Abstract
Mitochondrial serine hydroxymethyltransferase (SHMT), combined with glycine decarboxylase, catalyzes an essential sequence of the photorespiratory C2 cycle, namely, the conversion of two molecules of glycine into one molecule each of CO2, NH4+, and serine. The Arabidopsis (Arabidopsis thaliana) mutant shm (now designated shm1-1) is defective in mitochondrial SHMT activity and displays a lethal photorespiratory phenotype when grown at ambient CO2, but is virtually unaffected at elevated CO2. The Arabidopsis genome harbors seven putative SHM genes, two of which (SHM1 and SHM2) feature predicted mitochondrial targeting signals. We have mapped shm1-1 to the position of the SHM1 gene (At4g37930). The mutation is due to a G → A transition at the 5′ splice site of intron 6 of SHM1, causing aberrant splicing and a premature termination of translation. A T-DNA insertion allele of SHM1, shm1-2, and the F1 progeny of a genetic cross between shm1-1 and shm1-2 displayed the same conditional lethal phenotype as shm1-1. Expression of wild-type SHM1 under the control of either the cauliflower mosaic virus 35S or the SHM1 promoter in shm1-1 abrogated the photorespiratory phenotype of the shm mutant, whereas overexpression of SHM2 or expression of SHM1 under the control of the SHM2 promoter did not rescue the mutant phenotype. Promoter-β-glucuronidase analyses revealed that SHM1 is predominantly expressed in leaves, whereas SHM2 is mainly transcribed in the shoot apical meristem and roots. Our findings establish SHM1 as the defective gene in the Arabidopsis shm1-1 mutant.
Photorespiration is caused by the dual affinity of Rubisco for both CO2 and molecular oxygen (Bowes et al., 1971; Ogren and Bowes, 1971; Bowes and Ogren, 1972; Ogren, 1984). Whereas the carboxylation of the acceptor ribulose 1,5-bisphosphate (RuBP) leads to the production of two molecules of 3-phosphoglycerate (two C3 moieties) that can both be reconverted into RuBP by the Calvin cycle, the addition of molecular oxygen to RuBP (oxygenation) yields one molecule of 3-phosphoglycerate and one molecule of 2-phosphoglycolate (a C2 unit). The regeneration of 2-phosphoglycolate to 3-phosphoglycerate involves a reaction sequence that is known as the oxidative C2 cycle or the photorespiratory carbon cycle (termed C2 cycle hereafter). This cycle involves three organelles (chloroplasts, peroxisomes, and mitochondria) and one molecule each of CO2 and NH4+ are liberated during the conversion of two molecules of 2-phosphoglycolate into one molecule of 3-phosphoglycerate. These CO2 and NH4+ molecules have to be refixed by Rubisco and the glutamine synthase/GOGAT system, respectively.
The C2 cycle was elucidated in the 1970s and the enzymatic steps involved as well as some salvage pathways are well established (Leegood et al., 1995; Douce and Neuburger, 1999; Wingler et al., 1999, 2000). Mutants in the C2 cycle have contributed much to our understanding of this important biochemical pathway (Somerville and Ogren, 1982; Leegood et al., 1995; Somerville, 2001). These photorespiratory mutants display a conditional lethal phenotype, which means they are unable to thrive at ambient conditions whereas apparently they are not affected in conditions that suppress photorespiration, such as high CO2 (Somerville and Ogren, 1982; Blackwell et al., 1988).
The Arabidopsis (Arabidopsis thaliana) Ser hydroxymethyltransferase (SHMT) mutant stm was one of the first photorespiratory mutants described by Somerville and Ogren (1981) and was later renamed shm to avoid confusion with the mutant shoot meristemless (Barton and Poethig, 1993). The defective gene in shm, hereafter termed shm1-1, has not been identified until now.
In the initial study of this Arabidopsis mutant, no SHMT activity could be determined in shm1-1 leaf mitochondria and foliar Gly levels under photorespiratory conditions were 40-fold higher in shm1-1 in comparison to the wild type (Somerville and Ogren, 1981). These two observations indicated that a defective mitochondrial SHMT gene accounts for the photorespiratory phenotype of the shm1-1 mutant.
Together with the Gly decarboxylase complex, SHMT is involved in the reversible interconversion of Ser and Gly and both enzymes are closely associated with each other. During the operation of the C2 cycle, one molecule of Gly is first decarboxylated and subsequently deaminated in the Gly decarboxylase complex yielding CO2, NH4+, and the C1 donor, 5,10-methylene tetrahydrofolate (THF), which is used by SHMT to transfer the activated C1 unit onto another molecule of Gly (Douce and Neuburger, 1999). In leaves of C3 plants, mitochondrial SHMT is predominantly involved in the C2 cycle, as evidenced by the conditional lethal photorespiratory phenotype of shm1-1 (Somerville and Ogren, 1981). Very recently, however, a weak shm1 allele was isolated, which we will address as shm1-3 (Moreno et al., 2005). Homozygous shm1-3 mutants exhibit chlorotic lesions but are viable in ambient conditions (Moreno et al., 2005). Because of its compromised C2 cycle, shm1-3 overproduces reactive oxygen species and is thus more susceptible to salt stress and pathogens (Moreno et al., 2005).
