fruit architecture and the ability of the bacteria to reach the different tissues in the fruit. For instance, maximum expression levels are normally observed in the placenta, probably because it constitutes a diffusion barrier in the apoplastic network of the fruit. Consequently, interpretation of the spatial expression patterns obtained by agroinjection should take these considerations into account.
Xenoprotein Expression: Recombinant Antibodies
Production of xenoproteins in edible fruits has important biotechnological implications particularly for the production of recombinant products with oral therapeutic activity (Walmsley and Arntzen, 2003). Despite the advantages offered by fruits, their use as production platforms is often hampered by low yields and poor protein stability. Xenoprotein production at high yields requires construct selection and optimization, preferably in the same tissue/organ in which the final production is intended, and therefore efficient transient expression systems are much needed. Technologies for fruit transient expression available so far (i.e. biolistics) fail to render sufficient yields for, for example, western evaluation.
We are particularly interested in the production of IgA antibodies in fruits. IgAs are candidates for oral delivered microbicides as they play a role in the passive protection of mucosa against pathogen invasion (Corthesy, 2002). Two chicken IgA antibodies, n8 and n10, both selected from an anti-Eimeria-enriched recombinant library (Wieland et al., 2006), were chosen for fruit agroinjection. Previous expression studies in N. benthamiana leaves indicated that n8 and n10, despite sharing a common constant frame, show drastic differences in expression levels (Wieland, 2004). We used agroinjection as a method to study differential antibody stability directly in the fruit.
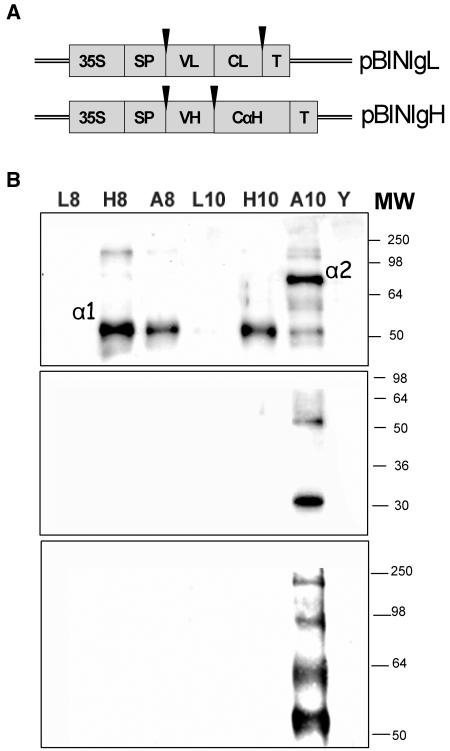
Agrobacterium cultures carrying antibody heavy chains (HCs; HC8 or HC10) and light chains (LCs; LC8 or LC10) under the control of 35S promoter (Fig. 3A) were agroinjected, either separately or in combination. In the latter case, high cotransformation rates will ensure coexpression of HCs and LCs, rendering assembled IgAs. Antibody expression in fruits was monitored by western blot detecting HCs (top section), LCs (middle section), and complexed IgAs (bottom section; Fig. 3B). Here, it can be observed that LCs do not accumulate when expressed alone (middle section, lanes L8 and L10). Conversely, HCs injected without partner LC render a single specific fragment (α1) of aproximately 55 kD (top section, lanes H8 and H10), therefore smaller than the expected 75 kD of chicken αHC. Interestingly, when HC10 and LC10 were coinfiltrated, LC10 became detectable (middle section, A10 lane), and the anti-αHC antibody detected a high molecular mass band (α2; lane A10, top section) whose mobility is compatible with a full-size chicken αHC. The presence of these two characteristic major bands (α1 and α2) was also observed in many chicken IgAs produced in N. benthamiana (Wieland, 2004). Taken together, the results indicate that chicken antibody chains require the presence of a cognate chain for stabilization. LCs are apparently not stable when expressed alone, whereas HCs are probably degraded into a proteolytic product (α1) in the absence of cognate LC. The presence of assembled IgA antibodies is shown in Figure 3B (bottom section) under nonreducing conditions. As can be observed, coexpression of HC and LC from n10 antibody rendered IgA complexes detected as high molecular mass bands (max 200 kD approximately). The complex pattern of bands found for n10 under nonreducing conditions has been described for other plant-made antibodies (Sharp and Doran, 2001) and probably reveals the presence of degradation products. No IgA complexes were detected in the case of n8 antibody (Fig. 3B, bottom section, lane A8). The simplest explanation for this is that LC8 is not stable when expressed in plants. This also will explain the absence of a full-size α2 band when HC8 and LC8 are coinfiltrated (Fig. 3B, top section, lane A8). However, other explanations involving HC8/LC8 mutual compatibility cannot be excluded.
