Abstract
The functions of plant glutathione S-transferases (GSTs) under normal growth conditions are poorly understood, but their activity as detoxification enzymes has been harnessed in agriculture for selective weed control. Herbicide safeners protect monocot crops from herbicide injury but have little effect on weedy monocot or dicot species. Protection by safeners is associated with expression of herbicide-metabolizing enzymes including GSTs, but the basis for selective action of safeners between monocots and dicots is not known. To address this question we have studied the response of Arabidopsis (Arabidopsis thaliana) to various safeners. Benoxacor, fenclorim, and fluxofenim did not protect Arabidopsis from herbicide injury but did induce RNA expression of the glutathione-conjugate transporters encoded by AtMRP1, AtMRP2, AtMRP3, and AtMRP4. These safeners also induced the organ-specific expression of AtGSTU19 and AtGSTF2, two previously characterized Arabidopsis GSTs from different classes of this enzyme family. RNA hybridization, immunoblot, and reporter gene analyses indicated expression of AtGSTU19 induced by safeners predominated in roots. To test the hypothesis that increased expression of AtGSTU19 would be sufficient to provide tolerance to chloroacetamide herbicides, a chimeric gene was produced containing the open reading frame for this GST driven by a constitutive promoter. Plants containing this transgene had a modest increase in AtGSTU19 protein, predominantly in roots, but this had no effect on tolerance to chloroacetamide herbicides. The localized induction of GSTs by safeners in roots of Arabidopsis may explain why these compounds are unable to provide herbicide tolerance to dicot plant species.
Glutathione S-transferases (GSTs) are found in plants, animals, fungi, and some bacteria, yet their role in plant biology is poorly understood. Most GSTs catalyze the conjugation of glutathione (GSH) to a variety of electrophilic substrates. However, some GSTs can function as GSH peroxidases and ligandins, making it difficult to assign specific roles for individual GST enzymes (Edwards et al., 2000). Extensive work in vertebrate systems has demonstrated the involvement of GSTs in metabolism of xenobiotics and mitigation of the effects of oxidative stress (Sheehan et al., 2001). In particular, GSTs are responsible for the metabolism of toxic compounds in the mammalian liver, including many drugs.
Thirty years of research has clearly demonstrated that plant GSTs play a similar role, contributing to the metabolism of xenobiotics and endogenous toxic compounds within plant cells (Sandermann, 1994). Recent studies have revealed the diversity of the GST super family in higher plants (McGonigle et al., 2000; Wagner et al., 2002), and documented the ability of GSTs from higher and lower plant species alike to metabolize xenobiotics (Pflugmacher et al., 2000). Plant GSTs can detoxify a variety of agrichemicals (Cole et al., 1997; Schröder, 1997; Cole and Edwards, 2000). By conjugating herbicides to GSH, GSTs effectively reduce the toxicity of these compounds and target them for degradation in the vacuole or apoplast (Marrs, 1996). Tolerance of certain monocot plants to herbicides is correlated with expression of herbicide-detoxifying GSTs in the foliage of these species (Hatton et al., 1999).
Safeners are synthetic compounds that enhance herbicide tolerance in selected monocot crops without impairing herbicide susceptibility in target weeds (Davies and Caseley, 1999). It is thought that safeners reduce herbicide toxicity in crops by inducing the expression of GSTs capable of conjugating the herbicide to GSH. GST-mediated metabolism of herbicides in maize (Zea mays; Dixon et al., 1997a, 1997b), sorghum (Sorghum bicolor; Gronwald and Plaisance, 1998), wheat (Triticum aestivum; Cummins et al., 1997; Pascal and Scalla, 1999), rice (Oryza sativa; Wu et al., 1999; Deng and Hatzios, 2002a, 2002b), and barley (Hordeum vulgare; Scalla and Roulet, 2002) can be selectively increased by treatment with herbicide safeners. The utility of safeners in agriculture depends on them not enhancing herbicide tolerance in target weeds. However, the basis for selectivity of safeners is unknown and has been identified as an important question in several recent reviews (Davies and Caseley, 1999; Abu-Qare and Duncan, 2002; Hatzios, 2003; Riechers et al., 2003).
It is well established that safeners induce the expression of GSTs in cereals, but few studies have examined safener action in dicot species. Induction of GSTs by safeners in pea (Pisum sativum; Edwards, 1996) and Arabidopsis (Arabidopsis thaliana; DeRidder et al., 2002) has demonstrated that dicot species can perceive and respond to safeners. The objective of this study was to gain insight into the basis for safener selectivity. We examined the response of Arabidopsis seedlings to safener treatment and characterized the expression of two GSTs in response to safeners. We provide evidence that localization of GST expression to certain plant organs or tissues may be an important factor determining safener efficacy. These results indicate that in Arabidopsis induction of GSTs with activity against herbicides does not occur in the appropriate tissues at a high enough level to confer herbicide tolerance and may account for the selective action of safeners in grass crops.
RESULTS
Effect of Safeners on Herbicide Toxicity in Arabidopsis
Chloroacetamide herbicides are a group of nonselective herbicides currently used in conjunction with safeners for weed control in grass crops. These herbicides are used in maize, sorghum, and rice and are usually applied just before or soon after the emergence of crop seedlings. Following chloroacetamide application, most susceptible weeds fail to emerge. After seedling emergence, the herbicide may enter through roots, shoots, or cotyledons and is transported via the xylem, resulting in accumulation of the herbicide in acropetal portions of the plant where it inhibits synthesis and maintenance of membranes (Ahrens, 1994; Böger et al., 2000). Symptoms of chloroacetamide injury in emerged plants include slightly cupped or crinkled leaves and shortened leaf midribs (Ahrens, 1994). Stunted root development has also been reported, although poorly documented.
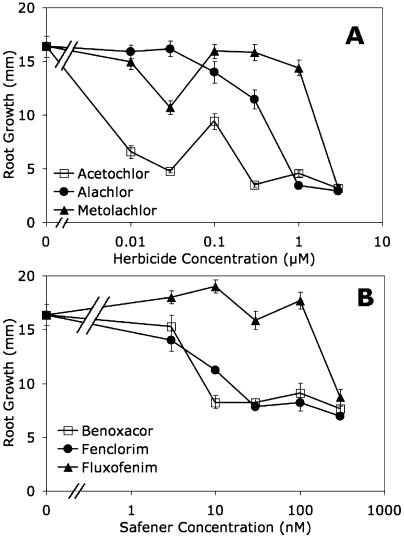
To assess the toxicity of chloroacetamides to Arabidopsis, seeds were germinated on media containing a range of concentrations of alachlor, acetochlor, or metolachlor. Symptoms of herbicide injury included malformation of leaves and inhibition of root growth. Acetochlor was most inhibitory of root growth followed by alachlor and metolachlor (Fig. 1A). Herbicide toxicity assays were also conducted with soil-grown plants that were sprayed with alachlor or metolachlor. In these studies, 5-d-old Arabidopsis seedlings were given a foliar application of herbicide at concentrations and rates similar to those commonly used to control weeds (0.5–8.0 g L−1 active ingredient [ai]). Two weeks after treatment, typical symptoms of chloroacetamide injury (growth inhibition and leaf deformation) were noted in seedlings exposed to herbicide concentrations of at least 4 g L−1 ai (Fig. 2). These experiments established appropriate conditions to assess the susceptibility of Arabidopsis to herbicides supplied either in agar medium or by foliar application.
