Abstract
Salicylic acid (SA) has been proposed to antagonize jasmonic acid (JA) biosynthesis and signaling. We report, however, that in salicylate hydroxylase-expressing tobacco (Nicotiana tabacum) plants, where SA levels were reduced, JA levels were not elevated during a hypersensitive response elicited by Pseudomonas syringae pv phaseolicola. The effects of cotreatment with various concentrations of SA and JA were assessed in tobacco and Arabidopsis (Arabidopsis thaliana). These suggested that there was a transient synergistic enhancement in the expression of genes associated with either JA (PDF1.2 [defensin] and Thi1.2 [thionin]) or SA (PR1 [PR1a-β-glucuronidase in tobacco]) signaling when both signals were applied at low (typically 10–100 μm) concentrations. Antagonism was observed at more prolonged treatment times or at higher concentrations. Similar results were also observed when adding the JA precursor, α-linolenic acid with SA. Synergic effects on gene expression and plant stress were NPR1- and COI1-dependent, SA- and JA-signaling components, respectively. Electrolyte leakage and Evans blue staining indicated that application of higher concentrations of SA + JA induced plant stress or death and elicited the generation of apoplastic reactive oxygen species. This was indicated by enhancement of hydrogen peroxide-responsive AoPR10-β-glucuronidase expression, suppression of plant stress/death using catalase, and direct hydrogen peroxide measurements. Our data suggests that the outcomes of JA-SA interactions could be tailored to pathogen/pest attack by the relative concentration of each hormone.
Plant responses to abiotic and biotic stress are, to a great extent, coordinated by the production of chemical signals (for review, see Feys and Parker, 2000). Salicylic acid (SA) was first associated with the induction of pathogenesis-related (PR) proteins and the establishment of systemic acquired resistance (SAR; Ryals et al., 1996). SA also contributes to the hypersensitive response (HR)-associated resistance (Delaney et al., 1994; Mur et al., 1997, 2000) via mechanisms that include the potentiation of reactive oxygen species (ROS) generation and cell death (Mur et al., 1997, 2000; Shirasu et al., 1997). Mutant screens and transgenic plants expressing salicylate hydroxylase to reduce SA levels (NahG [Gaffney et al., 1993]; SH-L [Bi et al., 1995]) have been used to characterize the SA signal transduction chain (Dong, 2001). The allelic series npr1, nim1, and sai1 in Arabidopsis (Arabidopsis thaliana; Bowling et al., 1994; Delaney et al., 1995; Shah et al., 1997) did not express high levels of PR proteins following SA treatment or pathogenic challenge. NPR1 was found to be an ankyrin repeat-containing protein (Cao et al., 1997) that interacts with TGA transcription factors to activate PR gene expression (Fan and Dong, 2002; Després et al., 2003). Mutants also have been isolated that exhibit constitutive activation of PR proteins and resistance: cpr1, cpr5 (Bowling et al., 1994, 1997), cpr6 (Clarke et al., 1998), and 16 constitutive immunity mutants (Maleck et al., 2002).
Jasmonic acid (JA) has been associated with responses to abiotic and biotic stresses (Wasternack and Hause, 2002), including wounding, insect herbivory, and resistance against necrotrophic pathogens such as Alternaria brassicicola and Botrytis cinerea (Penninckx et al., 1998; Thomma et al., 1998). JA is a key signal in the SA-independent induced systemic resistance elicited by rhizosphere biocontrol bacteria (Pieterse et al., 1996; van Loon et al., 1998). Volatile jasmonates have also emerged as important semiochemicals, attracting predators and parasitoids to herbivorous adult insects and larvae (Birkett et al., 2000). Furthermore, JA regulates the synthesis of terpenoid and indole semiochemicals (for review, see Agrawal, 2000).
Jasmonates are part of a large family of oxylipin products of linolenic acid (LN; C18:3) and probably hexadecatrienoic acid (C16:3) metabolism (Schaller et al., 2005). JA, methyl jasmonate, and the JA precursor 12-oxophytodienoic acid (OPDA) all induce a range of wound-inducible genes such a proteinase inhibitors (PI), which have insect antifeedant activity (Ryan, 1990), and antifungal proteins such as Thi (thionins) and PDF1.2 (defensin; Penninckx et al., 1998; Vignutelli et al., 1998). Application of JA has also been found to potentiate the oxidative burst (Kauss et al., 1994). Mutants in the jasmonate signal transduction chain have been isolated (for review, see Turner et al., 2002), the best characterized of which is coi1, which is insensitive to JA and is mutated in the F-box component of an E3 ubiquitin-ligase (Xie et al., 1998; Devoto et al., 2002). The JA precursors, OPDA and dinor-oxophytodenoic acid (dnOPDA) can act via separate COI1-dependent and -independent pathways to control defense gene expression (Stintzi et al., 2001). Constitutive JA-signaling mutants have been isolated carrying elevated levels of JA (cet mutants [constitutive expression of Thi2.1 gene; Hilpert et al., 2001]; cev1 [constitutive expression of vsp1; Ellis et al., 2002b]).
Considerable evidence suggests that SA- and JA-signaling pathways are antagonistic. Application of acetylsalicylate (aspirin) inhibited PI transcript accumulation (Doherty et al., 1988; Peña-Cortes et al., 1993; Doares et al., 1995) and SA suppressed basic (JA-regulated) PR gene expression in tobacco (Nicotiana tabacum; Niki et al., 1998). Predictably, such antagonism impacts on insect grazing as shown with exogenous application of the SA mimic, benzothiadiazole, to tomato (Lycopersicon esculentum) increasing grazing by corn earworm (Heliocoverpa zea; Stout et al., 1999) or beet armyworm (Spodoptera exigua; Thaler et al., 1999). Also, tobacco and cucumber (Cucumis sativus) plants exhibiting SAR displayed evidence of increased insect feeding or fecundity (Moran, 1998; Preston et al., 1999). Genetic manipulation of plants, via transgenes or mutagenesis, has also suggested an antagonistic relationship between SA and JA signaling. For instance, overexpression of a Rho-related GTP-binding protein in tobacco resulted in SA production at wound sites with a concomitant suppression of PI expression (Sano et al., 1994), while in the Arabidopsis eds4 mutant, which is reduced in SA biosynthesis, PDF1-2 expression could be induced at lower concentrations of methyl jasmonate (Gupta et al., 2000). Such antagonism has been linked to a SA-mediated suppression of JA biosynthesis (Peña-Cortes et al., 1993; Doares et al., 1995; Harms et al., 1998). The most conclusive evidence for this was provided by Spoel et al. (2003), who demonstrated a large increase in JA levels in pathogen-challenged Arabidopsis NahG transgenic lines. SA has been suggested to suppress the expression of the JA biosynthetic enzymes lipoxygenase-2 (Spoel et al., 2003) and allene oxide synthase (Laudert and Weiler, 2002). The converse-antagonistic relationship of JA on SA signaling has been less well studied, but it has been shown that JA could suppress acidic PR protein expression (Niki et al., 1998), and in mutant coi plants, SA-mediated gene expression and defenses were augmented (Kloek et al., 2001; Li et al., 2004).