Figure 3.
Expression of full IgA antibodies in tomato fruits. A, Schematic structure of pBINIgL and pBINIgH constructs. Black arrows represent cloning sites for phage display-derived variable sequences. B, Western analysis of tomato fruits agroinfiltrated with IgA antibody chains. Two phage display-derived clones (n8 and n10) were assayed. Tomatoes were infiltrated with cultures containing pBIN-IgL (L8 and L10 lanes), pBIN-IgH (H8 and H10 lanes), or coinfiltrated with a combination of HCs and LCs (A8 = L8 + H8; A10 = L10 + A10). Blots were decorated with anti-chicken IgH (alpha-specific) antibody (top section), anti-chicken LC (middle section), or anti-chicken IgY whole-molecule recognizing the native structure of chicken antibody (IgY and IgA share the same IgL in chicken).
The cotransformation efficiency of the system is remarkable as demonstrated by the mutual stabilization effect found between HCs and LCs of n10 antibody. The differential idiotype stability found in the case of n8 and n10 has also been described for antibodies produced in mammalian systems (Bentley et al., 1998) and underlines the need for selection of stable antibodies prior to plant stable transformation. This is, to our knowledge, the first report of full-size antibodies being expressed in fruits. Our observations therefore confirm that agroinjection can contribute to expand the possibilities of fruit-based xenoprotein production by providing a fast and efficient in fruit selection step.
Fruit VIGS
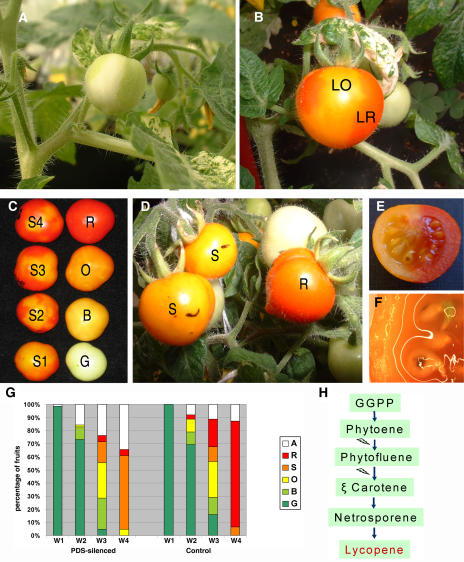
VIGS has emerged as a powerful tool for functional genomics. A Tobacco rattle virus (TRV)-based system (pTRV1/2) has been proven effective in tomato plants previously (Liu et al., 2002). In the original pTRV1/2 protocol, leaves from young plants are agroinfiltrated with pTRV1 and pTRV2, simultaneously. Upon infiltration, reconstructed viruses move systemically, expanding the silencing signal through the plant. We reasoned that fruit agroinjection could represent a shortcut to whole-plant VIGS for the study of gene function in fruit-specific processes. To test the efficiency of agroinjection as a delivery system for fruit VIGS, we agroinjected fruits at different developmental stages with a combination of pTRV1 and TRV2-tPDS, the latter containing a fragment of phytoene desaturase (PDS), a key enzyme in the carotene biosynthesis route. Silencing of PDS was previously shown to induce a photobleaching phenotype in leaves (Ratcliff et al., 2001; Liu et al., 2002) due to chlorophyll degradation. In the case of tomato fruits, it is known that mutations in the carotenoid biosynthesis gene phytoene synthase produce yellow fruit coloration due to the accumulation of flavonoids (chalconaringenin) and the absence of red pigment lycopene, which is normally produced downstream in the carotenoid biosynthesis pathway (Fig. 4H; Fray and Grierson, 1993). A similar yellow/orange phenotype has been reported when the isoprenoid biosynthesis route was chemically inhibited with fosmidomycin (Rodriguez-Concepcion et al., 2001). Accordingly, effective PDS silencing in tomato fruits should result in an orange fruit phenotype.