Figure 1.
Effect of safeners and herbicides on Arabidopsis root growth. Seedlings were germinated on solid half-strength Murashige and Skoog media containing various concentrations of herbicides (A) or safeners (B). Root length of at least 10 seedlings was measured after 7 d. Error bars indicate se.
Figure 2.
Chloroacetamide injury to Arabidopsis plants. Five-day-old Arabidopsis seedlings were treated with alachlor (0.4 g L−1 ai) by foliar application. Two weeks after treatment, control (A) and herbicide-treated (B) plants were photographed and examined for growth inhibition and developmental abnormalities. Typical symptoms of chloroacetamide injury were observed, including stunted growth, cupped and crinkled leaves, and shortened leaf midribs.
Experiments were also carried out to evaluate the influence of safener treatment on the toxicity of chloroacetamides to Arabidopsis seedlings. The safeners tested enhance herbicide tolerance in different cereal crops and have been shown to induce the expression of several GST genes in Arabidopsis (DeRidder et al., 2002). The morpholine safener benoxacor and the oxime ether safener fluxofenim protect maize and sorghum, respectively, from chloroacetamide injury (Gronwald and Plaisance, 1998; Davies and Caseley, 1999). Fenclorim, a pyrimidine, enhances chloroacetamide detoxification in rice (Wu et al., 1999; Deng and Hatzios, 2002b). A variety of methods were used to treat Arabidopsis with safeners, including seed treatment, foliar application, and addition to hydroponic growth medium. Safeners did not inhibit growth or development under these conditions, consistent with these compounds being generally regarded as nonphytotoxic. However, when seeds were germinated on agar medium containing safeners, severe inhibition of root growth and cotyledon development was observed with as little as 10 nm benoxacor or fenclorim (Fig. 1B). In contrast, fluxofenim showed toxicity only at concentrations above 100 nm (Fig. 1B). It should be noted that the concentrations of safeners that cause toxicity to seedlings on agar medium are much lower than those used to induce GSTs in older seedlings.
Because of the toxicity of safeners to young seedlings, it was not possible to evaluate whether treatment of seeds with safeners could attenuate the effects of herbicides on root growth and shoot morphology. However, root growth was not affected when older seedlings (>7 d old) were exposed to safeners, and so these conditions were used to determine if safeners could protect Arabidopsis from herbicide injury. Regardless of how Arabidopsis plants were exposed to safeners, we were unable to demonstrate any protective effect of these compounds on Arabidopsis plants (data not shown). Previous studies have shown that safeners enhance the expression of GST RNA, protein, and enzyme activity in Arabidopsis, (DeRidder et al., 2002). However, in spite of the increased abundance of GSTs, safeners fail to protect Arabidopsis plants from herbicide injury.
Effect of Safeners on Multidrug Resistance-Related Protein GSH-Conjugate Transporters
GSTs function within a well-characterized three-phase detoxification system that is the primary mechanism of xenobiotic metabolism in plants (Davies and Caseley, 1999). Detoxification of many agrichemicals requires the combined activities of all components of this system. Metabolism of chloroacetamide herbicides does not require modification by phase I enzymes but does involve GST-mediated conjugation (Coleman et al., 1997). Therefore, low activity of phase II or phase III components may limit the ability of Arabidopsis plants to detoxify these herbicides at a rate sufficient to confer tolerance. We have shown previously that GSH levels (phase II) in Arabidopsis increase up to 3-fold after exposure to safeners, a response similar to that observed in grass crops (DeRidder et al., 2002). Multidrug resistance-related proteins (MRPs) comprise a subclass of ATP-binding cassette transporters in plants that, in addition to other transport activities, are involved in phase III of this system as transporters of GSH conjugates (Kolukisaoglu et al., 2002; Martinoia et al., 2002). To our knowledge, the response of these genes to herbicide safeners has not been investigated in Arabidopsis seedlings, although safeners have been shown to induce the expression of specific MRPs in Arabidopsis cell suspension cultures (Sánchez-Fernández et al., 1998).
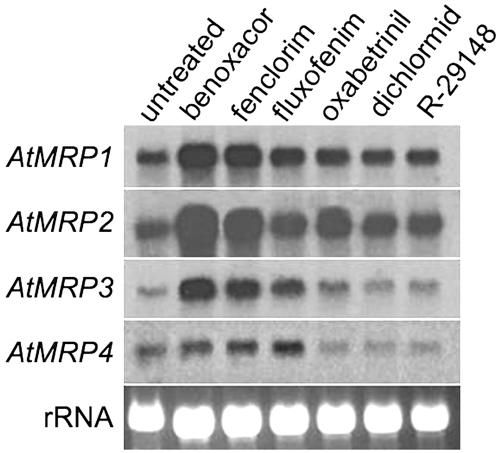
To study the effect of safeners on RNA expression of Arabidopsis MRPs, cDNA probes for four characterized transporters were hybridized to RNA isolated from seedlings treated with various safeners (Fig. 3). The highest level of sequence identity among these probes was 85% (between AtMRP1 and AtMRP2) and the experimental conditions were chosen to prevent cross-hybridization to other MRP transcripts. All four MRP genes were expressed under control conditions. Benoxacor and fenclorim induced high expression of AtMRP1, AtMRP2, and AtMRP3, whereas induction of AtMRP4 was greatest with fluxofenim. Of the four genes, AtMRP3 appeared to be most responsive to safeners, while AtMRP4 was induced only slightly by some of the safeners. Overall, the MRP genes responded to safeners in a pattern similar to that observed for other components of the detoxification system, such as GSTs and GSH content (DeRidder et al., 2002). Specifically, benoxacor, fenclorim, and fluxofenim were the most effective inducers of gene expression, often in that order. Other safeners had either no or a small effect on the expression of detoxification genes. These results suggest that the safeners benoxacor, fenclorim, and fluxofenim can induce all components of the three-phase detoxification system in Arabidopsis and increase the capacity to metabolize xenobiotics.
Figure 3.