All of these data notwithstanding, the simultaneous exhibition of SA- and JA-mediated phenomena has been noted. Tobacco plants exhibiting SAR as induced by either Tobacco mosaic virus or benzothiadiazole did not affect populations of aphids (Myzus nicotianae), white flies (Bemisia argentifolii), or leaf miners (Liriomyza; Ajlan and Potter, 1992; Inbar et al., 1998). Both SA and JA marker genes are expressed in cpr5, cpr6 (Clarke et al., 1998), cet (Hilpert et al., 2001), cpr22 (Yoshioka et al., 2001), and hrl1 (hypersensitive response-like lesions 1; Devadas et al., 2002) Arabidopsis mutants. In tobacco, Tobacco mosaic virus-induced expression of a PDF1.2-β-glucuronidase (GUS) transgene correlated, both locally and systemically, with that of SA-regulated PR1 transcripts (Mitter et al., 1998). Further, in a range of other experimental systems, contributions from both SA and JA signal transduction chains have been shown to be required. These include increased resistance to Peronospora parasitica Noco2 in the Arabidopsis cpr6 mutant (Clarke et al., 2000), resistance to B. cinerea in wild-type Arabidopsis (Audenaert et al., 2002; Govrin and Levine, 2002), cell death induced by the mycotoxin Fumonisin B1 (Asai et al., 2000), and semiochemical production in Arabidopsis (van Poecke and Dicke, 2002). Other studies have suggested that synergistic SA and JA interactions enhance PR1 (Xu et al., 1994) and Orn decarboxylase (Imanishi et al., 2000) gene expression.
Our interest in these apparently conflicting data sets arose from our observed spatially and temporally coincident synthesis of SA and JA (Kenton et al., 1999; Mur et al., 2000). In this paper we show that JA was not elevated in SH-L (salicylate hydroxylase expressing = NahG) transgenic tobacco plants. In both Arabidopsis and tobacco, reciprocal SA and JA antagonism of gene expression was dose dependent and, at lower concentrations, synergistic effects were observed. With high doses of both SA and JA cell death was initiated, which was influenced by apoplastic oxidative stress. Thus, we propose the existence of greater subtlety in SA + JA interactions than simple antagonism that could explain the widely differing observations on various plants made with pathogens, insects, or mutants.
RESULTS
JA Accumulation Is Not Elevated in 35S-SH-L Tobacco Plants Challenged with Avirulent Bacteria
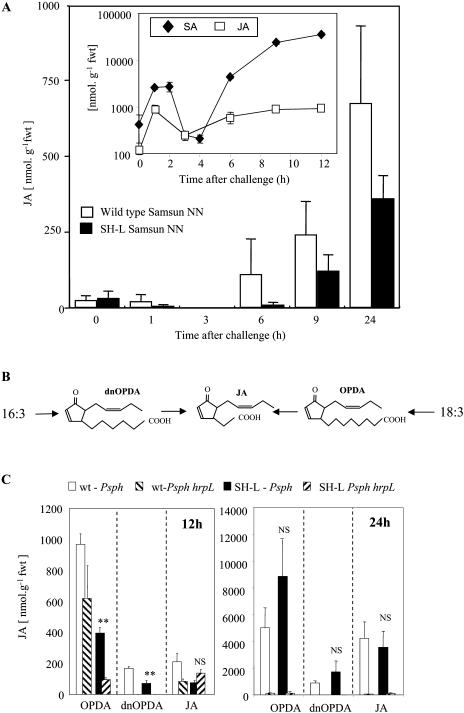
In the course of previous studies on tobacco undergoing HR following infiltration with avirulent Pseudomonas syringae pathovars we have observed that both JA and SA exhibited similar accumulation kinetics (Fig. 1, inset; Kenton et al., 1999; Mur et al., 2000). To further investigate, this JA accumulation was measured using an ELISA-based approach and a JA-specific antibody, and levels were compared between wild type and SH-L-expressing tobacco plants both infected with the avirulent P. syringae pv phaseolicola. Although SH-L expression has been shown to effectively reduce SA accumulation (Mur et al., 2000), no statistically significant differences in JA levels were observed between wild-type and SH-L plants (Fig. 1).
Figure 1.
Octadecanoid rises in wild type and salicylate hydroxylase (SH-L)-expressing transgenic tobacco plants following challenge with P. syringae pv phaseolicola. A, JA accumulation in wild type and salicylate hydroxylase (SH-L)-expressing (SH-L) transgenic Samsun NN tobacco at various times following challenge with the avirulent bacterium P. syringae pv phaseolicola detected using JA anti-sera. Inset, SA and JA accumulation in wild-type tobacco cv Samsun NN following challenge with P. syringae pv phaseolicola. All data are presented as mean (n = 3) nmol g−1 fresh weight (fwt) ± se. B, Alternative routes to the synthesis of JA are indicated. Lipoxygenation of chloroplastic C16:3 or C18:3 lipids leads ultimately to the formation of dnOPDA and OPDA, respectively. These are transported to the peroxisome where the acyl chain is shortened by successive rounds of β-oxidation to form JA. C, OPDA, dnOPDA, and JA accumulation in wild-type (wt) tobacco Samsun NN and SH-L transgenic Samsun NN tobacco plants at 12 and 24 h following inoculation with P. syringae pv phaseolicola (Psph) and a HR noneliciting P. syringae pv phaseolicola hrpL strain (Psph hrpL) detected using GC-MS. All data are presented as mean (n = 3) nmol g−1 fwt ± se. Significant differences (P < 0.01) between levels in wild-type and SH-L plants are indicated by two asterisks. NS, Nonsignificant differences.
JA can be derived from either OPDA or dnOPDA (Fig. 1B), precursors which themselves are able to induce defense gene expression and contribute to JA-mediated effects (Stintzi et al., 2001). Thus, we also assessed the influence of salicylate hydroxylase on the levels of these cyclopentenone JA precursors, as well as on JA; in this instance using gas chromatography-mass spectroscopy (GC-MS; Fig. 1C). At 12 h following inoculation with P. syringae pv phaseolicola both OPDA and dnOPDA levels were reduced in SH-L transgenic (P < 0.01) compared to wild-type plants. There was no significant difference in levels of OPDA in wild-type plants infected with P. syringae pv phaseolicola and the HR noninducing hrpL mutant 12 h after inoculation. However, by 24 h significant increases in both OPDA and dnOPDA (P < 0.001) were seen when challenging with the HR-eliciting strain compared to hrpL controls. Comparing wild-type and SH-L tobacco plants, there were no significant differences in the levels of JA at 12 or 24 h or in those of OPDA and dnOPDA at 24 h.
JA and SA Can Act Synergistically and Antagonistically to Influence Defense Gene Expression in Arabidopsis and Tobacco Plants
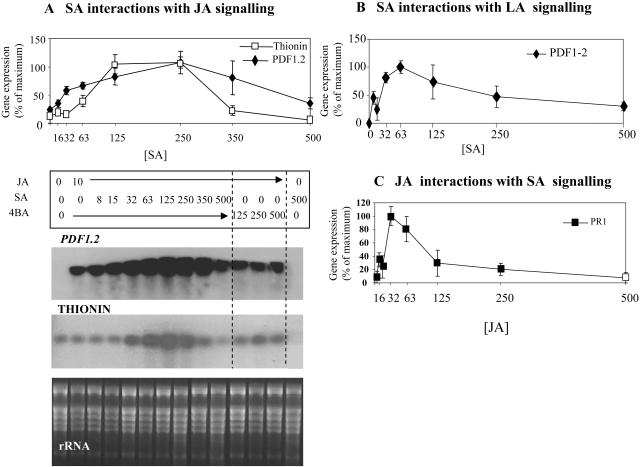
Even with simultaneous synthesis of SA and JA during a HR elicited in tobacco (Fig. 1, inset), suppression/antagonism could still be exhibited at the level of gene expression. Equally, it could be that the relative concentrations of SA and JA are important in determining gene expression levels (Imanishi et al., 2000). To investigate these possibilities, leaf panels of transgenic PR1a-GUS tobacco plants were injected with different concentrations of SA and JA and assayed for GUS activity after 24 h (Fig. 2). GUS activity was significantly (P < 0.001) up-regulated by SA but not JA, yet addition of 10 μm JA to 100 or 250 μm SA enhanced GUS activity (Fig. 2A). Maximal activity at 24 h occurred in the presence of 250 μm SA and 10 μm JA, and represented a >3-fold increase over that observed with 250 μm SA alone. However, the addition of 250 μm JA to 100 or 250 μm SA reduced PR1a-GUS expression compared to controls. Such data suggested distinct concentration-dependent effects of SA + JA combinations. At 48 h PR1a-GUS expression (data not shown) was markedly reduced with all SA + JA coincubations when compared to SA alone, suggesting that the synergistic effects observed at 24 h were transient.