Figure 4.
PDS silencing in tomato. A, Systemically (leaf-infiltrated) PDS-silenced plant showing photobleaching phenotype in leaves and fruits. B, Mature fruit from systemically (leaf-infiltrated) PDS-silenced plant showing red (LR) and yellow/orange (LO) sectors. C, Example of color evolution during ripening of Micro Tom fruits: G, green; B, breaker; O, yellow/orange; R, red; S, yellow/orange fruits showing different degrees of red pigmented sectors (ranging from S1 to S4). D, Fruits agroinjected with pTRV1/2-tPDS (S) or pTRV1 alone (R) showing drastic differences in red pigmentation at maturity. E, Longitudinal section of a mature tomato from a PDS-silenced plant showing internal red-yellow sectors. F, Close up of E showing viviparism in the yellow sector. G, Evolution of color in a group of 140 tomatoes agroinjected either with pTRV1/2-tPDS (left) or control pTRV1 (right) Agrobacterium cultures. Color was recorded for every tomato during 4 weeks (W1 to W4). Color categories were defined as in C. Number of tomatoes in every category is shown as a percentage of the total number of fruits. S category includes silenced fruits as well as a small number of nonsilenced fruits that were rapidly turning into red from the orange stage. H, Schematic representation of lycopene synthesis route in tomato.
We conducted two PDS-VIGS strategies. On one hand, we performed direct fruit agroinjection to assess its potential as a shortcut for functional gene analysis. In parallel, we followed systemic VIGS using standard inoculation procedures (Liu et al., 2002), aiming to compare and eventually validate the silencing phenotypes obtained with agroinjection.
For systemic VIGS, cotyledons and first leaves from six 2-week-old plants were extensively agroinfiltrated with a TRV1/2-tPDS mix. Five of the plants developed silencing symptoms in the leaves. PDS silencing was also evident in fruits as white sectors in several young fruits in four of the plants (Fig. 4A). At maturity, green sectors turned temporally yellow/orange and immediately developed into red, whereas white sectors remained yellow/orange, a clear sign of impaired lycopene accumulation (Fig. 4B). In total, 66% of the fruits from the four fruit-silenced plants (roughly 44% of all fruits in the experiment) showed silencing symptoms (n = 54), with yellow/orange sectors expanding between 10% and 100% of the whole fruit surface.
For local VIGS experiments, a total of 140 green fruits at different developmental stages (ranging from 7–24 DPA) were agroinjected, 71 of them with pTRV1/2-PDS mix and the remaining 69 using a control pTRV1 plasmid. Color changes were recorded, with color evolution divided in standard stages (Green, Breaker, Yellow/Orange, and Red; see Fig. 4C). An additional intermediate stage was defined in our experiments, named as S, corresponding to fruits at the yellow/orange stage showing also some red sectors. Control fruits that were scored as S developed rapidly into red, whereas most (61%) of pTRV1/2-PDS tomatoes remained arrested in S stage. The extension of red sectors in S-arrested tomatoes differed among fruits (ranging from S1 to S4 as depicted in Fig. 4C). Fruits arrested at S stage resembled those obtained with systemic PDS-VIGS (Fig. 4B), and therefore we concluded that they were locally silenced in PDS. Figure 4G shows the color evolution during the 4-week experiment. Only 4% of the TRV1/2-PDS fruits (three out of 71) developed into fully red tomatoes, in contrast with the 80% of the controls that turned red (95% if abscised fruits are excluded). Interestingly, the only three TRV1/2-PDS tomatoes that turned red were in late mature-green stage when injected and probably received the silencing signal too late to arrest lycopene accumulation. The remaining 34% of the fruits abscised prior to reaching maturation. This fraction was composed mainly by very young fruits (between 1 and 2 weeks post anthesis), which apparently could not cope with the injury/stress caused during manipulation. It is worth noticing that, excluding abscised fruits, 95% of the TRV1/2-tPDS tomatoes that remained attached to the plant until the end of the experiment (26 d) showed PDS silencing symptoms (S arrest). Occasionally, nontreated fruits growing in the same truss as agroinjected fruits developed also yellow sectors similar to those found in systemic silenced fruits, indicating systemic transmission of silencing signals from fruit to fruit (data not shown).