RNA expression of Arabidopsis MRP genes. Arabidopsis seedlings growing in liquid culture were exposed to various safeners for 24 h and total RNA was extracted. Twenty micrograms of total RNA for each treatment were hybridized with probes for each of four characterized MRP genes in Arabidopsis.
Organ-Specific Expression of Arabidopsis GSTs in Response to Safeners
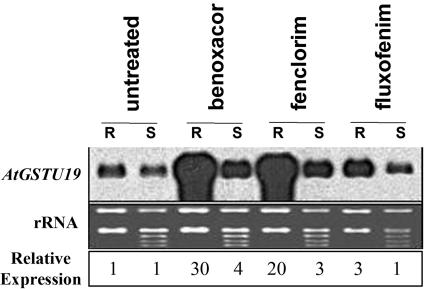
AtGSTU19, a tau-class Arabidopsis GST, is induced by herbicide safeners and can conjugate chloroacetamide herbicides (alachlor, acetochlor, and metolachlor) to GSH in vitro (DeRidder et al., 2002). Nevertheless, treatment of Arabidopsis plants with safeners did not increase their tolerance to these herbicides. This observation, coupled with the fact that chloroacetamide herbicides accumulate in and cause damage to shoot tissues (Böger et al., 2000), prompted us to examine whether expression of this GST was localized to specific tissues. Under control conditions, RNA expression of AtGSTU19 was slightly higher in roots than shoots (Fig. 4). The response of AtGSTU19 to safeners was much greater in roots, where benoxacor increased RNA levels 30-fold compared to only 4-fold in shoots. Similar results were seen with fenclorim, whereas fluxofenim produced a modest induction only in roots. These results are consistent with immunoblot experiments showing root-specific induction of AtGSTU19 in response to safeners (DeRidder et al., 2002).
Figure 4.
Organ-specific expression of AtGSTU19. Three-week-old Arabidopsis seedlings grown in hydroponic medium were treated for 24 h with three safeners (100 μm), and total RNA was extracted from roots (R) and shoots (S). RNA blots (5 μg per lane) of these samples were probed with radiolabeled full-length AtGSTU19 cDNA. The RNA expression was quantified using a phosphorimager, and relative expression of AtGSTU19 in roots and shoots is shown. Expression was calculated in roots and shoots separately and is reported relative to the control samples.
Reporter Gene Analysis of AtGSTU19 Induction by Safeners
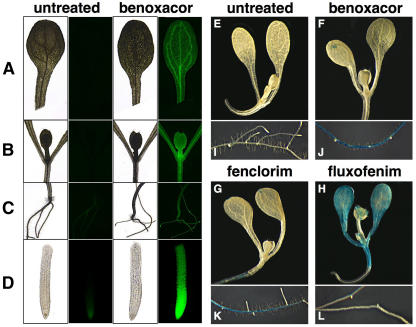
To analyze AtGSTU19 expression in more detail, the AtGSTU19 promoter was used to construct a green fluorescent protein (GFP) reporter gene (Reichel et al., 1996). The 2-kb promoter sequence used in these experiments was considered likely to contain most, if not all, sequences necessary for regulated expression of AtGSTU19. Transgenic plants carrying this chimeric reporter gene were grown in liquid culture for 7 d and then treated with 100 μm benoxacor for 24 h. Representative examples of the expression of AtGSTU19∷GFP are shown in Figure 5 (A–D). Expression of GFP was relatively low in untreated seedlings. Exposure of the transgenic plants to benoxacor resulted in high levels of expression of the GFP reporter, most noticeably in the root apex (Fig. 5D). The induction of GFP expression in roots was so pronounced that exposure time for images of roots had to be reduced to one-quarter of that used for other tissues. These results are consistent with RNA and protein expression analyses that show AtGSTU19 induction by safeners mainly in the roots. GFP expression was also induced by benoxacor in shoot vasculature and leaf primordia. Variation in the intensity of AtGSTU19∷GFP expression was observed between independent transgenic lines, but the localization was consistent.
Figure 5.
Reporter gene expression driven by GST gene promoters in safener-treated Arabidopsis seedlings. A to D, One-week-old seedlings carrying the AtGSTU19∷GFP transgene were treated with 100 μm benoxacor for 24 h. GFP expression in cotyledons (A), emerging leaves (B), root-shoot transition zone (C), and root apex (D) were recorded in vivo using fluorescence microscopy (emission 510 nm). Bright-field images of untreated and benoxacor-treated plants are shown next to the corresponding fluorescent images. Exposure time for roots was 0.5 s, and 2.0 s for all other tissues. E to L, Arabidopsis seedlings carrying the AtGSTF2∷GUS transgene were grown in liquid medium for 7 d and treated with benoxacor (F and J), fenclorim (G and K), or fluxofenim (H and L) for 24 h. Shoots (E–H) and roots (I–L) were photographed after staining for GUS activity.
Expression of AtGSTF2 in Response to Safeners
We also examined expression of AtGSTF2, an Arabidopsis phi-class GST, using transgenic Arabidopsis expressing the β-glucuronidase (GUS) reporter gene under the control of the AtGSTF2 promoter (Fig. 5, E–L). No expression was observed in untreated seedlings. After treatment with benoxacor or fenclorim, expression in shoots was detectable only in hydathodes, whereas expression in the roots was much more pronounced (Fig. 5, F, G, J, and K). Interestingly, GUS expression was not observed in lateral roots or lateral root primordia, in contrast to the expression of AtGSTU19∷GFP (Fig. 5C). However, seedlings exposed to fluxofenim showed clear induction of the reporter in shoot tissues, with little expression in roots (Fig. 5, H and L). These results indicate that regulation of GST gene expression by safeners is complex and can be controlled by different signaling pathways. Furthermore, while benoxacor induces the expression of both AtGSTF2 and AtGSTU19, it does so in distinct tissues.
Effect of Ectopic AtGSTU19 Expression on Herbicide Tolerance
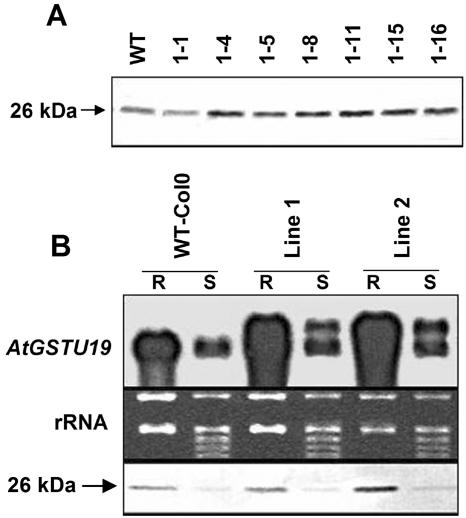
Expression of AtGSTU19 predominates in roots following safener treatment. However, chloroaectamide herbicides are rapidly translocated to acropetal regions of the shoot (Böger et al., 2000). The relatively low expression of AtGSTU19 in shoots may be insufficient to alleviate herbicide toxicity. Increased expression of AtGSTU19 in shoots may provide tolerance to the chloroacetamide herbicides that accumulate in this tissue. To test this hypothesis, the open reading frame (ORF) of AtGSTU19 was expressed in Arabidopsis under the control of a constitutive promoter. Immunoblot analysis of seven transgenic lines revealed that expression of AtGSTU19 was increased in seedlings but only modestly in most lines (Fig. 6A).