Figure 2.
SA interactions with JA or LN on PR1a-GUS expression in transgenic tobacco. GUS activity in explants of transgenic tobacco at 24 h following treatment with water or 10, 100, 250 μm SA with either 0, 10, 100, 250 μm JA (A) or 0, 10, 100, 250 μm α-LN (B). Data are given as mean (n = 6) GUS activity; pmol 4-methylumbelliferone min−1 explant ± se.
Given that JA biosynthesis has been proposed to be a target for SA antagonism and that cyclopentenones (OPDA and dnOPDA) induce defense genes, the effects of applying the octadecanoid precursor LN (C18:3) was examined (Fig. 2B). Supplying LN alone failed to significantly (P < 0.001) induce PR1a-GUS. However, LN enhanced SA induction of PR1a-GUS expression but only with 250 μm SA and 10 μm LN. Application of 250 μm LN with both 100 and 250 μm SA suppressed PR1a-GUS activity.
These tobacco experiments were extended by examining defense gene transcription in Arabidopsis explants, which were treated with combinations of different SA and JA concentrations. The defense genes PDF1.2 and Thi2.1 have been shown to be regulated by JA (Penninckx et al., 1996; Vignutelli et al., 1998), and by 12 h their expression was induced in Arabidopsis by treatment with 10 μm JA (Fig. 3A). However, coapplication of 10 μm JA with concentrations of SA up to 250 μm enhanced the accumulation of both PDF1.2 and Thi2.1 transcripts. With higher SA concentrations, PDF1.2 and Thi2.1 transcript levels declined. The defense-inactive SA isomer, 4-hydroxybenzoic acid (4BA), failed to synergize with JA, and SA alone did not induce PDF1.2 or Thi2.1 expression.
Figure 3.
Effect of SA interactions with JA or LN on PDF1.2, Thi, and PR1 expression in explants of Arabidopsis ecotype Columbia (Col-0). A, PDF1.2 and Thi2.1 transcript accumulation in Arabidopsis Col-0 explants (the central inner 0.5-cm diameter core of a 1-cm disc; see “Materials and Methods”) at 12 h following treatment with 10 μm JA and increasing concentrations of SA (from 8–500 μm) or 4BA (from 125–500 μm), or 500 μm SA alone. The ethidium bromide-stained gel showing ribosomal RNA (rRNA) is presented to demonstrate equal loading. Northern results for two biological replicates for each experiment were scanned by densitometry to obtain numerical values. Values for each replicate were expressed as a percentage of the maximum value within the same replicate. The graph gives the mean percentage for each treatment and the range between replicates. B, Graphical representation of PDF1.2 transcript accumulation in Arabidopsis explants at 12 h following treatment with 10 μm LN and increasing concentrations of SA (from 15–500 μm), or 4BA (from 125–500 μm), or 500 μm SA alone. C, PR1 transcription in Arabidopsis explants at 12 h following treatment with 10 μm SA and increasing concentrations of JA or LN (from 8–500 μm) or only with JA, LN (125–500 μm), or 500 μm SA alone. Graphs B and C were derived as described in A.
To further examine the possible suppressive effects of SA on JA biosynthesis or cyclopentenone signaling, this experiment was repeated using LN rather than JA. As in tobacco, LN proved to be a less effective inducer of JA-mediated defense genes (Fig. 3B, presented as a plot of densitometric data derived from radiographs of northern blots). This reduced efficacy is likely to be due to only a fraction of LN being metabolized to JA. However, coapplication of LN with increasing concentrations of SA up to 63 μm elevated PDF1.2 expression. At >63 μm SA PDF1.2 transcript accumulation was suppressed. By 24 h following treatment gene expression was also reduced indicating that synergistic SA + JA were time dependent (data not shown).
The converse SA + JA interaction was investigated using the SA-inducible PR1 gene. When treating Arabidopsis explants with 10 μm SA (the minimum concentration at which we have observed PR1 expression; Bi et al., 1995), increasing concentrations of JA up to 32 μm JA had a synergistic effect on the transcription of PR1 (Fig. 3C). At JA concentrations above 32 μm PR1 transcription was less elevated and at ≥125 μm JA the synergistic effect declined until, with 500 μm, no JA effect was observable. As in tobacco, when LN was substituted for JA, this proved to be less effective at synergizing SA-mediated PR1 transcription than JA (data not shown). Taken together, these data strongly suggest that, as with PR1a-GUS in tobacco, SA and JA can transiently synergize defense gene expression, whereas antagonism is a feature of higher concentrations or the prolonged coaccumulation of both signals irrespective of concentration.
PR1 and PDF1.2 transcript accumulation was examined in npr1-1 and coi1 mutant backgrounds to examine the contribution of these signaling components to the synergistic mechanism(s; Fig. 4). Treatments were limited to those that in preliminary experiments (data not shown) had been found to maximally enhance SA and JA gene expression (50 μm SA, 50 μm JA at 12 h). In agreement with our previous observations, in wild-type plants JA enhanced SA-induced PR1 transcription while SA enhanced JA-induced PDF1.2. In the npr1-1 background PR1 transcription was not induced by SA or combined SA + JA treatment, and JA induced PDF1.2 but transcript levels were not enhanced in the presence of SA, contrasting with the observations made in wild-type plants. As expected, in the coi1-1 mutant SA induced PR1 and JA failed to induce PDF1.2 transcription but JA synergism of SA-induced PR1 expression was lacking in a coi1-1 background. Thus, synergism is influenced by established JA- and SA-signaling pathways.
Figure 4.
Effect of SA interactions with JA on PDF1.2 and PR1 transcript accumulation in Arabidopsis npr1-1 and coi1-1. PR1 and PDF1.2 transcription in explants (the central inner 0.5-cm diameter core of a 1-cm disc) from Arabidopsis wild-type Col-0, npr1-1, and coi1-1 in untreated controls and at 12 h following treatment with 50 μm JA and 50 μm SA, either alone or in combination. The ethidium bromide-stained gel showing ribosomal RNA (rRNA) is presented to demonstrate equal loading.