Deleterious side effects of fruit agroinjection appeared mainly in young fruits, both silenced and controls, and consisted in growth arrest, premature ripening, and abscission. To minimize side effects, we restricted the temporal window of treatment to green fruits between 20 and 25 DPA (at the beginning of mature green stage), giving time to silencing signals to take effect on developmental processes occurring from this point (ripening) but minimizing shedding off. Under these conditions, efficiency of PDS silencing was maintained at levels ranging between 87% and 91% in two different experiments (n = 24), with fruit abscission reduced to 4% and 8%, respectively. We also observed that concentration of Agrobacterium cultures could be reduced to optical density = 0.3 without significant changes in the efficiency of silencing (data not shown). It is worth noting that PDS-silenced fruits often showed viviparous seed germination. In fruits silenced systemically, where often PDS-silenced sectors divide the fruit in two clearly defined parts, it was particularly noteworthy that premature seed germination was restricted to the yellow half of the fruit (Fig. 4, E and F). Reduced dormancy has been described before in the abscisic acid (ABA)-deficient sitiens mutant (Groot and Karssen, 1992). Since the precursors of ABA synthesis are derived from the carotene route, viviparous seeds could result from a reduction of ABA levels in PDS-silenced fruits as a consequence of the inhibition of carotene biosynthesis.
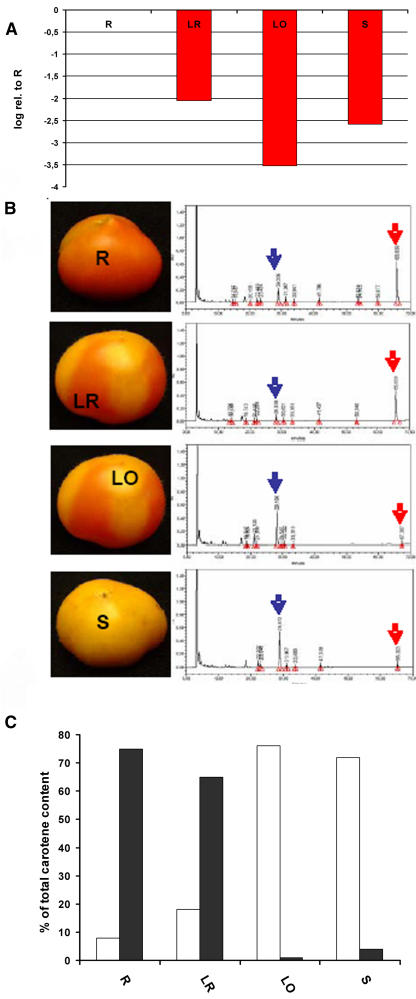
Further characterization of the PDS-silenced phenotype was carried out both in agroinjected and systemically silenced fruits. PDS mRNA levels were measured by quantitative PCR in silenced and nonsilenced fruit pericarp. As shown in Figure 5A, a significant reduction on PDS mRNA levels was observed in all silenced samples when compared with control red pericarp from the same age. Yellow/orange tissue from systemic PDS-silenced fruits showed very low levels of mRNA accumulation, indicating a very effective silencing. Slightly lower inhibition levels were found in locally PDS-silenced fruits. Interestingly, red sectors in systemic PDS-silenced tomatoes also showed up to 4 times reduction in PDS mRNA when compared with nonsilenced controls, without effects in tissue color.
Figure 5.