Figure 6.
Ectopic expression of AtGSTU19 in transgenic plants. A, One-week-old seedlings of wild type (WT) and seven independent transgenic lines were grown in liquid culture, and total soluble protein was extracted from whole seedlings. Following SDS-PAGE separation of protein (15 μg per lane), gels were blotted and probed with AtGSTU19 antisera. A 26-kD protein (AtGSTU19 subunit) was recognized in all samples. B, Wild type and two independent transgenic lines grown on half-strength Murashige and Skoog agar medium. RNA was extracted separately from roots (R) and shoots (S), resolved on an agarose gel, and blotted to a membrane. The RNA blot (top section) was hybridized with a radiolabeled AtGSTU19 cDNA. The transgenic GSTU19 mRNA is of higher molecular mass than the endogenous mRNA and appears as a discrete band in both root and shoot samples due to a longer 3′-untranslated region. For immunoblot analysis (bottom section), soluble protein was extracted separately from root and shoot tissues, resolved by SDS-PAGE (15 μg per lane), and probed with polyclonal antibody raised against recombinant AtGSTU19 (26 kD).
Organ-specific expression of the transgene was examined in two transgenic lines (Fig. 6B). The RNA from the transgene can be distinguished from that of the endogenous gene because it has a larger 3′-untranslated region. In the two transgenic lines that were examined in detail, AtGSTU19 RNA expression was approximately double that seen in nontransgenic plants (Fig. 6B, top section). Somewhat surprisingly, expression of the transgene was much higher in roots than shoots, paralleling the RNA expression of the endogenous AtGSTU19 gene. If both transcripts were translated with similar efficiency, this should result in an approximate doubling of AtGSTU19 protein, with higher expression in roots. Immunoblot studies confirmed that expression of AtGSTU19 was slightly higher in transgenic plants and predominated in roots as it does in nontransgenic plants (Fig. 6B, bottom section).
The tolerance to chloroacetamide herbicides of transgenic plants with elevated expression of AtGSTU19 was investigated. In the first experiment, three overexpression lines and wild-type Arabidopsis seedlings were germinated on media containing the herbicides alachlor, metolachlor, or acetochlor. There were no significant differences between lines in root length or dry weight after 1 week (data not shown). The experiment was performed twice with similar results. In a second experiment, Arabidopsis plants were grown in potting mix and 5-d-old seedlings were sprayed with alachlor or metolachlor and observed for signs of herbicide toxicity for 2 weeks following treatment. In two independent experiments there was no evidence of differential herbicide tolerance in any of the transgenic lines (data not shown).
DISCUSSION
In monocot crops, herbicide tolerance can be enhanced by safeners that induce herbicide-metabolizing enzymes. To be effective, safeners must act selectively on the crops they protect, i.e. not safen weed species. This study seeks to define differences between plants that are protected by safeners and those that are not, in order to understand why safeners are ineffective at providing herbicide tolerance to weedy species.
There are at least three possible explanations for why safeners did not protect Arabidopsis plants from herbicide damage. First, components of the three-phase detoxification system other than GSTs may not respond to safeners and thus limit the capacity of Arabidopsis to metabolize herbicides. Second, treatment of Arabidopsis and other dicots with safeners may not induce expression of GSTs in tissues where their activity is required to protect plants from herbicide damage. Finally, GSTs induced by safeners in dicots may not have activity with herbicide substrates.
In monocot species, safeners have been shown to enhance the activity of multiple components of the three-phase detoxification system such as cytochrome P450 monooxygenases, GSTs, GSH, and GSH-conjugate transporters (Davies and Caseley, 1999; Theodoulou et al., 2003). In Arabidopsis, GSTs, GSH (DeRidder et al., 2002), and the RNA expression of four GSH-conjugate transporters (Fig. 3) are induced by safeners. While these components may play a vital role in safener-mediated herbicide metabolism, their induction is unable to protect Arabidopsis from herbicide injury and may be irrelevant if expression of herbicide-metabolizing GSTs is not localized to appropriate tissues. While organ-specific induction of GSH and GS-conjugate transporters was not examined here, studies with transgenic tobacco (Nicotiana tabacum) plants have shown that expression of a maize GST in shoots is sufficient to confer tolerance to metolachlor, suggesting that expression of other components of the detoxification system may not limit herbicide metabolism in dicots (Jepson et al., 1997).
Many studies suggest that tissue-specific expression of GSTs may be an important factor that determines herbicide tolerance in safener-treated plants. Most grass crops display a significant level of tolerance to many chloroacetamide and thiocarbamate herbicides, even without safener treatment. This is correlated with the constitutive expression of GSTs capable of metabolizing these herbicides in roots and shoots (Jablonkai and Hatzios, 1993; Dixon et al., 1997a, 1997b; Davies and Caseley, 1999). Upon treatment of grass crops with safeners, GSTs able to conjugate these herbicides are induced predominantly in shoot tissues. This response is associated with a high level of protection against herbicide damage in maize (Irzyk and Fuerst, 1993; Holt et al., 1995; Dixon et al., 1998), wheat (Cummins et al., 1997; Riechers et al., 1997; Pascal and Scalla, 1999; Xu et al., 2002), rice (Deng and Hatzios, 2002a, 2002b), and sorghum (Dean et al., 1990; Gronwald and Plaisance, 1998).
In contrast to grass crops, most monocot weeds and dicot species have little or no innate tolerance to chloroacetamide or thiocarbamate herbicides (Davies and Caseley, 1999). Safener-induced expression of GSTs in dicots is observed mainly in roots and is not correlated with increased herbicide tolerance. For example, the maize safener dichlormid enhanced GST activity toward atrazine in pea seedlings, but only in roots (Edwards, 1996). We have obtained similar results with Arabidopsis. RNA hybridization (Fig. 4) and immunoblot (DeRidder et al., 2002) experiments indicated that safeners induced the expression of AtGSTU19 preferentially in roots. Reporter gene studies demonstrate this expression of AtGSTU19 is concentrated in the root apex (Fig. 5) with lower expression in the shoot. This pattern of expression may reflect the concentration of safeners in these tissues. Studies in rice have demonstrated that tolerance to the herbicide pretilachlor is correlated with the accumulation of fenclorim in shoots (Scarponi et al., 2003). However, similar studies on dicot plants have not been reported, to our knowledge. In addition to shoot damage, chloroacetamides severely inhibited root growth in young, unsafened Arabidopsis seedlings. Although AtGSTU19 could participate in detoxification of the herbicide in roots, it is expressed at a low level under control conditions. Safeners increase the expression of AtGSTU19, but they are also toxic to germinating seedlings (Fig. 1). This made it impossible to test the capacity of safeners to protect against herbicide injury under these conditions.