JA and SA Synergize to Induce Cell Death
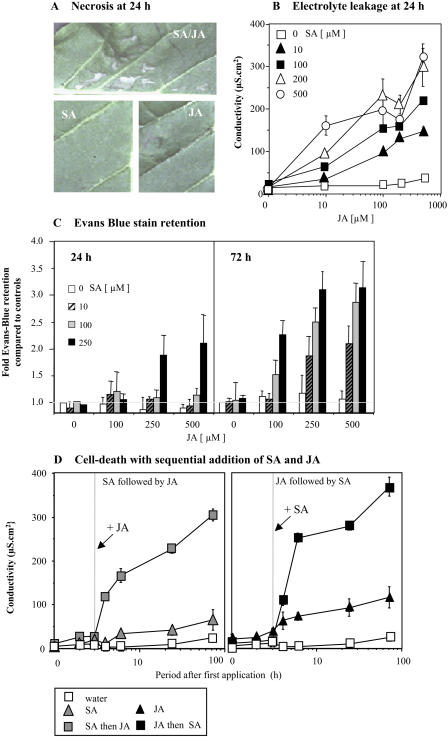
It was noted that when inoculating tobacco plants with 500 μm SA in combination with 500 μm JA, infiltrated areas exhibited patches of necrosis after 24 h (Fig. 5A). This necrosis was not observed when inoculating with either SA or JA alone (or if 500 μm 4BA was substituted for SA in combination with JA; data not shown).
Figure 5.
SA-JA-mediated initiation of plant stress or cell death in tobacco. A, Visible cell death in tobacco leaf panels at 24 h following injection with 0.5 mm SA and 0.5 mm, either alone or in combination. B, Changes in the conductivity (electrolyte leakage) of solutions bathing explants (1-cm diameter cores) of tobacco at 24 h following treatment with various concentrations of JA (0, 10, 100, 200, and 500 μm) with SA (0, 10, 100, 200, and 500 μm). Results are given as mean conductivity change; μS cm2 (n = 6) ± se. C, Retention of Evans blue stain in explants at 24 and 72 h following treatment with 0, 100, 200, and 500 μm JA in combination with 0, 100, 200, or 500 μm SA. Stain retention is expressed as fold increase in mean staining of untreated explants (0 μm SA + JA) at corresponding time points (n = 6) ± se. D, Changes in the conductivity (electrolyte leakage) of solutions bathing explants (1-cm diameter cores) of tobacco in untreated samples, following treatment with 200 μm SA or treatment with 200 μm SA to which 200 μm JA was added after 2 h, as well as the converse scenario where explants were treated with 200 μm JA and 200 μm JA to which 200 μm SA was added at 2 h. Results are given as mean (n = 6) conductivity change, μS cm2 ± se.
To further investigate this phenomenon, leaf discs were treated with various concentrations of SA and JA, and the conductivity of the bathing solution (as a measure of plant stress or death) was measured after 24 h (Fig. 5B). High concentrations of neither JA nor SA (500 μm) resulted in marked electrolyte leakage from the tobacco explants. However, with the exception of the 10 μm SA + 10 μm JA combination, all coapplications of the two signals resulted in a substantial increase in electrolyte leakage. To help distinguish between plant-explant stress and death, viability was estimated by retention of the Evans blue stain which indicates cell rupture (Fig. 5C). This suggested that at 24 h cell death was not observed except with 250 μm SA with 250 or 500 μm JA. However, by 72 h, increases in Evans blue staining were observed with lower concentrations of SA and JA.
Some authors have suggested that the timing of applying SA and/or JA influences the outcome of their interaction (e.g. Thaler et al., 2002). Such effects were assessed by monitoring of electrolyte leakage. Explants were pretreated with 100 μm SA or JA for 2 h before addition of the other signal (Fig. 5D). Compared with SA alone (or control), the addition of JA at 2 h triggered a rapid and substantial increase in the conductivity of the bathing solution. Electrolyte leakage was approximately 5-fold higher than observed with SA alone. In the JA pretreatment experiment, JA alone induced a small increase in electrolyte leakage when compared to control, which was consistent with the stress-related changes previously reported (Kenton et al., 1999). When SA was added, cell death was markedly enhanced so that, by 4 h post SA addition, electrolyte leakage was approximately 3-fold higher than that observed with JA alone (Fig. 5D). Similarly enhanced cell death (as determined by Evans blue retention) was observed with combinations of JA and SA (100 μm each). As with synergistic effects on PR1 and PDF1.2 gene transcription, increased cell death was not observed in npr1-1 and coi1 mutants (data not shown).
JA-Induced Gene Expression and Cell Death Is Mediated by Hydrogen Peroxide and Potentiated by SA
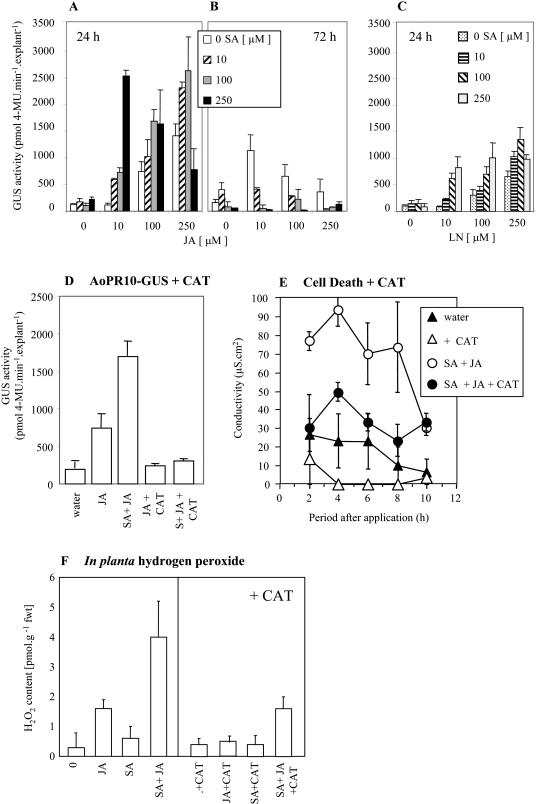
Plant cell death has been linked with the generation of oxidative stress (Levine et al., 1994), therefore SA + JA effects on our marker for oxidative stress, the AoPR10-GUS transgene (Bi et al., 1995), were assessed (Fig. 6, A and B). Unlike PR1a-GUS, the AoPR10-GUS transgene was activated by JA alone but, as previously reported (Mur et al., 1996), not by SA. Nevertheless, addition of SA augmented JA-induced AoPR10-GUS expression at 24 h except at the highest concentrations (250 μm of each). At 72 h, application of any concentration of SA appeared to antagonize JA-mediated AoPR10-GUS expression (Fig. 6B). Predictably, application of LN also induced AoPR10-GUS expression, although less than with JA (Fig. 6C), while coapplication with 100 and 250 μm SA enhanced LN-induced AoPR10-GUS expression. To establish if SA + JA effects on AoPR10-GUS expression were due to oxidative events, the effects of coinfiltrating catalase (CAT) with JA alone, or in combination with SA (100 μm each), were investigated (Fig. 6D). CAT suppressed both JA-induced and SA-synergized AoPR10-GUS expression at 12 h, thereby substantiating our hypothesis.
Figure 6.
SA interactions with jasmonates on AoPR10-GUS expression in transgenic tobacco and the generation of oxidative stress. AoPR10-GUS activity in explants of transgenic tobacco at 24 (A) and 72 h (B) following treatment with water (0) or 10, 100, or 250 μm SA with either 0, 10, 100, or 250 μm JA or at 24 h with 0, 10, 100, or 250 μm α-LN (C). D, AoPR10-GUS activity in tobacco leaves at 24 h following injection of water; 100 μm JA alone or a combination of 100 μm SA + 100 μm JA and also100 μm JA with 50 units mL−1 catalase (CAT) or 100 μm SA + 100 μm JA with CAT. E, Changes in the conductivity (electrolyte leakage) of solutions bathing tobacco explants (1-cm diameter cores) from leaf panels previously inoculated with water and 50 units mL−1 CAT or 250 μm JA + 250 μm SA with or without and 50 units mL−1 CAT. Results are given as mean (n = 6) conductivity change, μS cm2. F, H2O2 content in one third of tobacco leaves at 12 h following injection either with water (0), 100 μm JA, 100 μm SA, or 100 μm JA + 100 μm SA compared to another third of the leaf inoculated where 50 units mL−1 CAT was added to either water (0), 100 μm JA, 100 μm SA, or 100 μm JA + 100 μm SA. For each sample the H2O2 content of the untreated third of the same leaf was subtracted from the value obtained for the treated parts. Results are given as mean (n = 6) samples H2O2 content (pmol g−1 fwt) ± se.