Molecular studies of the shm1-1 mutant revealed that SHM transcripts of apparently normal length accumulated in the mutant, although these transcripts were more abundant at elevated CO2 conditions in the mutant than in the wild type (Beckmann et al., 1997). In silico analyses showed that the Arabidopsis genome harbors seven SHM genes, two of which encode gene products that are predicted to be targeted to the mitochondria (McClung et al., 2000; Bauwe and Kolukisaoglu, 2003). AtSHM1 appears to encode the major SHMT isozyme in Arabidopsis leaves and its transcript accumulation is controlled by light and the circadian clock (McClung et al., 2000). Thus, AtSHM1 was considered a good candidate for the defective gene in the shm1-1 mutant (McClung et al., 2000), but this hypothesis has not been directly tested.
In this study, we report on the positional cloning and the molecular characterization of the defective gene in shm1-1, on the isolation of a new allele, shm1-2, and on the complementation of the shm1-1 mutant with the wild-type SHM1 allele. In addition, we show that the gene encoding the second putatively mitochondrial-targeted SHM isozyme in Arabidopsis, SHM2, is predominantly expressed in roots and the shoot apical meristem (SAM), whereas SHM1 encodes the major isoform in leaves. Surprisingly, expression of SHM2 in shm1-1 under the control of either the 35S or the SHM1 promoter failed to complement the photorespiratory shm phenotype, indicating that either SHM2 does not encode a fully functional SHMT protein or the protein is not targeted to mitochondria. Our findings unequivocally demonstrate that At4g37930 (AtSHM1) is crucial for plant growth in ambient air and for proper function of the C2 cycle.
RESULTS
Positional Cloning of the Defective Gene in the shm1-1 Mutant
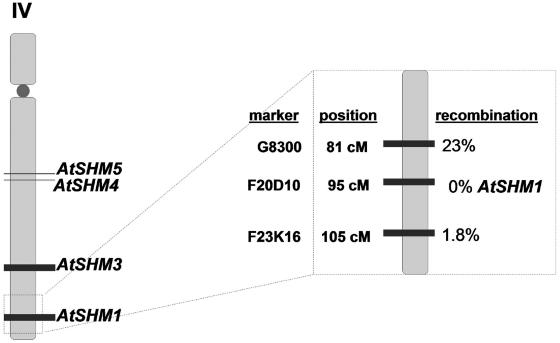
Somerville and Ogren (1981) have demonstrated that the Arabidopsis mutant shm1-1 lacks mitochondrial SHMT activity and therefore displays a photorespiratory phenotype. The Arabidopsis genome encodes seven putative SHMT proteins, two of which (AtSHM1 and AtSHM2) are presumably localized in the mitochondrial matrix as indicated by the presence of a putative mitochondrial targeting signal (McClung et al., 2000). The subcellular localization was predicted by computer algorithms and not further supported by experimental evidence (McClung et al., 2000; Bauwe and Kolukisaoglu, 2003). In addition, conceptual translations of genes in the Arabidopsis genomes are frequently hampered by the inclusion or omission of exon sequences, thus raising the possibility that additional, unrecognized mitochondrial SHMT isozymes are encoded by the Arabidopsis genome. To narrow down the number of candidates for the defective gene in shm1-1, the mutation was mapped using a cleaved amplified polymorphic sequence (CAPS) marker approach (Konieczny and Ausubel, 1993). To this end, a mapping population was developed from a genetic cross between shm1-1, which is in the ecotype Columbia (Col-0) background, and Landsberg erecta (Ler). For F2 individuals, 186 of 837 (22.1%) displayed a photorespiratory phenotype, and these plants were selected for the mapping procedure. Using all 186 F2 individuals showing the mutant phenotype, we mapped the shm1 locus to 95 cm on chromosome IV (Fig. 1) of the recombinant inbred map (Lister and Dean, 1993). AtSHM1 (At4g37930) is located at this map position and a CAPS marker (F20D10) was developed for this locus. No recombination of the marker F20D10 and the shm1 locus was observed (Fig. 1), indicating that AtSHM1 is deficient in shm1-1.
Figure 1.
Cartoon depicting the map position of the shm1-1 locus on the recombinant inbred map (Lister and Dean, 1993) according to our mapping data. A total of 186 F2 individuals of a cross between the shm1-1 mutant (background Col-0) and Ler showing a photorespiratory phenotype were scored for the cosegregation of 23 CAPS markers (see Supplemental Table II) with the photorespiratory phenotype as described in “Materials and Methods.”