Effect of PDS silencing in tomato fruits. A, Relative abundance of PDS mRNA in pericarp from silenced tomatoes. Samples are defined as in Figure 4: LR, red sectors of systemically silenced tomatoes; LO, yellow/orange sectors of systemically silenced tomatoes; S, pericarp from pTRV1/2-tPDS-agroinjected tomatoes arrested at S stage. Relative mRNA levels were calculating using pricarp from TRV1-agroinjected red tomatoes (R) as a reference for the calculations. B, Carotene chromatographic profiles of the same samples as in A. C, Relative levels of lycopene (black bars) and the PDS substrate phytoene (white bars) in pericarp samples. Metabolite levels are given as a percentage of the total carotenoid content in every sample.
Finally, the carotenoid profile of the different samples was also determined (Fig. 5B). As expected, PDS-silenced pericarp produced low levels of lycopene, accumulating instead the PDS substrate phytoene (Fig. 5C). Carotene profiles correlated with PDS mRNA levels except for red pericarp in systemic PDS-silenced fruits, where lycopene accumulates at similar levels than control red tissue despite the lower PDS mRNA levels. This suggests that PDS mRNA levels need to reach a certain threshold in order to trigger lycopene accumulation.
Final Remarks
We have analyzed here the potential of agroinjection for transient expression in tomato fruits. As with any invasive methodology, agroinjection carries certain limitations that should be kept in mind in the design of experiments. The massive presence of Agrobacterium cells in the fruit can induce side effects that should be minimized, e.g. reducing culture concentration and/or incubation times when possible. Appropriate control treatments including agroinfiltrated fruits should be included in any experimental design. With the appropriate controls in place, we have shown that agroinjection is a useful tool for fruit biology. It functions as a fast-construct testing methodology, in the study of promoter regulation, as exemplified with pHSP70B∷GUS reporter fusion, in the study xenoprotein expression and stability, as shown in the production of IgA antibodies, and, finally, as a shortcut in VIGS functional gene analysis Moreover, agroinjection may be very helpful when assaying fruit gene constructs that may interfere with plant developmental processes.
While this manuscript was in preparation, Fu and collaborators published a description of virus-induced gene silencing in tomato fruits using several pTRV1/2 delivery methods, which included syringe infiltration of the fruits (Fu et al., 2005). In their report, the authors showed that local fruit infiltration (cv Lichum and cv Ailsa Craig) with VIGS vectors encoding LeEIL and LeEIN2 genes from the ethylene perception route resulted in green, ripening-impeded fruit sectors. Our results reported here on the possibility of establishing a VIGS system in tomato fruits are fully supported by the results shown by Fu et al. (2005). Interestingly, the green/red sectorization produced by the manipulation of ethylene route differed from the yellow/red sectorization reported here, beautifully indicating that accumulation of the flavonoid chalconaringenin is probably an ethylene-dependent event. A relevant contribution of our system is the total fruit infiltration obtained with agroinjection of small-sized Micro Tom fruits, which can contribute to an increase in the efficiency of the silencing. This is an important consideration because adaptation of fruit VIGS to gene functional screenings requires a strong and reliable system that maximizes both the percentage of silenced fruits and the silenced surface in each fruit, so that the requirement for silencing markers (Chen et al., 2004) can be eliminated.
MATERIALS AND METHODS
Agrobacterium-Based Transient Transformation
Agrobacterium cultures (5 mL) were grown overnight from individual colonies at 28°C in YEB medium plus selective antibiotics, transferred to 50 mL induction medium (0.5% beef extract, 0.1% yeast extract, 0.5% Peptone, 0.5% Suc, 2 mm MgSO4, 20 μm acetosyringone, 10 mm MES, pH 5.6) plus antibiotics, and grown again overnight. Next day, cultures were recovered by centrifugation, resuspended in infiltration medium (10 mm MgCl2, 10 mm MES, 200 μm acetosyringone, pH 5.6; optical density = 1.0 unless stated otherwise), and incubated at room temperature with gentle agitation (20 rpm) for a minimum of 2 h. Cultures were combined when required, collected with a syringe, and injected in the fruits as described below. In methylene blue experiments, cells were incubated for 5 min in infiltration medium containing 0.05% methylene blue, recovered by centrifugation, washed twice with infiltration medium, and agroinjected.