We also used CAT infiltration to test whether SA + JA-mediated cell death was influenced by ROS production. CAT was coinjected with SA + JA, and the resulting electrolyte leakage from explants was measured (Fig. 6E). The induction of electrolyte leakage when simply infiltrating with water suggested that the injection process resulted in plant stress. However, when JA and SA were coincubated, substantial additional electrolyte leakage occurred and this effect was markedly inhibited (at least within the first 6 h) by the addition of CAT. These data implicated hydrogen peroxide (H2O2) in the cell death induced by JA and SA.
To further establish a role of ROS, H2O2 levels in planta were measured following treatment with 100 μm JA and 100 μm SA and assayed after 6 h (Fig. 6F). Treatment with JA alone induced accumulation of H2O2 (confirmed as such by CAT controls) to approximately 2-fold that observed in controls. In contrast, SA induced little if any additional H2O2 accumulation in accordance with the failure of SA to induce AoPR10-GUS. However, H2O2 levels in tissue treated with both JA and SA were substantially elevated compared to those observed when applying JA alone.
DISCUSSION
SA Does Not Always Inhibit JA Biosynthesis and Signaling
In mammalian systems, the derivation of proinflammatory eicosanoids from arachidonic acid (C20:4) is inhibited by aspirin (acetylsalicylate) via transacetylation of the biosynthetic cyclooxygenase enzymes (Vane, 1971). Possibly due to some parallels between this and the formation of jasmonates from LN (C18:3), the inhibitory effects of salicylates on JA biosynthesis and signaling have been extensively investigated. It has been repeatedly demonstrated that aspirin, SA, and SA mimics suppress JA-mediated gene expression and associated phenomena (e.g. Peña-Cortés et al., 1993; Niki et al., 1998; Stout et al., 1999; Thaler et al., 1999). As exogenously applied jasmonates reverse the inhibitory effects of SA application, this effect has been linked to the suppression of JA biosynthesis (e.g. Sivasankar et al., 2000). Most tellingly in this regard, Spoel et al. (2003) showed that Arabidopsis plants expressing NahG exhibited a significant increase in JA following inoculation with the virulent strain P. syringae pv tomato DC3000. Since we had observed the coproduction of SA and JA in HR lesions in tobacco, we predicted that the reduction of SA levels in SH-L (=NahG) tobacco lines would result in elevated JA levels. Surprisingly, we failed to observe any statistically significant difference in the patterns of JA synthesis during the first 12 h of the developing HR following inoculation with the avirulent strain P. syringae pv phaseolicola (Fig. 1A). Similarly, levels of the JA precursors OPDA and dnOPDA, which can themselves act as signaling molecules (Danon et al., 2005), were unchanged in SH-L-expressing tobacco. An analogous situation was noted by Dhondt et al. (2002), where cell death elicited by the fungal elicitor β-megaspermin in tobacco was associated with both SA and JA synthesis, and JA levels were not altered in NahG plants. Interestingly, Heck et al. (2003) also failed to observe increases in JA levels in transgenic Arabidopsis NahG plants or in sid2 mutants (which are mutated in the SA-biosynthetic gene isochorismate synthase) following inoculation with the avirulent Pst DC3000 avrRpt2 strain. In the interaction of Xanthomonas campestris pv vesicatoria and tomato, the use of NahG plants has shown that JA synthesis can be dependent on SA accumulation (O'Donnell et al., 2003). In this context, it may be relevant that OPDA levels were significantly lower in 35S-SH-L tobacco plants at 12 h following challenge with P. syringae pv phaseolicola (Fig. 1C).
In attempting to rationalize these data, we note that in both the Heck et al. (2003) and Spoel et al. (2003) studies, elevated JA levels were measured during compatible interactions involving P. syringae pv tomato DC3000. Hence, SA + JA interactions could be substantially different during the HR compared to developing disease scenarios. Indeed, the antagonism of JA signaling by SA may be a requirement of the pathogenic processes of this bacterial strain.
Varying SA and JA Interactions Are Observed at Different Relative Concentrations
In examining the effects of synchronously produced SA and JA, workers have noted either synergistic (Xu et al., 1994; Imanishi et al., 2000) or antagonistic (Peña-Cortés et al., 1993) interactions. Such data argue that the nature of SA + JA interactions is specific for a particular gene or set of genes, or that there exists a degree of as yet unsuspected subtlety. It was notable that studies reporting antagonistic interactions following exogenous application of SA tended to use concentrations of >0.5 mm, while lower levels (e.g. 0.1 mm) had either no effect (Peña-Cortés et al., 1993; Thaler et al., 2002) or exhibited synergistic effects on gene expression (Imanishi et al., 2000). Thus, in examining the effects of SA and JA on gene expression it was clearly important to test a range of concentrations of each signal. In Arabidopsis, PDF1.2 and Thi2.1 are established marker genes for JA signaling and have been reported to exhibit variable, apparently contradictory responses to SA + JA interaction. For instance, in cpr6 × NahG Arabidopsis plants, constitutive expression of PDF1.2 was increased (Clarke et al., 1998), while in the hrl1 mutant, constitutive expression of PDF1.2 was reduced in crosses with npr1-1 or NahG transgenics (Devadas et al., 2002). This ambivalent response to SA by PDF1.2 was also reflected in our data. JA-induced PDF1.2 expression could be enhanced by concentrations of SA up to approximately 350 μm but reduced at higher levels. Thus, the different trends observed in different mutants could be dependent on the relative concentrations of JA and SA or the modifications of each respective signaling cascade. It was perhaps significant that in hrl1, where synergistic effects on JA-mediated gene expression were observed, SA levels were lower than those measured for cpr6 (Devadas et al., 2002). When considering the concentration of SA and JA used in our experiments, it should be noted that we infiltrated our solutions into the apoplastic space. Hence, it is not possible to directly compare effects with other studies where SA and JA were applied as a spray or foliar drench. Moreover, in our own studies, we cannot relate apoplastic concentrations to SA and JA levels within the cell. We consider that the effects seen (synergy → antagonism → oxidative stress → death) cannot be precisely linked to the SA + JA concentrations that we applied but do reflect the consequences of differing cellular levels of these signals.
Examining the effect of JA on SA-mediated signaling also suggested the existence of both synergistic and antagonistic effects. In tobacco, we observed a synergistic increase in PR1a-GUS expression at the lower range of SA and JA concentrations, while at higher concentrations GUS activity was suppressed. Similarly, PR1 transcript accumulation in Arabidopsis in response to 10 μm SA was increasingly enhanced by the addition of JA up to 125 μm, over which expression was reduced. Clark et al. (2000) also reported that in cpr6 PR1, expression was dependent on synergy between npr1-1, jar1 (a JA-signaling mutant), as well as ein3 (an ethylene-signaling mutant).