A Mutation in the 5′ Splice Site of Intron 6 of AtSHM1 Causes Aberrant Splicing of the SHM1 mRNA in shm1-1
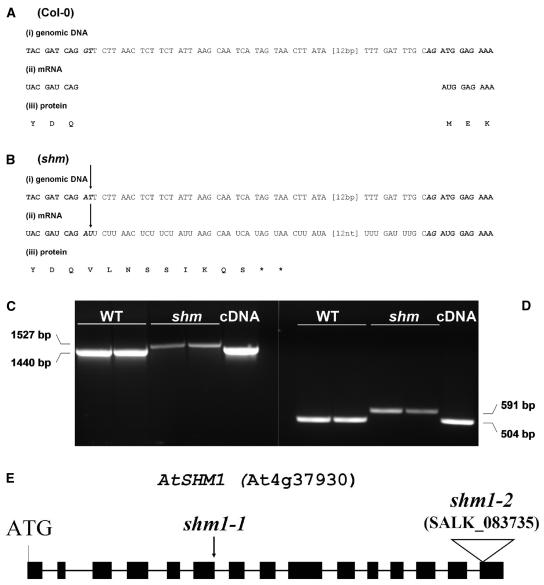
Genetic mapping strongly indicated that AtSHM1 is the affected gene in shm1-1. Therefore, the SHM1 gene was sequenced in the mutant and a G → A transition was detected in the consensus sequence of the 5′ splice donor site of intron 6 in the mutant allele (Fig. 2B). To check whether the mutation of the consensus splice site would cause mis-splicing of the mRNA, the corresponding mRNA from the shm1-1 mutant was amplified by reverse transcription (RT)-PCR and sequenced. Sequencing demonstrated that the mutation led to aberrant splicing of intron 6 during shm1-1 mRNA maturation (Fig. 2B), producing a slightly longer shm1-1 mRNA (Fig. 2, C and D). Although intron 6 spans 87 bp and would thus not cause a translational frameshift in the mutant, it also contains several in-frame translational stop codons (Fig. 2B). Hence, a premature termination of AtSHM1 mRNA translation is to be expected, which would account for the absence of mitochondrial SHMT activity in shm1-1.
Figure 2.
Comparison between the genomic DNA (i), the mRNA (ii), and the derived protein sequence (iii) of wild-type (A) and shm1-1 mutants (B). Only the sequence between the end of exon 6 (start-ATG + 1,272 bp) and the beginning of exon 7 (start-ATG + 1,376 bp) is shown. Bold, Exon; italics, intron; bold italics, conserved splice motif. The arrows indicate the point mutation in shm1-1. Asterisk (*), Translational stop. C, RT-PCR products from wild-type and shm1-1 mRNA obtained with primers prSHM4 and prSHM9 (see Table I). D, Primers prSHM5 and prSHM6 (see Table I). Two independent RNA preparations of each line were taken for the RT-PCR reactions. From left to right, Two lanes wild-type (Col-0); two lanes shm1-1 mutant; one lane cDNA. The RT-PCR fragment sizes are indicated beside the image. Plasmid containing the SHM1 expressed sequence tag clone 148C5T7 was used as a positive control in the PCR reaction. E, Cartoon showing AtSHM1 gene structure and the shm1-2 T-DNA insertion site in the last of the 15 SHM1 exons. As a reference, the point mutation in shm1-1 is indicated by an arrowhead.
A Presumptive Loss-of-Function T-DNA Allele shm1-2 Also Exhibits a Photorespiratory Phenotype
We have isolated a T-DNA insertion allele for AtSHM1 from the SALK collection (SALK_083735), hereafter termed shm1-2, which was tested for allelism with shm1-1. The T-DNA insertion in shm1-2 is located in the last of the 15 exons of the SHM1 gene (Fig. 2E). The shm1-2 T-DNA allele has recently been used for an allelism test with a third, weak shm allele (referred to as shm1-3 in this study; originally designated shmt1-1 by Moreno et al. (2005). According to Moreno et al. (2005), shm1-2 never reached maturity and exhibited a chlorotic and dwarf phenotype. In our hands, shm1-2 lines homozygous for the T-DNA insertion uniformly showed a photorespiratory phenotype of chlorosis at ambient CO2 levels that was fully rescued at 3% CO2 (Fig. 3, B and C), which could also be inferred from the description by Moreno et al. (2005). SHMT activity in crude leaf extracts of both shm1-1 and shm1-2 was approximately 10% of the wild type (Table I). This indicates that (1) AtSHM1 is the predominant SHMT isoform in leaves and (2) shm1-1 and shm1-2 are loss-of-function alleles. Homozygous shm1-2 was crossed to shm1-1 and the resulting F1 progeny uniformly exhibited a photorespiratory phenotype (Fig. 3E) and similar total SHMT activity to both mutant parental lines (Table I). A T-DNA loss-of-function mutant of SHM2 (SALK _095881) that lacked detectable SHM2 mRNA and presumably lacked SHM2 protein function did not display the conditional lethal photorespiratory phenotype at ambient conditions (data not shown), indicating that SHM2 is not functionally equivalent to SHM1, although the SHM2 gene product is predicted to be targeted to the mitochondrial matrix (McClung et al., 2000; Bauwe and Kolukisaoglu, 2003).
Figure 3.
Phenotypes of representative Col-0 wild-type (A), shm1-2 (C), shm1-1 (D), and shm1-1 × shm1-2 F1 (E) plants after 20 d in ambient air. Plants had been grown at 3% CO2 for 1 week before they were shifted to air. B, Representative shm1-2 individual that was constantly grown at 3% CO2. Please note that all images represent the same scale.
Table I.