Agroinjection was performed as follows. Tomato fruits (Solanum lycopersicum cv Micro Tom) at different stages of development were infiltrated using a 1-mL syringe with a 0.5-×16-mm needle (BD Pastipak). Needle was introduced 3 to 4 mm in depth into the fruit tissue through the stylar apex, and the infiltration solution was gently injected into the fruit. The total volume of solution injected varied with the size of the fruit, with a maximum of 600 μL in mature green tomatoes. The progress of the process could be followed by a slight change in color in the infiltrated areas. Once the entire fruit surface has been infiltrated, some drops of infiltration solution begin to show running off the hydathodes at the tip of the sepals. Only completely infiltrated fruits were used in the experiments. Tomatoes at developmental stages beyond breaker did not infiltrate completely using this method and therefore were not included in the experiments. For tomato leaf agroinfiltration, needles were removed and Agrobacterium cultures were introduced in the intercellular spaces as described earlier (Liu et al., 2002).
Plasmids and Bacterial Strains
For reporter gene analysis, pBIN-YFP/GUS and pHSP70B∷GUS plasmids were used. pBIN-YFP/GUS is a pBIN derivative carrying 35S Cauliflower mosaic virus∷YFP and 35S Cauliflower mosaic virus∷GUS constructs in tandem. Plasmid pHSP70B∷GUS contained a 1.98-kb fragment of Arabidopsis (Arabidopsis thaliana) genomic DNA upstream of the ATG codon of the AtHSP70B gene (Sung et al., 2001), cloned in pGREEN backbone. Plasmids were transferred to Agrobacterium strains LBA4404, C58C1, and MOG101 with no significant differences observed in the levels of transient transformation between the different strains.
For chicken IgA expression, two series of plasmids were used. pBIN-IgL series are pBIN derivatives containing 35S promoter and murine kappa light signal peptide, which incorporate chicken IgL chains n8 and n10 as SalI/XbaI restriction fragments selected from phage display libraries cloned in pCHICK3 phagemid vector (Wieland, 2004). In a similar fashion, pBIN-IgH series contain murine kappa light signal peptides and Cα1 to 4 constant regions from chicken IgαH, and incorporate chicken VH regions n8 and n10 as SalI/SstI restriction fragments selected from pCHICK3-phage display libraries. Agrobacterium C58C1 cultures carrying pBIN-IgL and pBIN-IgH plasmids were either infiltrated separately or coinfiltrated (ratio 1:1) in tomato fruits.
For PDS silencing experiments, previously described pTRV1 and pTRV2-tPDS plasmids were agroinjected (Liu et al., 2002). The version of pTRV2-tPDS vector used here had its PDS intron sequences removed (S. Prat, personal communication).
Detection of Xenoprotein Expression
Histochemical detection of GUS activity was performed as described (Jefferson, 1987). Negative controls, consisting in nonagroinjected tomatoes, pTRV1-agroinjected tomatoes, and pBIN-YFP/GUS-agroinjected tomatoes collected and fixed 30 min after agroinjection, did not render significant blue staining. Quantitative glucuronidase activity assay method was adapted from Jefferson (1987). Briefly, tomato slices (approximately 100 mg) were homogenized in 100 μL GUS extraction buffer, debris cleared by centrifugation, and 10 μL of the resulting supernatant incubated with 190 μL of GUS assay buffer at 37°C. At different time intervals, 10-μL aliquots of each reaction were stopped with 90 μL of 1 m sodium carbonate. The A550 was determined using TECAN microtiter plate spectofluorimeter. For GUS time-course analysis, homogenization step was omitted and instead tomato slices were weighted and incubated directly in GUS assay buffer.
YFP expression was detected under UV light using binocular lens. Confocal images from fresh tissue were taken with a Leika DMIRE2 confocal microscopy.