The bulk of the evidence indicates that high levels of GST expression in shoot tissues is essential for safeners to mitigate herbicide toxicity. In particular, the coleoptile sheath that shields the emerging hypocotyl of germinating monocot crops is known to be important for the action of safeners and herbicides (Hickey and Krueger, 1974; Scott-Craig et al., 1998; Davies and Caseley, 1999; Riechers et al., 2003). Proteomic studies in diploid wheat have shown that the coleoptile contains high levels of GSTs involved in safener-mediated herbicide detoxification (Zhang and Riechers, 2004). This organ is not present in dicots. Further evidence for the importance of GST expression in shoots comes from maize; a chloroacetamide-sensitive cultivar was deficient in ZmGST27 RNA in leaves, whereas herbicide tolerant lines expressed this GST in both roots and leaves (Rossini et al., 1998). When this maize GST was expressed in leaves of transgenic tobacco plants, it conferred a high level of tolerance to metolachlor compared to nontransgenic plants, demonstrating that GST expression in shoots can confer herbicide tolerance to dicots (Jepson et al., 1997). It is interesting that induction of AtGSTU19 by benoxacor occurs in tissues that include the shoot meristem (Fig. 5), where chloroacetamides are thought to inhibit actively growing tissue. However, the magnitude of this response may be insufficient for protection from herbicide injury. Alternatively, expression of other key components involved in herbicide detoxification, such as MRPs, may be lacking in the meristems.
To test the hypothesis that tissue localization and level of GST expression are important determinants of safener selectivity, we produced transgenic Arabidopsis plants that overexpressed AtGSTU19 in all tissues. The transgene did not produce a dramatic increase in abundance of AtGSTU19, as observed in immunoblots (Fig. 6). Plants with the highest level of transgene expression had approximately double the normal level of AtGSTU19 protein. These plants did not show any increased tolerance to alachlor, acetochlor, or metolachlor compared to nontransgenic plants. This result may be due to the relatively small increase in AtGSTU19 in transgenic plants and the low level of AtGSTU19 protein in shoot tissues (Fig. 6). Despite the fact that a constitutive promoter was used, expression of the transgene predominated in roots and paralleled that of the endogenous AtGSTU19 gene. This result may reflect the properties of the constitutive promoter and could be addressed by using another transgene with the AtGSTU19 ORF under the control of a different promoter. Experiments are under way to produce transgenic plants that express AtGSTU19 at higher levels in Arabidopsis shoots.
To protect plants from herbicide injury, safeners should induce GSTs able to conjugate herbicide substrates in the appropriate tissue. Although AtGSTF2 is highly induced in shoots by the safener fluxofenim (Fig. 5), this does not protect Arabidopsis from herbicide injury. If AtGSTF2 has a low activity with herbicide substrates, safener-induced expression of this protein will likely have no effect on herbicide tolerance.
Based on RNA and protein expression studies as well as reporter gene experiments, it appears that regulation of AtGSTU19 expression by benoxacor is largely controlled at the transcriptional level. Similar conclusions about safener-regulated gene expression have been drawn from studies with monocot species (Riechers et al., 2003). However, a recent proteomics study showed that RNA expression of several Arabidopsis GSTs was induced by benoxacor, but the only GST protein that increased was AtGSTU19 (Smith et al., 2004). A recent study by Zhang and Riechers (2004) indicated that two closely related GSTs induced by safeners in wheat coleoptiles may undergo differential posttranslational modification. These results indicate that control of GST expression by safeners is complex and may involve regulation at multiple levels. Interestingly, analysis of Arabidopsis GSTs by two-dimensional SDS-PAGE and tandem mass spectrometry has revealed the presence of multiple isoforms of AtGSTU19 following benoxacor treatment, suggesting that posttranslational control of GST expression may be important in Arabidopsis (data not shown).
Safeners have a dramatic effect on the expression of genes encoding detoxification proteins in plants. Although synthetic, safeners take advantage of extant response mechanisms in plants. It is possible that safeners mimic compounds produced by other organisms in natural environments, such as allelochemicals. Release of these compounds into the environment suppresses the growth and development of neighboring plants, thereby reducing competition (Olofsdotter et al., 2002). Plants may counter by inducing mechanisms that detoxify these compounds. It would be interesting to examine the effect of known allelochemicals on the expression of GSTs in plants and to see whether safeners can alleviate the toxicity of these compounds to seed germination and plant growth.
CONCLUSION
The results of this study show that in Arabidopsis safeners such as benoxacor, fenclorim, and fluxofenim effectively induce the expression of several components of the three-phase detoxification system necessary for herbicide metabolism. Interestingly, the expression of herbicide-metabolizing GSTs by safeners is specific to some organs and tissues, and this may be critical for the selectivity of these compounds. This study establishes Arabidopsis as a useful system for the evaluation of safener-regulated gene expression in a dicot species and has led to a hypothesis to account for differences in the efficacy of safeners between plant species.
MATERIALS AND METHODS
Chemicals
Analytical grade (95%–99% pure) safeners and herbicides were provided by the following companies: Syngenta, benoxacor, oxabetrinil, fluxofenim, fenclorim, R-29148, dichlormid, and metolachlor; and Monsanto, alachlor and acetochlor. Stock solutions (100 mm) of herbicide safeners and herbicides were prepared in acetone and stored at −20°C.
Plant Material and Safener or Herbicide Treatments
For experiments in liquid culture, seedlings of Arabidopsis (Arabidopsis thaliana L. Heynh. ecotype Columbia) were grown for 7 d in media containing half-strength Murashige and Skoog balanced salt solution, 10 g L−1 Suc, and Gamborg's vitamin solution under sterile conditions (Murashige and Skoog, 1962). Plants were grown under continuous soft-white fluorescent lighting with gentle shaking on a rotary shaker at 25°C. Cultures of 7-d-old Arabidopsis seedlings were treated with safeners (100 μm) for 24 h. An equal volume of acetone, which was used as solvent for safeners and has been shown to have no effect on GST RNA levels or enzyme activity (Rossini et al., 1998), was used in control treatments. After treatment, plant tissue was harvested, frozen in liquid nitrogen, and stored at −70°C until use.