SHMT activity in Arabidopsis leaf extracts
Total SHMT activity was assayed in leaf extracts from the wild type (Col-0), the shm1-1 mutant, two independent primary shm1-1-35S:SHM1 transformants (2T1 and 3T1), homozygous shm1-2, and F1 progeny from a cross between shm1-1 and shm1-2. Results represent the means of 12 replicates ±se that were obtained in four independent experiments.
| Genetic Background | SHMT Activity |
|---|---|
| nmol mg−1 min−1 | |
| Col-0 | 1.64 ± 0.07 |
| shm1-1 | 0.18 ± 0.01 |
| 2T1 | 1.60 ± 0.06 |
| 3T1 | 1.49 ± 0.08 |
| shm1-2 | 0.20 ± 0.02 |
| shm1-2 × shm1-1 | 0.22 ± 0.02 |
SHM1 and SHM2 Are Not Redundant
To assess whether the failure of SHM2 to complement a lack of SHM1 function in shm1-1 is due to different expression patterns of SHM1 and SHM2, we overexpressed both genes in shm1-1 under the control of the strong constitutive cauliflower mosaic virus (CaMV) 35S promoter. In addition, we performed a promoter-swap experiment. The wild-type full-length cDNAs encoded by the SHM1 (expressed sequence tag 148C5T7) and SHM2 (C104687; Arabidopsis Biological Resource Center [ABRC]), were expressed either under the control of the constitutive CaMV 35S promoter or approximately 1 kb of their own proximal promoters, or the promoter of the respective other isoform (promoter swap) in stably transformed shm1-1 mutants. Figure 4 shows representative individuals of all transformants (Fig. 4, B–D and F–H), as well as empty-vector (Fig. 4E) and wild-type (Fig. 4A) controls after growth for 28 d in ambient air. SHM2 expression failed to rescue the conditional lethal phenotype, regardless of the promoter employed (Fig. 4, D and F). In contrast, expression of SHM1 under the control of the constitutive CaMV 35S promoter or its endogenous promoter restored growth (Fig. 4, B and C) and total foliar SHMT activity to wild-type levels (Table I). However, transformation of shm1-1 with pSHM2:SHM1 did not complement the mutant (Fig. 4G), indicating that the expression pattern and/or strength of the SHM2 promoter are not sufficient to permit complementation of the mutant phenotype.
Figure 4.
Complementation analysis of shm1-1 with chimeric SHM constructs and by constitutive overexpression of SHM1 and SHM2. A, Col-0 control, shm1-1 mutant plants were transformed with CaMV 35S:SHM1 (B), pSHM1:SHM1 (C), pSHM1:SHM2 (D), empty-vector pH2GW7.0 vector only (E), CaMV 35S:SHM2 (F), pSHM2:SHM1 (G), and pSHM2:SHM2 (H). Representative T2 individuals are shown after growth for 28 d in ambient air. Please note that the images in A and B are presented at 1.5× lower magnification.
To further test this hypothesis, the Escherichia coli uidA reporter gene, encoding β-glucuronidase (GUS), was fused to the SHM1 and SHM2 promoter fragments used in the complementation study described above and the reporter gene constructs were transformed into Arabidopsis. While pSHM1 mediated strong GUS activity in the entire shoot, including leaves (Fig. 5A), little GUS activity was detected in the shoots of plants carrying pSHM2:GUS, in which GUS activity was restricted to the roots, the SAM, and the first true leaf (Fig. 5, B and C). GUS expression from pSHM2 was not observed in mature, fully expanded leaves. The reporter gene data are supported by AtSHM2 transcript profiles generated using the digital northern tool of GENEVESTIGATOR (Zimmermann et al., 2004), indicating that AtSHM2 is expressed only at very low levels in photosynthetic tissues (data not shown). In addition, transcript coresponse analysis using the Arabidopsis Co-Response Database (Steinhauser et al., 2004a, 2004b) showed that only AtSHM1 transcripts, but not those of other putative SHM genes in Arabidopsis, show transcriptional coresponse with genes encoding enzymes of the photorespiratory pathway, such as phosphoglycolate phosphatase or hydroxypyruvate reductase (data not shown).
Figure 5.
Localization of SHM1 and SHM2 promoter activity. T2 progeny of stably transformed wild-type plants carrying pSHM1:GUS (A) or pSHM2:GUS (B and C) were stained for GUS activity as described in “Materials and Methods.”
DISCUSSION
The Arabidopsis mutant shm1-1 was isolated in the early 1980s and since then it has been clear that insufficient mitochondrial SHMT activity accounted for the photorespiratory phenotype of the mutant (Somerville and Ogren, 1981). However, the molecular identity of the shm1-1 mutation has remained elusive and it had been hypothesized that a locus required for SHMT activity rather than an SHMT structural gene was affected in shm1-1 (Beckmann et al., 1997; McClung et al., 2000).
This hypothesis seemed evident, since SHM1 steady-state transcript levels were increased in the shm1-1 mutant under nonphotorespiratory conditions (Beckmann et al., 1997) and because no apparent difference in transcript size between the mutant and the wild type was observed (Beckmann et al., 1997). Furthermore, the products of at least two SHM genes, SHM1 and SHM2, were predicted to be targeted to the mitochondria (McClung et al., 2000). Thus, both genes could potentially play a role in the C2 cycle, making functional redundancy of the two mitochondrial SHMT isoforms likely.