ROS Generation Is a Feature of Synergistic Gene Expression and Cell Death
Several Arabidopsis mutants have been identified that exhibit the constitutive coactivation of SA- and JA-signaling pathways and also exhibit cell death, for example, cet2, cet9 (Hilpert et al., 2001), and hrl1 (Devadas et al., 2002). We observed that necrotic lesions could be elicited in tobacco by injection with >250 μm SA and JA, but only when added in combination (Fig. 5A). Data from electrolyte leakage, as compared to that of Evans blue staining, indicated that plant stress was a notable feature even of lower concentrations (Fig. 5B). Both SA and JA alone have been observed to potentiate the oxidative burst (Kauss et al., 1994; Kauss and Jeblick, 1995; Mur et al., 2000), thus increased plant stress could have occurred from the activation of dual potentiation mechanisms. To investigate this hypothesis, the effect of SA + JA combinations was assessed on AoPR10-GUS expression as an easily assayable potentiation and oxidative stress marker (Mur et al., 1996, 2004). AoPR10-GUS expression proved to be induced by the application of JA alone, a feature which has been noted with other PR10 genes (Jwa et al., 2001). However, JA has been shown to activate the synthesis of polyamines, which serve as substrates for apoplastic polyamine oxidase to generate H2O2 (Walters, 2003). As we suppressed JA-induced AoPR10-GUS activity with CAT it was likely that the transgene was primarily responsive to H2O2 (Fig. 6D). Significantly, SA + JA-synergized AoPR10-GUS activity was suppressed with catalase, suggesting that coapplication of these signals increased oxidative stress. This hypothesis was confirmed by direct measurements of in planta H2O2 content, indicating that SA and JA copotentiation of the oxidative burst could be a facet of the synergistic mechanism. A synergized oxidative burst would have considerable effects on defense gene expression, as some genes whose transcription is regulated by SA or JA have been shown to be also modulated by H2O2 (Chen et al., 1993; Orozco-Cardeñas et al., 2001).
SA + JA-induced cell death could also be reduced by the coapplication of CAT (Fig. 6E), indicating that increased oxidative stress was also instrumental in this process. In this context, it may be significant that JA- and SA-signaling pathways are both required for some forms of cell death (Asai et al., 2000) and that observable cell death in tobacco occurs at approximately 6 h in the P. syringae pv phaseolicola-tobacco interaction, when both SA and JA are present (Fig. 1, inset). Further, in SH-L plants the oxidative burst recovered to wild-type levels only when JA synthesis had been initiated (Mur et al., 2000). Clearly, many other signals regulate the HR, but such correlative observations suggest that synergistic SA + JA interactions influencing the generation of oxidative stress represent one facet.
How far oxidative cellular stress is intrinsic to the antagonism mechanism remains to be clearly established. Northern-blot results suggested that antagonism occurred in PDF1.2 and Thi2.1 expression between 350 and 500 μm SA with 10 μm JA (Fig. 3A) where significant electrolyte leakage, but no Evans blue staining, was observed (Fig. 5). However, PR1 transcription was suppressed with 125 μm JA and 10 μm SA (Fig. 3C), where minimal electrolyte linkage was noted (Fig. 5B). Thus, the data suggest that although antagonism need not always be linked to increased plant stress, this remains a factor that should be considered in studies examining SA + JA effects.
Components in SA + JA Antagonistic and Synergistic Mechanism(s)
Several signaling components have been suggested to be part of the mechanism through which SA antagonizes JA signaling. One could be MAPK4 as the mpk4 mutant exhibited constitutive exhibition of SAR as well as SA- and npr1-1-dependent suppression of PDF1.2 and Thi2.1 gene expression (Petersen et al., 2000). The transcription factor WRKY70 also plays a key role in the induction of SA- and the suppression of JA-dependent genes (Li et al., 2004). The action of WRKY70 is partially mediated by NPR1-1, which must be also considered part of the antagonism mechanism (Clarke et al., 1998; Spoel et al., 2003). However, npr1-1-dependent PDF1.2 expression seen in hrl-1 (Devadas et al., 2002) and our data suggested another role for NPR1 in SA + JA synergistic effects on PDF-1.2 expression (Fig. 4) and cell death. Further, NPR1-1 has been shown to be instrumental in some cell death mechanisms (Aviv et al., 2002) and affected the pattern of necrosis in hrl1 (Devadas et al., 2002). Taken together, these observations suggest that NPR1 is an important transcriptional switch, regulating SA signaling as well as influencing JA responses and modulating cell death probably by regulating antagonistic/synergistic mechanisms.
Surprisingly our data suggested that COI1 influenced both PR1 transcription and electrolyte leakage. Although Fumonsin-B1-induced cell death was reduced in the jai1 (JA-insensitive) Arabidopsis mutant (Asai et al., 2000), in hrl1-1 and cet1, cet3 and cet4.1 the exhibition of cell death was not reduced by the coi1-1 mutation (Devadas et al., 2002; Nibbe et al., 2002). Indeed, in hrl1-1 × coi1 double mutants cell death was exaggerated (Devadas et al., 2002), possibly indicating an antagonistic role for JA on cell death in this mutant. Clearly, further investigations are required to establish the contribution of individual JA-signaling components to the synergism mechanism.
A question remains as to the relevance of synergistic signal interactions to resistance responses. Often simultaneous activation of signaling pathways has no additive effects with resistance patterns to discrete pathogens and pests being maintained (e.g. van Wees et al., 2000), although there are instances where both SA- and JA-signaling pathways are required (e.g. Ellis et al., 2002a). It seems likely that variably employed synergistic/antagonistic mechanisms, which need involve not only SA and JA but also, for example, ethylene (Penninckx et al., 1998; Tuominen et al., 2004), may represent positive and negative feedback loops allowing the tailoring of the plant response to a particular stress (Maleck and Dietrich, 1999).
MATERIALS AND METHODS
Plant Materials and Chemicals
The derivation of AoPR10-GUS and PR1a-GUS Samsun NN transgenic lines is described in Mur et al. (1996, 1997). Tobacco (Nicotiana tabacum) plants were grown under 16-h-light period at 23°C ± 2°C and used for experimentation at 5 weeks following germination. Arabidopsis (Arabidopsis thaliana) plants were grown at 20°C ± 2°C under an 8-h-light period and used at approximately 4 weeks following germination. Plants were illuminated with 55 W (Osram, Sylvania) high-frequency lighting tubes (4,580 lumen output), supplemented by 2 × 30 W clear-tube cooled lighting. Light fluence rates at the top of the plants always exceeded 100 μmol m−2 s−1. All chemicals were purchased from Sigma Pharmaceuticals.
Bacterial Inoculation and Chemical Treatments of Plants
Tobacco plants were inoculated with avirulent bacterial pathogens as described in Mur et al. (2000). Data presented in Figure 1A were derived using an inoculum of 2 × 108 bacterial cells mL−1 while those in Figure 1C used 2 × 106 cells mL−1. Tobacco plants were treated with various concentrations of SA and JA by injection into leaf panels. If assaying for GUS activities or Evans blue staining, the injected area was marked with a felt-tipped pen and assayed, following coring with a 1-cm cork borer (1 core = 0.785 cm2) at set times. When assaying for electrolyte leakage in tobacco or Arabidopsis, injected areas were immediately cored and placed in 24-well plates (Nalge Nunc International). When examining SA + JA effects on gene transcription in Arabidopsis, chemicals were added to the solution (1 cm3) bathing 1-cm diameter explants in 24-well plates. The solutions were vacuum infiltrated into the explants by placement in a vacuum chamber and evacuating the air for 5 min, using a pump. After 12 h, an inner core (using a 0.5-cm diameter cork-borer) was taken from the 1-cm diameter explant and the outer ring, consisting of the original wound site, was discarded. RNA was immediately extracted only from the inner core. This avoided contributions to the results made by wound-associated gene expression. Individual RNA samples represented pooled preparations from four explants.