Solid medium was prepared by adding 8 g L−1 of agar. Herbicides or safeners were added to the media after autoclaving when the media had cooled to 50°C. Arabidopsis seedlings were germinated on media containing various concentrations of herbicide or safener and grown under similar conditions as described for plants grown in liquid medium. Plant growth was measured and tissue harvested after 5 to 7 d of growth.
For experiments examining the effect of herbicides or safeners on plants grown in soil, Arabidopsis seeds were sown in Metromix 360 potting mixture (Scotts). Seeds were germinated in a mist room with natural lighting for 4 d, and then transferred to a growth room under a 16-h photoperiod at a light intensity of 100 μmol s−1 m−2 and at 23°C to 25°C.
To determine the lowest concentration of herbicide that produced visible symptoms of injury, herbicides were applied as a foliar spray to 3-week-old plants, and plants were monitored for signs of phytotoxicity for 14 d. The normal field application rate is 7 to 30 g L−1 at 50 gallons ha−1 for alachlor, and 6 to 20 g L−1 at 50 gallons ha−1 for metolachlor (Ahrens, 1994). For this experiment a range of concentrations of both herbicides (0.1, 0.2, 0.5, 1.0, 2.0, 4.0, and 8.0 g L−1 of ai, or approximately 2–30 mm) was tested. The herbicides were dissolved in 2 mL acetone prior to adding water to a final volume of one liter. A nonionic chemical surfactant was used at 0.25% (v/v) to maximize contact of the herbicide with the plant surface. The control treatment contained 0.2% acetone and 0.25% nonionic chemical surfactant. The herbicides alachlor and metolachlor were applied at a rate of 50 gallons ha−1 using a TeeJet spray nozzle (yellow type 8002 0.2 gallons min−1) at 40 psi and a speed of 3 mph. For subsequent experiments, 4.0 g L−1 ai (approximately 15 mm) was used for both herbicides as this was the lowest concentration that reproducibly reduced shoot size and altered leaf morphology. To examine the effect of safeners on herbicide toxicity, 3-week-old Arabidopsis plants were treated with safeners (100 μm) by foliar application to run off once per day for 4 consecutive days. Metolachlor or alachlor (15 mm) was then applied as a foliar spray and plants were monitored for signs of toxicity for 14 d.
Immunoblot Analysis
Preparation of protein extracts and immunoblot analyses were carried out as previously described (DeRidder et al., 2002). Protein samples were resolved on 12% polyacrylamide SDS gels, and blots of these gels were reacted with polyclonal antibodies raised against purified AtGSTU19-19.
RNA Hybridization Analysis and PCR
RNA was isolated from plant tissues as described (Carpenter and Simon, 1998). RNA samples were separated in 1.2% denaturing agarose gels, blotted onto a nitrocellulose membrane, and cross-linked to the membrane by UV irradiation. Twenty-five nanograms of cDNA was labeled with 32P-dATP using a Decaprime kit (Ambion) and used as a probe in subsequent RNA hybridization studies.
The hybridization probe for AtGSTU19 was a full-length cDNA excised from the pRL2 vector and gel purified. Full-length cDNA clones for the AtMRP genes were used as templates to produce probes by PCR. The primers used were AtMRP1, 5′-CATCTGTAAAACCAGTTGAAAATGG and 5′-TTCTGCATGACGGCTAATGAAG; AtMRP2, 5′-GATCAAACAGCGGAACAACCA and 3′-CTTCCATTCTGCATGACAGCAAAC; AtMRP3, 5′-CTCGTTTTCGGAATCTATTTTGCC and 5′-TGCTGTTGCTTCTATCTTTCTTGAGG; and AtMRP4, 5′-CCAATCCAATGGCTCAGATTTG and 5′-TGGAGATTTGTAGCCTTTGCTGAGT. The PCR products were recovered from agarose gels and labeled as described above. Radioactive probes were purified on Sephadex G-50 columns prior to hybridization to ensure specificity. For RNA hybridization studies examining the effect of safeners on the expression of AtMRP genes, 20 μg of total RNA per sample was resolved on 1.2% agarose gels, blotted onto nylon membranes (Hybond NX, Amersham Pharmacia), and cross-linked to the membrane by UV irradiation. The hybridization probes were denatured at 96°C, added to the hybridization tube containing the blot, and incubated at 42°C overnight. Hybridization buffer included 5× sodium chloride/sodium phosphate/EDTA, 0.5% (v/v) SDS, 5× Denhardt's solution, 100 μg mL−1 boiled salmon sperm DNA, and 50% (v/v) formamide. After hybridization, blots were washed twice at room temperature in 2× SSC containing 0.1% SDS for 10 min, followed by a wash at 42°C in 0.2× SSC containing 0.1% SDS for 15 min.
Preparation of GFP Reporter Gene Construct
The binary vector pGPTV-bar (Becker et al., 1992) was modified to contain a GFP reporter gene instead of the GUS gene. The polylinker region, GUS ORF, and nos polyA sequences were excised from pGPTV-bar using EcoRI and HindIII and replaced with an EcoRI-HindIII fragment excised from the binary vector pCK-GFP-S65C (Reichel et al., 1996) containing a translational leader sequence, the ORF for GFP, and cauliflower mosaic virus polyA sequence. The resulting binary vector was named pBAR-GFP-BDR. A 2-kb fragment containing the AtGSTU19 promoter was obtained by PCR using primers that added EcoRI sites at both ends (5′-TCTTTCTCGAATTCTTTGGTTGGG and 5′-ATAAGCACTCGAATTCCACAGTAGC). This was cloned into the EcoRI site upstream of the GFP ORF in pBAR-GFP-BDR. A plasmid containing this promoter in the correct orientation relative to the GFP ORF was identified and transferred into Agrobacterium strain GV3850. This was used to produce transgenic Arabidopsis plants as described (Clough and Bent, 1998). Transgenic T3 plants were identified by selecting glufosinate-resistant seedlings. T3 lines homozygous for a single active T-DNA insert were used for further experiments. Transgenic plants containing the AtGSTF2∷GUS reporter gene have been described previously (Smith et al., 2003).
GFP Expression Analysis
For qualitative analysis of GFP expression in response to safeners, Arabidopsis seedlings were grown in liquid culture for 7 d and treated with the safener benoxacor (100 μm, 24 h) as previously described. Following treatment, live seedlings were viewed under an epifluorescence microscope (Nikon Microscope Eclipse E800) at either 10× magnification (for cotyledons, transition zone, and emerging leaves) or 20× magnification (for roots). Fluorescence from chlorophyll was minimized by the GFP filter. Bright field and fluorescent images were taken of specific tissues. Exposure time for fluorescent images was 2 s except for roots, which were taken with 0.5 s exposure.