This study provides unequivocal evidence that mutation in the shm1-1 mutant indeed affects SHM1 and that a second putative mitochondrial SHMT encoded by SHM2 cannot complement loss of SHM1 function. SHM1 and SHM2 are highly similar at the nucleotide and amino acid levels (McClung et al., 2000; Bauwe and Kolukisaoglu, 2003) and are likely to represent the products of a duplication event: Arabidopsis has undergone multiple rounds of polyploidization and a recent estimate is that 27% of the gene pairs formed by polyploidization persist in the genome (Blanc and Wolfe, 2003). However, the majority of these gene pairs have undergone functional divergence (Blanc and Wolfe, 2004), as is apparently the case for SHM1 and SHM2.
Whereas the conditional lethal photorespiratory phenotype of the shm1-1 mutant could be cured by expression of wild-type SHM1 under the control of either its own or the constitutive viral CaMV 35S promoter, SHM1 expression from the SHM2 promoter failed to rescue the shm1-1 mutant phenotype (Fig. 4). SHM2 expression in the mutant background was not able to rescue the mutant, regardless of the promoter used to drive SHM2 expression (Fig. 4). In addition, strong GUS activity was detected in leaves when GUS expression was driven by the SHM1 promoter, whereas GUS expression driven by the SHM2 promoter was restricted to roots, the SAM, and the first true leaves (Fig. 5). Probing SHM1 and SHM2 promoter activities provides a straightforward explanation as to why SHM1 expression driven by the SHM2 promoter could not cure the photorespiratory phenotype of shm1-1 (Fig. 4): The SHM2 promoter lacks activity in rosette leaves, where photorespiration takes place. Thus, in the case of SHM1 and SHM2, functional divergence has apparently occurred at the promoter level. It can thus be unambiguously concluded that AtSHM1 is the SHMT coding gene involved in the C2 cycle. Surprisingly, however, SHM2 was not able to complement the shm1-1 phenotype even under the control of a strong promoter. Apparently, functional divergence of SHM1 and SHM2 has also occurred at the level of enzymatic activity or subcellular targeting; either SHM2 does not encode a fully functional SHMT protein or the SHM2 gene product is not targeted to the mitochondrial matrix. Further studies on the subcellular localization and activity of the SHMT2 protein are in progress to resolve this question.
Together with the recent identification of the peroxisomal Ala:glyoxylate aminotransferase (Liepman and Olsen, 2001, 2003) and d-glycerate 3-kinase (Boldt et al., 2005), the molecular identification of the defective gene in shm1-1 completes the identification of all genes encoding enzymes known to be involved in the photorespiratory C2 cycle. However, the C2 cycle also requires transporters that catalyze the transport of photorespiratory intermediates across the membranes of chloroplasts, peroxisomes, and mitochondria. With the exception of the plastidic oxoglutarate/malate and Glu/malate translocators (Weber et al., 1995; Weber and Flügge, 2002; Renné et al., 2003) none of these transporters are known (Linka and Weber, 2005). It remains a challenge for the future to identify all genes involved in the C2 cycle.
MATERIALS AND METHODS
Seed Material
Seeds of the shm1-1 mutant (CS8010) and of the SALK T-DNA insertion line SALK_083735 (shm1-2) were obtained from the ABRC.
Plant Growth
Seeds were sterilized as described by Clough and Bent (1998) and germinated at room temperature in a 12-h/12-h light/dark cycle on one-half-strength Murashige and Skoog medium in 3% CO2 at a photon flux density of approximately 100 μmol m−2 s−1. Plantlets were transferred to soil after the first four primary leaves had emerged and the growth cycle was allowed to complete under the same conditions.
Mapping of the shm1-1 Locus
The shm1-1 mutant (Col-0) was crossed to Ler, the F1 was self-fertilized, and the resulting F2 mapping population (837 F2 individuals) was grown for 7 weeks at 1,300 μL mL−1 CO2. The population was transferred to ambient conditions, the photorespiratory phenotype was scored 4 d after the transfer, and the cosegregation of the shm1-1 phenotype with 23 CAPS markers (obtained from The Arabidopsis Information Resource [TAIR]; see also www.arabidopsis.org, unless stated otherwise; see Supplemental Table II) was determined.
Constitutive Overexpression of SHM1 and SHM2 and Complementation Studies with Chimeric SHM1 and SHM2 Expression Constructs
For constitutive overexpression of wild-type SHM1 in the shm1-1 mutant background, a BamHI-KpnI fragment of expressed sequence tag 148C5T7 (GenBank accession no. T75910; ABRC), encoding the full-length SHM1 cDNA, was cloned into a modified pGREENII bar vector (Hellens et al., 2000) in which the multiple cloning site was replaced by the EcoRI-HindIII fragment of the 35S promoter/nopaline synthase terminator cassette derived from pBIN-AR (Höfgen and Willmitzer, 1990). The RT-PCR product corresponding to the full-length SHM2 cDNA was cloned in pGEMT easy, the resulting EcoRI fragment was cloned in pENTR-A vector, and then moved to the modified pB7GWIWG2I destination vector. shm1-1 mutants were transformed with the construct by the floral-dip method (Clough and Bent, 1998). The T1 progeny were grown at 0.3% CO2 for 14 d before the plantlets were selected for the bar marker by spraying them with a Basta solution (0.025% [w/v] phosphinotricine, 0.1% [v/v] Tween 20) twice a week. The Basta-resistant plants were then surveyed for the absence of the photorespiratory phenotype after transfer to ambient air.