Estimations of Cell Viability by Electrolyte Leakage and Evans Blue Staining
Electrolyte leakage in 1-cm diameter cores was determined as described in Mur et al. (1997). Cell death was estimated by retention of Evans blue stain by 1-cm cores as stated in Mur et al. (2000).
Northern Hybridization
RNA extraction, northern blotting, and hybridization were undertaken as described in Draper et al. (1988). Probes for PR1 (At2g19990) and Thi2.1 (At1g77260) were obtained from The Arabidopsis Information Resource (http://www.arabidopsis.org). Autoradiographs were quantified using ImageQuant Software (Molecular Dynamics), which qualified pixel intensity over a designated area.
Octadecanoid and SA Measurements
JA measurements shown in Figure 1A were carried out as stated in Kenton et al. (1999) using anti-JA antibodies. Other measurements of JA and OPDA and dnOPDA were undertaken using GC-MS as described in Stenzel et al. (2003). For GC-MS, approximately 0.5 g homogenized sample from Pseudomonas syringae pv. phaseolicola-challenged tobacco was extracted with 10 mL methanol. [2H6] JA and [2H6] OPDA were added as internal standards for GC-MS. Mean values are presented from three independent extraction/separation procedures. Data for SA levels have been previously presented (Mur et al., 2000) and the assay protocol used is described in Bi et al. (1995).
In Planta H2O2 Measurements
H2O2 accumulation was determined following the TiCl4-based technique developed by Brennan and Frenkel (1977). H2O2 accumulation was expressed in terms of an increase in levels compared to controls in the same leaf. Thus, one half of a tobacco leaf was infiltrated with water (control) and the other with either SA or JA alone or a combination of both. As each assay required 0.5 g of plant tissue, large, fully expanded leaves of 5-week-old tobacco plants were used. Such leaves were easy to rapidly infiltrate (typically 2 min), which appeared to aid in synchronizing the generation of the oxidative burst. Each leaf piece was ground in 1 mL acetone, and a 0.5-mL aliquot taken, to which 75 μL of freshly prepared 20% TiCl4 (v/v in 11 m HCl) was added followed by 150 μL of NH4OH. The resulting precipitate was collected by microcentrifugation (5 min, 11,000g) and the supernatant carefully discarded. The pellet was resuspended in 400 μL 1 m H2SO4, to which 180 μL of acetone was added. Any insoluble material was removed by centrifugation (5 min at 11,000g). The absorbance of the supernatant was measured at 405 nm with a 630-nm reference (to deduct reading due to turbidity). The concentration of the samples was estimated from a standard curve, where 0.1 mm to 1 mm H2O2 was added to extracts from controls and complexed with titanium as stated. The levels of H2O2 within challenged leaves (micromole/gram fresh weight−1) were calculated as for SA levels in Bi et al. (1995). Each experiment was repeated at least six times to generate the data presented.
Repetition and Statistical Analysis
Each experiment was undertaken at least three times to generate similar data to that presented in this paper. The exception was the northern-hybridization data in Figures 3 and 4, which were repeated only once, giving similar results. All other data was tested for significance by ANOVA using Minitab version 13.
Acknowledgments
We thank Prof. John Draper (University of Wales [UW] Aberystwyth, UK) for his support during the initial phase of this project. Prof. John Mansfield (Wye College, Imperial, UK) kindly provided P. syringae pv phaseolicola, Prof. Xinian Dong (Duke University, Durham, NC) the Arabidopsis npr1-1 mutant, Prof. John Turner (University of East Anglia, Norwich, UK) coi1-1, and Prof. Willem Broekaert (Katholieke Universiteit Leuven, Belgium) the PDF1.2 cDNA. We especially appreciate the contribution made by Prof. Broekaert, Dr. Bart Thomma (Katholieke Universiteit Leuven, Belgium), and Dr. Aileen Smith (UW Aberystwyth, UK) in developing some of the ideas presented in this paper. We also wish to thank Dr. Shannon Clarke (University of Otago, Dunedin, New Zealand) for critically reading this manuscript and Pat Causton (UW Aberystwyth, UK) for growing and maintaining plant material. The pathogen work described in this paper was undertaken under DEFRA license PHL 123A/4324.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Luis A.J. Mur (lum@aber.ac.uk).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.072348.
References
- Agrawal AA (2000) Mechanisms, ecological consequences and agricultural implications of tri-trophic interactions. Curr Opin Plant Biol 3: 329–335 [DOI] [PubMed] [Google Scholar]
- Ajlan AM, Potter DA (1992) Lack of effect of tobacco mosaic virus-induced systemic acquired resistance on arthropod herbivores in tobacco. Phytopathology 82: 647–651 [Google Scholar]
- Asai T, Stone JM, Heard JE, Kovtun Y, Yorgey P, Sheen J, Ausubel FM (2000) Fumonisin B1-induced cell death in Arabidopsis protoplasts requires jasmonate-, ethylene-, and salicylate-dependent signaling pathways. Plant Cell 12: 1823–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audenaert K, De Meyer GB, Höfte MM (2002) Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid-dependent signaling mechanisms. Plant Physiol 128: 491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv DH, Rusterucci C, Iii BF, Dietrich RA, Parker JE, Dangl JL (2002) Runaway cell death, but not basal disease resistance, in lsd1 is SA- and NIM1/NPR1-dependent. Plant J 29: 381–391 [DOI] [PubMed] [Google Scholar]
- Bi YM, Kenton P, Mur L, Darby R, Draper J (1995) Hydrogen peroxide does not function downstream of salicylic acid in the induction of PR protein expression. Plant J 8: 235–245 [DOI] [PubMed] [Google Scholar]
- Birkett MA, Campbell CA, Chamberlain K, Guerrieri E, Hick AJ, Martin JL, Matthes M, Napier JA, Pettersson J, Pickett JA, et al (2000) New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc Natl Acad Sci USA 97: 9329–9334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling SA, Clarke JD, Liu Y, Klessig DF, Dong X (1997) The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell 9: 1573–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling SA, Guo A, Cao H, Gordon AS, Klessig DF, Dong X (1994) A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell 6: 1845–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan T, Frenkel C (1977) Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol 59: 411–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X (1997) The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88: 57–63 [DOI] [PubMed] [Google Scholar]
- Chen Z, Silva H, Klessig DF (1993) Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 262: 1883–1886 [DOI] [PubMed] [Google Scholar]
- Clarke JD, Liu Y, Klessig DF, Dong X (1998) Uncoupling PR gene expression from NPR1 and bacterial resistance: characterization of the dominant Arabidopsis cpr6-1 mutant. Plant Cell 10: 557–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JD, Volko SM, Ledford H, Ausubel FM, Dong X (2000) Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis. Plant Cell 12: 2175–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon A, Miersch O, Felix G, Camp RG, Apel K (2005) Concurrent activation of cell death-regulating signaling pathways by singlet oxygen in Arabidopsis thaliana. Plant J 41: 68–80 [DOI] [PubMed] [Google Scholar]