Overexpression of AtGSTU19 in Arabidopsis
The ORF of AtGSTU19, including approximately 15 bp upstream of the ATG, was amplified from a cDNA plasmid using primers (5′-AAATCTCTTTGTAAGCTTTAGCGAT and 5′-GAACCATATGACTAGTGAAACATATT) that contained restriction sites for HindIII and SpeI (underlined). After digestion with these enzymes, the ORF was inserted into the binary vector pE1779 (Ni et al., 1995). This vector contains a promoter for constitutive expression of the transgene in all tissues of the plant, a polyadenylation signal, and the bar gene for herbicide selection with Basta. The transgene mRNA was expected to be longer than the endogenous transcript of AtGSTU19 due to a longer, vector-encoded 3′-untranslated region. The recombinant plasmid was transferred into Agrobacterium and used to produce transgenic Arabidopsis plants. Eight independent T3 lines homozygous for the transgene at at least one locus were obtained and used for further experiments.
Acknowledgments
The authors thank Nahla El-Sherif for invaluable assistance with some experiments, Dr. Debbie Sherman for microscopy assistance, and Dr. Philip Rea for providing plasmids containing MRP-encoding cDNAs used in this study.
This work was supported in part by the U.S. Department of Agriculture (National Research Initiative program grant to P.B.G. and National Needs Fellowship to B.P.D.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Peter B. Goldsbrough (goldsbrough@purdue.edu).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.067199.
References
- Abu-Qare AW, Duncan HJ (2002) Herbicide safeners: uses, limitations, metabolism, and mechanisms of action. Chemosphere 48: 965–974 [DOI] [PubMed] [Google Scholar]
- Ahrens WH, editor (1994) Herbicide Handbook. Weed Science Society of America, Champaign, IL
- Becker D, Kemper E, Schell J, Masterson R (1992) New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Mol Biol 20: 1195–1197 [DOI] [PubMed] [Google Scholar]
- Böger P, Matthes B, Schmalfuß J (2000) Towards the primary target of chloroacetamides: new findings pave the way. Pest Manag Sci 56: 497–508 [Google Scholar]
- Carpenter CD, Simon AE (1998) Preparation of RNA. In J Martinez-Zapater, J Salinas, eds, Methods in Molecular Biology, Vol 82. Humana Press, Totowa, NJ, pp 85–89 [DOI] [PubMed]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cole D, Cummins I, Hatton P, Dixon D, Edwards R (1997) Glutathione transferases in crops and major weeds. In KK Hatzios, ed, Regulation of Enzymatic Systems Detoxifying Xenobiotics in Plants. Kluwer Academic Publishers, Amsterdam, pp 139–154
- Cole DJ, Edwards R (2000) Secondary metabolism of agrochemicals in plants. In T Roberts, ed, Metabolism of Agrochemicals in Plants. John Wiley and Sons, Hoboken, NJ, pp 108–134
- Coleman JOD, Blake-Kalff MMA, Davies TGE (1997) Detoxification of xenobiotics by plants: chemical modification and vacuolar compartmentation. Trends Plant Sci 2: 144–151 [Google Scholar]
- Cummins I, Cole DJ, Edwards R (1997) Purification of multiple glutathione transferases involved in herbicide detoxification from wheat (Triticum aestivum L.) treated with the safener fenchlorazole-ethyl. Pestic Biochem Physiol 59: 35–49 [Google Scholar]
- Davies J, Caseley JC (1999) Herbicide safeners: a review. Pestic Sci 55: 1043–1058 [Google Scholar]
- Dean JV, Gronwald JW, Eberlein CV (1990) Induction of glutathione S-transferase isozymes in sorghum by herbicide antidotes. Plant Physiol 92: 467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng F, Hatzios KK (2002. a) Purification and characterization of two glutathione S-transferase isozymes from indica-type rice involved in herbicide detoxification. Pestic Biochem Physiol 72: 10–23 [Google Scholar]
- Deng F, Hatzios KK (2002. b) Characterization and safener induction of multiple glutathione S-transferases in three genetic lines of rice. Pestic Biochem Physiol 72: 24–39 [Google Scholar]
- DeRidder BP, Dixon DP, Beussman DJ, Edwards R, Goldsbrough PB (2002) Induction of glutathione S-transferases in Arabidopsis by herbicide safeners. Plant Physiol 130: 1497–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D, Cole DJ, Edwards R (1997. a) Characterisation of multiple glutathione transferases containing the GST I subunit with activities toward herbicide substrates in maize (Zea mays). Pestic Sci 50: 72–82 [Google Scholar]
- Dixon DP, Cole DJ, Edwards R (1997. b) Characterization and regulation of multiple glutathione transferases in corn (Zea mays). Plant Physiol (Suppl) 114: 155–156 [Google Scholar]
- Dixon DP, Cole DJ, Edwards R (1998) Purification, regulation and cloning of a glutathione transferase (GST) from maize resembling the auxin-inducible type-III GSTs. Plant Mol Biol 36: 75–87 [DOI] [PubMed] [Google Scholar]
- Edwards R (1996) Characterisation of glutathione transferases and glutathione peroxidases in pea (Pisum sativum). Physiol Plant 98: 594–604 [Google Scholar]
- Edwards R, Dixon DP, Walbot V (2000) Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci 5: 193–198 [DOI] [PubMed] [Google Scholar]
- Gronwald JW, Plaisance KL (1998) Isolation and characterization of glutathione S-transferase isozymes from sorghum. Plant Physiol 117: 877–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton PJ, Cummins I, Cole DJ, Edwards R (1999) Glutathione transferases involved in herbicide detoxification in the leaves of Setaria faberi (giant foxtail). Physiol Plant 105: 9–16 [Google Scholar]
- Hatzios KK (2003) Herbicide safeners: effective inducers of plant defense gene-enzyme systems. Phytoparasitica 31: 3–7 [Google Scholar]
- Hickey JS, Krueger WA (1974) Alachlor and 1,8-naphthalic anhydride effects on corn coleoptiles. Weed Sci 22: 250–252 [Google Scholar]
- Holt DC, Lay VJ, Clarke ED, Dinsmore A, Jepson I, Bright SWJ, Greenland AJ (1995) Characterization of the safener-induced glutathione S-transferase isoform-II from maize. Planta 196: 295–302 [DOI] [PubMed] [Google Scholar]