Fruit-expressed chicken IgAs were detected following western-blot standard procedures. Placenta and locular frozen tissues were ground in N2 (l), extracted in 1×phosphate-buffered saline 1:1 (v/w) in the presence of plant protease inhibitor cocktail (Sigma), and cleared by centrifugation. Total protein content was estimated with Bio-Rad Dc protein assay (Bio-Rad). Tomato cleared extracts (10 μg of protein per sample) were separated by SDS-PAGE. For the separation of individual Ig chains, samples were boiled in the presence of Laemmli-running buffer containing 0.1 m dithiothreitol and run in standard 12% acrylamide gels. For the detection of IgA complexes, samples were run in Bio-Rad TX 5% to 12% gradient gels without reducing agent. Gels were transferred to PVDA membranes following standard procedures. LCs and HCs were detected using goat anti-chicken LC and goat anti-chicken IgA alpha-specific antibody, respectively (Bethyl). A rabbit anti-chicken IgY whole-molecule antibody (Sigma) was also used for the detection of IgA complexes. Peroxidase-conjugated secondary antibodies were detected with ECL system (Amersham).
Analysis of Fruit Affected in Carotenoid Biosynthesis
Relative abundance of PDS mRNA in pericarp samples was determined by quantitative reverse transcription-PCR. RNA samples from tomato pericarp were prepared with RNAeasy plant mini kit using on-column RNAse-free DNAse Set treatment (Qiagen), and copied to cDNA with Superscript II reverse transcriptase (Invitrogen). Primers PDSF1 (TCATCAACCTTCCGTGCTTC) and PDSR1 (AACATCCCTTGCCTCCAGC) rendering a 141-bp amplicon were mixed with SYBER GREEN PCR master mix (Applied Biosystems) in appropriated proportions. A tomato actin amplicon was used as internal standard for quantifications. Samples were amplified in triplicate with ABI PRISM 7000 sequence detection system and analyzed with ABI PRISM 7000 SDS software.
For carotene content analysis, tomato pericarp samples (200 mg) from silenced and nonsilenced fruits were ground in N2 (l), extracted, and analyzed as described (Fraser et al., 2000).
Acknowledgments
We thank M.D. Gomez for her help with microscopy techniques and Dr. M.J. Rodrigo for her assistance in carotene measurements. VIGS vectors were supplied by Dr. Dinesh-Kumar (Yale University), and HSP70B:GUS construct was kindly provided by Prof. Maule (John Innes Center, UK).
This work was supported by Generalitat Valenciana (project no. GV04B–28) and the Spanish Ministry of Science and Education (Ramón y Cajal Program).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Antonio Granell (agranell@ibmcp.upv.es).
References
- Aparicio F, Thomas CL, Lederer C, Niu Y, Wang DW, Maule AJ (2005) Virus induction of heat shock protein 70 reflects a general response to protein accumulation in the plant cytosol. Plant Physiol 138: 529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley KJ, Gewert R, Harris WJ (1998) Differential efficiency of expression of humanized antibodies in transient transfected mammalian cells. Hybridoma 17: 559–567 [DOI] [PubMed] [Google Scholar]
- Chen JC, Jiang CZ, Gookin TE, Hunter DA, Clark DG, Reid MS (2004) Chalcone synthase as a reporter in virus-induced gene silencing studies of flower senescence. Plant Mol Biol 55: 521–530 [DOI] [PubMed] [Google Scholar]
- Corthesy B (2002) Recombinant immunoglobulin A: powerful tools for fundamental and applied research. Trends Biotechnol 20: 65–71 [DOI] [PubMed] [Google Scholar]
- D'Aoust MA, Lerouge P, Busse U (2004) Efficient and reliable production of pharmaceuticals in alfalfa. In R Fischer, S Schillberg, eds, Molecular Farming. Wiley-VCH Verlag GmbH & Co., Weinheim, Germany
- Fraser PD, Pinto MES, Holloway DE, Bramley PM (2000) Application of high-performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. Plant J 24: 551–558 [DOI] [PubMed] [Google Scholar]
- Fray RG, Grierson D (1993) Identification and genetic-analysis of normal and mutant phytoene synthase genes of tomato by sequencing, complementation and co-suppression. Plant Mol Biol 22: 589–602 [DOI] [PubMed] [Google Scholar]