Chimeric constructs were generated by Gateway technology (Invitrogen). A PstI and a NotI restriction site were added to the N and C terminus of the SHM1 cDNA; a PstI and an EcoRI site were added to the N and C terminus of the SHM2 cDNA, respectively, using the primers: SHM1PstI Fwd (5′CCATTTTGTTATTTCTGCAGTCTCTTCTCTCTCGTTCATG), SHM1NotI Rev (5′-ATATCTCGAGTGCGGCCGCCCTTAGTTCTTGTACTTCATGGTTTC), SHM2PstI Fwd (5′-AATCGCACTCACTGCAGAGAAACAGAGAAGACGATAGAT), and SHM2NotI Rev (5′-ATATCTCGAGTGCGGCCGCCCGCTACTCTTTGTATCTCATCGTCT CTTTC).
Upstream regions of 925 bp from the SHM1 and 1,234 bp of the SHM2 gene were amplified by PCR on Col-0 genomic DNA using the primers: pSHM1BamHI (5′-CTTTTTTAATTGATCTGGATCCTTCACAAACATGCATGCACCATT-3′), pSHM1PstI (5′-CATGAACGAGAGAGAAGAGACTGCAGAAATAACAAAATTGG-3′), pSHM2PstI (5′-TCTTCTCTGTTTCTCTGCAGTGAGTGCGATTA-3′), and pSHM2EcoRI (5′-AATTGCTTCATTTTCGGAATTCCACAAGCTTCTTCTTTTTTTA-3′).
Following restriction digestion with the appropriate endonucleases, different combinations were ligated into the pENTR vector, transformed into Escherichia coli, and transferred to the modified pH2GW7.0 gateway vector (without 35S promoter) by LR clonase reactions. Agrobacterium tumefaciens strain AGL1 was transformed with the resulting plasmids by floral dip as described above. The T1 generation was grown at 3% CO2 for 14 d during selection for hygromycin-resistant individuals and Hyg plantlets were then assessed for the presence of the transgene by PCR (as described below) and for the photorespiratory phenotype as described above.
Screening of Transgenic Plant Populations by PCR
Genomic DNA was extracted from Arabidopsis (Arabidopsis thaliana) leaves as described by Edwards et al. (1991) and PCR analysis of transgenic progeny was conducted according to standard protocols using appropriate primer pairs.
To identify SHM1 T-DNA insertion mutants, we used the gene-specific primer Shmt1-TDNA rvs (5′-GTTACAGCTTTCATCATCCCACAC-3′) together with the T-DNA left-border-specific primer LBb1 (5′-GCGTGGACCGCTTGCTGCAACT-3′).
Promoter-uidA Fusions
For promoter-uidA fusions, the promoter regions (925 bp for pSHM1, 1,234 bp for pSHM2) were amplified using two-step PCR reactions: The first step was performed with specific primers containing 12 nucleotides of the attB sites (in capitals), as well as gene-specific nucleotides (lowercase): SHM1-B1guspro (5′-AAAAAGCAGGCTCCcttgatgtttcacaaacatgc-3′), SHM1-B2guspro (5′-AGAAAGCTGGGTCttttcgctaaacctctctct-3′), SHM2-B1guspro (5′-AAAAAGCAGGCTCCtcgagattaacaagcttctt-3′), and SHM2-B2guspro (5′-AGAAAGCTGGGTCttctctatctatcgtcttct-3′).
In the second PCR step, the universal attB adapter primers were used to amplify the product produced in step 1. The resulting PCR products were moved into pDONR207 by BP clonase reactions (Invitrogen). The promoters were then transferred to the pBGWFS7 destination vector using the LR clonase reaction. T1 populations were selected with Basta, as described above for the chimeric constructs and bar plants were examined for the presence of the transgene as described below.
Histochemical Analysis of GUS Activity
Plant tissues were incubated in GUS assay solution (50 mm sodium phosphate, pH 7.2, 0.5 mm potassium ferrocyanide, 0.5 mm potassium ferricyanide, 20% methanol, and 2 mm 5-bromo-4-chloro-β-glucuronide) at 37°C for 12 to 16 h, essentially as described by Jefferson et al. (1987; Jefferson, 1989). Slight vacuum was applied to facilitate substrate infiltration. Chlorophyll-containing tissue was cleared in 70% ethanol for photographic analysis.