- Irzyk GP, Fuerst EP (1993) purification and characterization of a glutathione S-transferase from benoxacor-treated maize (Zea mays). Plant Physiol 102: 803–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonkai I, Hatzios KK (1993) In-vitro conjugation of chloroacetanilide herbicides and atrazine with thiols and contribution of nonenzymatic conjugation to their glutathione-mediated metabolism in corn. J Agric Food Chem 41: 1736–1742 [Google Scholar]
- Jepson I, Holt DC, Roussel V, Wright SY, Greenland AJ (1997) Transgenic plant analysis as a tool for the study of maize glutathione S-transferases. In KK Hatzios, ed, Regulation of Enzymatic Systems Detoxifying Xenobiotics in Plants. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 313–323
- Kolukisaoglu HU, Bovet L, Klein M, Eggmann T, Geisler M, Wanke D, Martinoia E, Schulz B (2002) Family business: the multidrug-resistance related protein (MRP) ABC transporter genes in Arabidopsis thaliana. Planta 216: 107–119 [DOI] [PubMed] [Google Scholar]
- Marrs KA (1996) The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 127–158 [DOI] [PubMed] [Google Scholar]
- Martinoia E, Klein M, Geisler M, Bovet L, Forestier C, Kolukisaoglu U, Müller-Röber B, Schulz B (2002) Multifunctionality of plant ABC transporters: more than just detoxifiers. Planta 214: 345–355 [DOI] [PubMed] [Google Scholar]
- McGonigle B, Keeler SJ, Lan SMC, Koeppe MK, O'Keefe DP (2000) A genomics approach to the comprehensive analysis of the glutathione S-transferase gene family in soybean and maize. Plant Physiol 124: 1105–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–497 [Google Scholar]
- Ni M, Cui D, Einstein J, Narasimhulu S, Vergara CE, Gelvin SB (1995) Strength and tissue-specificity of chimeric promoters derived from the octopine and mannopine synthase genes. Plant J 7: 661–676 [Google Scholar]
- Olofsdotter M, Jensen LB, Courtois B (2002) Improving crop competitive ability using allelopathy: an example from rice. Plant Breed 121: 1–9 [Google Scholar]
- Pascal S, Scalla R (1999) Purification and characterization of a safener-induced glutathione S-transferase from wheat (Triticum aestivum). Physiol Plant 106: 17–27 [Google Scholar]
- Pflugmacher S, Schröder P, Sandermann H (2000) Taxonomic distribution of plant glutathione S-transferases acting on xenobiotics. Phytochemistry 54: 267–273 [DOI] [PubMed] [Google Scholar]
- Reichel C, Mathur J, Eckes P, Langenkemper K, Koncz C, Schell J, Reiss B, Maas C (1996) Enhanced green fluorescence by the expression of an Aequorea victoria green fluorescent protein mutant in mono- and dicotyledonous plant cells. Proc Natl Acad Sci USA 93: 5888–5893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechers DE, Irzyk GP, Jones SS, Fuerst EP (1997) Partial characterization of glutathione S-transferases from wheat (Triticum spp.) and purification of a safener-induced glutathione S-transferase from Triticum tauschii. Plant Physiol 114: 1461–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechers DE, Zhang Q, Xu FX, Vaughn KC (2003) Tissue-specific expression and localization of safener-induced glutathione S-transferase proteins in Triticum tauschii. Planta 217: 831–840 [DOI] [PubMed] [Google Scholar]
- Rossini L, Frova C, Mizzi L, Gorla MS (1998) Alachlor regulation of maize glutathione S-transferase genes. Pestic Biochem Physiol 60: 205–211 [Google Scholar]
- Sánchez-Fernández R, Ardiles-Díaz W, Van Montagu M, Inzé D, May MJ (1998) Cloning and expression analyses of AtMRP4, a novel MRP-like gene from Arabidopsis thaliana. Mol Gen Genet 258: 655–662 [DOI] [PubMed] [Google Scholar]
- Sandermann H (1994) Higher-plant metabolism of xenobiotics: the green liver concept. Pharmacogenetics 4: 225–241 [DOI] [PubMed] [Google Scholar]
- Scalla R, Roulet A (2002) Cloning and characterization of a glutathione S-transferase induced by a herbicide safener in barley (Hordeum vulgare). Physiol Plant 116: 336–344 [Google Scholar]
- Scarponi L, Del Buono D, Vischetti C (2003) Persistence and detoxification of pretilachlor and fenclorim in rice (Oryza sativa). Agronomie 23: 147–151 [Google Scholar]
- Schröder P (1997) Fate of glutathione S-conjugates in plants: degradation of the glutathione moiety. In KK Hatzios, ed, Regulation of Enzymatic Systems Detoxifying Xenobiotics in Plants. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 233–244
- Scott-Craig JS, Casida JE, Poduje L, Walton JD (1998) Herbicide safener-binding protein of maize: purification, cloning, and expression of an encoding cDNA. Plant Physiol 116: 1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D, Meade G, Foley VM, Dowd CA (2001) Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J 360: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AP, DeRidder BP, Guo WJ, Seeley EH, Regnier FE, Goldsbrough PB (2004) Proteomic analysis of Arabidopsis glutathione S-transferases from benoxacor- and copper-treated seedlings. J Biol Chem 279: 26098–26104 [DOI] [PubMed] [Google Scholar]
- Smith AP, Nourizadeh SD, Peer WA, Xu JH, Bandyopadhyay A, Murphy AS, Goldsbrough PB (2003) Arabidopsis AtGSTF2 is regulated by ethylene and auxin, and encodes a glutathione S-transferase that interacts with flavanoids. Plant J 36: 433–442 [DOI] [PubMed] [Google Scholar]
- Theodoulou FL, Clark IM, He XL, Pallett KE, Cole DJ, Hallahan DL (2003) Co-induction of glutathione S-transferases and multildrug resistance associated protein by xenobiotics in wheat. Pest Manag Sci 59: 202–214 [DOI] [PubMed] [Google Scholar]
- Wagner U, Edwards R, Dixon DP, Mauch F (2002) Probing the diversity of the Arabidopsis glutathione S-transferase gene family. Plant Mol Biol 49: 515–532 [DOI] [PubMed] [Google Scholar]
- Wu JR, Cramer CL, Hatzios KK (1999) Characterization of two cDNAs encoding glutathione S-transferases in rice and induction of their transcripts by the herbicide safener fenclorim. Physiol Plant 105: 102–108 [Google Scholar]
- Xu FX, Lagudah ES, Moose SP, Riechers DE (2002) Tandemly duplicated safener-induced glutathione S-transferase genes from Triticum tauschii contribute to genome- and organ-specific expression in hexaploid wheat. Plant Physiol 130: 362–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Riechers DE (2004) Proteomic characterization of herbicide safener-induced proteins in the coleoptile of Triticum tauschii seedlings. Proteomics 4: 517–523 [DOI] [PubMed] [Google Scholar]