- Fu D-Q, Zhu B-Z, Zhu H-L, Jiang W-B, Luo Y-B (2005) Virus-induced gene silencing in tomato fruits. Plant J 31: 299–308 [DOI] [PubMed] [Google Scholar]
- Goodin MM, Dietzgen RG, Schichnes D, Ruzin S, Jackson AO (2002) pGD vectors: versatile tools for the expression of green and red fluorescent protein fusions in agroinfiltrated plant leaves. Plant J 31: 375–383 [DOI] [PubMed] [Google Scholar]
- Groot SPC, Karssen CM (1992) Dormancy and germination of abscisic acid-deficient tomato seeds: studies with the sitiens mutant. Plant Physiol 99: 952–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jani D, Meena LS, Rizwan-ul-Haq QM, Singh Y, Sharma AK, Tyagi AK (2002) Expression of cholera toxin B subunit in transgenic tomato plants. Transgenic Res 11: 447–454 [DOI] [PubMed] [Google Scholar]
- Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5: 387–405 [Google Scholar]
- Kapila J, DeRycke R, VanMontagu M, Angenon G (1997) An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci 122: 101–108 [Google Scholar]
- Liu YL, Schiff M, Dinesh-Kumar SP (2002) Virus-induced gene silencing in tomato. Plant J 31: 777–786 [DOI] [PubMed] [Google Scholar]
- Ma Y, Lin SQ, Gao Y, Li M, Luo WX, Zhang J, Xia NS (2003) Expression of ORF2 partial gene of hepatitis E virus in tomatoes and immunoactivity of expression products. World J Gastroenterol 9: 2211–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marillonnet S, Thoeringer C, Kandzia R, Klimyuk V, Gleba Y (2005) Systemic Agrobacterium tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants. Nat Biotechnol 23: 718–723 [DOI] [PubMed] [Google Scholar]
- Ratcliff F, Martin-Hernandez AM, Baulcombe DC (2001) Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J 25: 237–245 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Concepcion M, Ahumada I, Diez-Juez E, Sauret-Gueto S, Lois LM, Gallego F, Carretero-Paulet L, Campos N, Boronat A (2001) 1-Deoxy-D-xylulose 5-phosphate reductoisomerase and plastid isoprenoid biosynthesis during tomato fruit ripening. Plant J 27: 213–222 [DOI] [PubMed] [Google Scholar]
- Sandhu JS, Krasnyanski SF, Domier LL, Korban SS, Osadjan MD, Buetow DE (2000) Oral immunization of mice with transgenic tomato fruit expressing respiratory syncytial virus-F protein induces a systemic immune response. Transgenic Res 9: 127–135 [DOI] [PubMed] [Google Scholar]
- Sharp JM, Doran PM (2001) Characterization of monoclonal antibody fragments produced by plant cells. Biotechnol Bioeng 73: 338–346 [DOI] [PubMed] [Google Scholar]
- Spolaore S, Trainotti L, Casadoro G (2001) A simple protocol for transient gene expression in ripe fleshy fruit mediated by Agrobacterium. J Exp Bot 52: 845–850 [DOI] [PubMed] [Google Scholar]
- Sung DY, Vierling E, Guy CL (2001) Comprehensive expression profile analysis of the Arabidopsis hsp70 gene family. Plant Physiol 126: 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley AM, Alvarez ML, Jin Y, Kirk DD, Lee SM, Pinkhasov J, Rigano MM, Arntzen CJ, Mason HS (2003) Expression of the B subunit of Escherichia coli heat-labile enterotoxin as a fusion protein in transgenic tomato. Plant Cell Rep 21: 1020–1026 [DOI] [PubMed] [Google Scholar]
- Walmsley AM, Arntzen CJ (2003) Plant cell factories and mucosal vaccines. Curr Opin Biotechnol 14: 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland W (2004) From phage display to plant expression: fulfilling prerequisites for chicken oral immunotherapy against coccidiosis. PhD thesis. Wageningen University, Wageningen, The Netherlands
- Wieland W, Orzaez D, Lammers A, Schots A (2006) Display and selection of chicken IgA phage fragments. Vet Immunol Immunopathol doi/10.1016/j.vetimm.2005.09.012 (in press) [DOI] [PubMed]
- Wroblewski T, Tomczak A, Michelmore R (2005) Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis. Plant Biotechnol J 3: 259–273 [DOI] [PubMed] [Google Scholar]
- Yang YN, Li RG, Qi M (2000) In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J 22: 543–551 [DOI] [PubMed] [Google Scholar]