Sequencing of Genomic DNA
Genomic DNA from wild-type and shm1-1 inflorescences was isolated using a urea-based buffer (Liu et al., 1995), 20 ng of the genomic DNA preparations were subjected to PCR amplification with different sets of AtSHM1-specific primers (see Supplemental Table I) according to standard protocols and the obtained fragments were subcloned into pGEMT-Easy (Promega). Standard dye-termination sequencing reactions containing 1 μg of vector with subcloned fragments and 30 pmol of primer were resolved on an ABI Prism 3100 sequencer.
Sequencing of SHM1 mRNA
RNA from wild-type and shm1-1 mutant Arabidopsis leaves was isolated by the Z6 buffer method (Logemann et al., 1987) and aliquots of the RNA preparations were reverse transcribed using the ImProm reverse transcriptase kit (Promega) following the manufacturer's instructions. The SHM1 cDNAs were amplified by PCR with two different primer sets, prSHM4-prSHM9 and prSHM5-prSHM6 (see Table I) and sequenced as described previously.
Assay of SHMT Activity
Crude extracts were prepared by grinding approximately 400 mg of leaf tissue in 300 μL of extraction buffer (50 mm phosphate buffer, 1 mm β-mercaptoethanol, and 2.5 mm EDTA) and the extracts were clarified by centrifugation at 20,000g for 10 min. SHMT activity was tested by following the conversion of radioactive carbon from Ser to methylene THF (Geller and Kotb, 1989). The assay was performed with 0.25 mm pyridoxal 5′ phosphate, 2 mm THF, 0.4 mm Ser [3-3H] Ser (33 Ci mmol−1), and the crude extract in a final volume of 100 μL. The enzyme assays were performed at 37°C for 20 min. Twenty-five microliters of the reaction mixture were streaked onto Whatman DE.81 paper. After drying the filter, unreacted Ser was removed by washing the filter three times for 20 min with 20 mL of water. The radioactivity associated with methylene THF was measured by liquid scintillation counting.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Veronica Maurino (University of Cologne) for the kind provision of the modified pGREENII vector that was used for the CaMV 35S-driven overexpression of the wild-type SHM1 gene in the mutants. Momoko Minakawa is also acknowledged for assistance with plant cultures.
This work was supported by the Deutsche Forschungsgemeinschaft (postdoctoral research fellowship to L.M.V. and grant no. WE2231/2–1 to A.P.M.W.), the National Science Foundation (grant no. MCB–0348074 to A.P.M.W.), and the U.S. Department of Agriculture (grant no. 2002–01392 to C.R.M.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with journal policy described in the Instructions for Authors (http://www.plantphysiol.org) is: Andreas P.M. Weber (aweber@msu.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.071399.
References
- Barton MK, Poethig RS (1993) Formation of the shoot apical meristem in Arabidopsis thaliana—an analysis of development in the wild-type and in the shoot meristemless mutant. Development 119: 823–831 [Google Scholar]
- Bauwe H, Kolukisaoglu U (2003) Genetic manipulation of glycine decarboxylation. J Exp Bot 54: 1523–1535 [DOI] [PubMed] [Google Scholar]
- Beckmann K, Dzuibany C, Biehler K, Fock H, Hell R, Migge A, Becker TW (1997) Photosynthesis and fluorescence quenching, and the mRNA levels of plastidic glutamine synthetase or of mitochondrial serine hydroxymethyltransferase (SHMT) in the leaves of the wild-type and of the SHMT-deficient stm mutant of Arabidopsis thaliana in relation to the rate of photorespiration. Planta 202: 379–386 [DOI] [PubMed] [Google Scholar]
- Blackwell RD, Murray AJS, Lea PJ, Kendall AC, Hall NP, Turner JC, Wallsgrove RM (1988) The value of mutants unable to carry out photorespiration. Photosynth Res 16: 155–176 [DOI] [PubMed] [Google Scholar]
- Blanc G, Wolfe KH (2003) A recent paleopolyploidy superimposed on older large-scale duplications in the Arabidopsis genome. Genome Res 13: 137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc G, Wolfe KH (2004) Functional divergence of duplicated genes formed by polyploidy during Arabidopsis evolution. Plant Cell 16: 1679–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldt R, Edner C, Kolukisaoglu U, Hagemann M, Weckwerth W, Wienkoop S, Morgenthal K, Bauwe H (2005) d-Glycerate 3-kinase, the last unknown enzyme in the photorespiratory cycle in Arabidopsis, belongs to a novel kinase family. Plant Cell 17: 2413–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes G, Ogren WL (1972) Oxygen inhibition and other properties of soybean ribulose 1,5-diphosphate carboxylase. J Biol Chem 247: 2171–2176 [PubMed] [Google Scholar]
- Bowes G, Ogren WL, Hageman RH (1971) Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun 45: 716–722 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Douce R, Neuburger M (1999) Biochemical dissection of photorespiration. Curr Opin Plant Biol 2: 214–222 [DOI] [PubMed] [Google Scholar]
- Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19: 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller AM, Kotb MY (1989) A binding assay for serine hydroxymethyltransferase. Anal Biochem 180: 120–125 [DOI] [PubMed] [Google Scholar]
- Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol 42: 819–832 [DOI] [PubMed] [Google Scholar]
- Höfgen R, Willmitzer L (1990) Biochemical and genetic analysis of different patatin isoforms expressed in various organs of potato (Solanum tuberosum). Plant Sci 66: 221–230 [Google Scholar]
- Jefferson RA (1989) The GUS reporter gene system. Nature 342: 837–838 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny A, Ausubel FM (1993) A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based marker. Plant J 4: 403–410 [DOI] [PubMed] [Google Scholar]
- Leegood RC, Lea PJ, Adcock MD, Häusler RE (1995) The regulation and control of photorespiration. J Exp Bot 46: 1397–1414 [Google Scholar]
- Liepman AH, Olsen LJ (2001) Peroxisomal alanine: glyoxylate aminotransferase (AGT1) is a photorespiratory enzyme with multiple substrates in Arabidopsis thaliana. Plant J 25: 487–498 [DOI] [PubMed] [Google Scholar]
- Liepman AH, Olsen LJ (2003) Alanine aminotransferase homologs catalyze the glutamate:glyoxylate aminotransferase reaction in peroxisomes of Arabidopsis. Plant Physiol 131: 215–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linka M, Weber AP (2005) Shuffling ammonia between mitochondria and plastids during photorespiration. Trends Plant Sci 10: 461–465 [DOI] [PubMed] [Google Scholar]
- Lister C, Dean C (1993) Recombinant inbred lines for mapping RFLP and phenotypic markers in Arabidopsis thaliana. Plant J 4: 745–750 [DOI] [PubMed] [Google Scholar]
- Liu Y-G, Mitsukawa N, Oosumi T, Whittier RF (1995) Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J 8: 457–463 [DOI] [PubMed] [Google Scholar]
- Logemann J, Schell J, Willmitzer L (1987) Improved method for the isolation of RNA from plant tissues. Anal Biochem 163: 16–20 [DOI] [PubMed] [Google Scholar]
- McClung CR, Hsu M, Painter JE, Gagne JM, Karlsberg SD, Salome PA (2000) Integrated temporal regulation of the photorespiratory pathway. Circadian regulation of two Arabidopsis genes encoding serine hydroxymethyltransferase. Plant Physiol 123: 381–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JI, Martin R, Castresana C (2005) Arabidopsis SHMT1, a serine hydroxymethyltransferase that functions in the photorespiratory pathway influences resistance to biotic and abiotic stress. Plant J 41: 451–463 [DOI] [PubMed] [Google Scholar]
- Ogren WL (1984) Photorespiration: pathways, regulation, and modification. Annu Rev Plant Biol 35: 415–442 [Google Scholar]
- Ogren WL, Bowes G (1971) Ribulose diphosphate carboxylase regulates soybean photorespiration. Nat New Biol 230: 159–160 [DOI] [PubMed] [Google Scholar]
- Renné P, Dreßen U, Hebbeker U, Hille D, Flügge UI, Westhoff P, Weber APM (2003) The Arabidopsis mutant dct is deficient in the plastidic glutamate/malate translocator DiT2. Plant J 35: 316–331 [DOI] [PubMed] [Google Scholar]
- Somerville CR (2001) An early Arabidopsis demonstration: resolving a few issues concerning photorespiration. Plant Physiol 125: 20–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville CR, Ogren WL (1981) Photorespiration-deficient mutants of Arabidopsis thaliana lacking mitochondrial serine transhydroxymethylase activity. Plant Physiol 67: 666–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville CR, Ogren WL (1982) Isolation of photorespiratory mutants in Arabidopsis thaliana. In M Edelman, RB Hallik, NH Chua, eds, Methods in Chloroplast Molecular Biology. Elsevier, Amsterdam, pp 129–138
- Steinhauser D, Junker BH, Luedemann A, Selbig J, Kopka J (2004. a) Hypothesis-driven approach to predict transcriptional units from gene expression data. Bioinformatics 20: 1928–1939 [DOI] [PubMed] [Google Scholar]
- Steinhauser D, Usadel B, Luedemann A, Thimm O, Kopka J (2004. b) CSB.DB: a comprehensive systems-biology database. Bioinformatics 20: 3647–3651 [DOI] [PubMed] [Google Scholar]
- Weber A, Flügge UI (2002) Interaction of cytosolic and plastidic nitrogen metabolism in plants. J Exp Bot 53: 865–874 [DOI] [PubMed] [Google Scholar]
- Weber A, Menzlaff E, Arbinger B, Gutensohn M, Eckerskorn C, Flügge UI (1995) The 2-oxoglutarate/malate translocator of chloroplast envelope membranes: molecular cloning of a transporter containing a 12-helix motif and expression of the functional protein in yeast cells. Biochemistry 34: 2621–2627 [DOI] [PubMed] [Google Scholar]
- Wingler A, Lea PJ, Leegood RC (1999) Photorespiratory metabolism of glyoxylate and formate in glycine-accumulating mutants of barley and Amaranthus edulis. Planta 207: 518–526 [Google Scholar]
- Wingler A, Lea PJ, Quick WP, Leegood RC (2000) Photorespiration: metabolic pathways and their role in stress protection. Philos Trans R Soc Lond B Biol Sci 355: 1517–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR: Arabidopsis microarray database and analysis toolbox. Plant Physiol 136: 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.