- Delaney TP, Friedrich L, Ryals JA (1995) Arabidopsis signal transduction mutant defective in chemically and biologically induced disease resistance. Proc Natl Acad Sci USA 92: 6602–6606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gutrella M, Kessmann H, Ward E, et al (1994) A central role of salicylic-acid in plant-disease resistance. Science 266: 1247–1250 [DOI] [PubMed] [Google Scholar]
- Després C, Chubak C, Rochon A, Clark R, Bethune T, Desveaux D, Fobert PR (2003) The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. Plant Cell 15: 2181–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devadas SK, Enyedi A, Raina R (2002) The Arabidopsis hrl1 mutation reveals novel overlapping roles for salicylic acid, jasmonic acid and ethylene signaling in cell death and defence against pathogens. Plant J 30: 467–480 [DOI] [PubMed] [Google Scholar]
- Devoto A, Nieto-Rostro M, Xie D, Ellis C, Harmston R, Patrick E, Davis J, Sherratt L, Coleman M, Turner JG (2002) COI1 links jasmonate signaling and fertility to the SCF ubiquitin-ligase complex in Arabidopsis. Plant J 32: 457–466 [DOI] [PubMed] [Google Scholar]
- Dhondt S, Gouzerh G, Muller A, Legrand M, Heitz T (2002) Spatio-temporal expression of patatin-like lipid acyl hydrolases and accumulation of jasmonates in elicitor-treated tobacco leaves are not affected by endogenous levels of salicylic acid. Plant J 32: 749–762 [DOI] [PubMed] [Google Scholar]
- Doares SH, Narvaez-Vasquez J, Conconi A, Ryan CA (1995) Salicylic acid inhibits synthesis of proteinase inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiol 108: 1741–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty HM, Selvendram RR, Bowles DJ (1988) The wound response of tomato plants can be inhibited by aspirin and related hydroxy-benzoic acid. Physiol Mol Plant Pathol 33: 377–384 [Google Scholar]
- Dong X (2001) Genetic dissection of systemic acquired resistance. Curr Opin Plant Biol 4: 309–314 [DOI] [PubMed] [Google Scholar]
- Draper J, Scott R, Armitage P, Walden R (1988) Plant Genetic Transformation and Gene Expression: A Laboratory Manual. Blackwell Scientific Publications, Oxford
- Ellis C, Karafyllidis I, Turner JG (2002. a) Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae. Mol Plant Microbe Interact 15: 1025–1030 [DOI] [PubMed] [Google Scholar]
- Ellis C, Karafyllidis I, Wasternack C, Turner JG (2002. b) The Arabidopsis mutant cev1 links cell wall signaling to jasmonate and ethylene responses. Plant Cell 14: 1557–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Dong X (2002) In vivo interaction between NPR1 and transcription factor TGA2 leads to salicylic acid-mediated gene activation in Arabidopsis. Plant Cell 14: 1377–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys BJ, Parker JE (2000) Interplay of signaling pathways in plant disease resistance. Trends Genet 16: 449–455 [DOI] [PubMed] [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J (1993) Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261: 754–756 [DOI] [PubMed] [Google Scholar]
- Govrin EM, Levine A (2002) Infection of Arabidopsis with a necrotrophic pathogen, Botrytis cinerea, elicits various defense responses but does not induce systemic acquired resistance (SAR). Plant Mol Biol 48: 267–276 [DOI] [PubMed] [Google Scholar]
- Gupta V, Willits MG, Glazebrook J (2000) Arabidopsis thaliana EDS4 contributes to salicylic acid (SA)-dependent expression of defense responses: evidence for inhibition of jasmonic acid signaling by SA. Mol Plant Microbe Interact 13: 503–511 [DOI] [PubMed] [Google Scholar]
- Harms K, Ramirez I, Peña-Cortes H (1998) Inhibition of wound-induced accumulation of allene oxide synthase transcripts in flax leaves by aspirin and salicylic acid. Plant Physiol 118: 1057–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck S, Grau T, Buchala A, Metraux JP, Nawrath C (2003) Genetic evidence that expression of NahG modifies defence pathways independent of salicylic acid biosynthesis in the Arabidopsis-Pseudomonas syringae pv. tomato interaction. Plant J 36: 342–352 [DOI] [PubMed] [Google Scholar]
- Hilpert B, Bohlmann H, op den Camp RO, Przybyla D, Miersch O, Buchala A, Apel K (2001) Isolation and characterization of signal transduction mutants of Arabidopsis thaliana that constitutively activate the octadecanoid pathway and form necrotic microlesions. Plant J 26: 435–446 [DOI] [PubMed] [Google Scholar]
- Imanishi S, Nakakita M, Yamashita K, Furata A, Utsuno K, Muramoto N, Kojima H, Nakamura K (2000) Aspirin and salicylic acid do not inhibit methyl jasmonate-inducible expression of a gene for ornithine decarboxylase in tobacco BY-2 cells. Biosci Biotechnol Biochem 64: 125–133 [DOI] [PubMed] [Google Scholar]
- Inbar M, Doostdar H, Sonoda RM, Leibee GL, Mayer RT (1998) Elicitors of plant defensive systems reduce insect densities and disease incidence. J Chem Ecol 24: 135–149 [Google Scholar]
- Jwa NS, Kumar Agrawal G, Rakwal R, Park CH, Prasad Agrawal V (2001) Molecular cloning and characterization of a novel jasmonate inducible pathogenesis-related class 10 protein gene, JIOsPR10, from rice (Oryza sativa L.) seedling leaves. Biochem Biophys Res Commun 286: 973–983 [DOI] [PubMed] [Google Scholar]
- Kauss H, Jeblick W (1995) Pretreatment of parsley suspension cultures with salicylic acid enhances spontaneous and elicited production of H2O2. Plant Physiol 108: 1171–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H, Jeblick W, Ziegler J, Krabler W (1994) Pretreatment of parsley (Petroselinum crispum L.) suspension cultures with methyl jasmonate enhances elicitation of activated oxygen species. Plant Physiol 105: 89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenton P, Mur LAJ, Atzorn R, Wasternack C, Draper J (1999) (−)-Jasmonic acid accumulation in tobacco hypersensitive response lesions. Mol Plant Microbe Interact 12: 74–78 [Google Scholar]
- Kloek AP, Verbsky ML, Sharma SB, Schoelz JE, Vogel J, Klessig DF, Kunkel BN (2001) Resistance to Pseudomonas syringae conferred by an Arabidopsis thaliana coronatine-insensitive (coi1) mutation occurs through two distinct mechanisms. Plant J 26: 509–522 [DOI] [PubMed] [Google Scholar]
- Laudert D, Weiler EW (2002) Allene oxide synthase: a major control point in Arabidopsis thaliana octadecanoid signaling. Plant J 15: 675–684 [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593 [DOI] [PubMed] [Google Scholar]
- Li J, Brader G, Palva ET (2004) The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 16: 319–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleck K, Dietrich RA (1999) Defense on multiple fronts: How do plants cope with diverse enemies? Trends Plant Sci 4: 215–219 [DOI] [PubMed] [Google Scholar]
- Maleck K, Neuenschwander U, Cade RM, Dietrich RA, Dangl JL, Ryals JA (2002) Isolation and characterization of broad-spectrum disease-resistant Arabidopsis mutants. Genetics 160: 1661–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitter N, Kazan K, Way HM, Broekaert WF, Manners JM (1998) Systemic induction of an Arabidopsis plant defensin gene promoter by tobacco mosaic virus and jasmonic acid in transgenic tobacco. Plant Sci 136: 169–180 [Google Scholar]
- Moran PJ (1998) Plant-mediated interactions between insects and a fungal plant pathogen and the role of plant chemical responses to infection. Oecologia 115: 523–530 [DOI] [PubMed] [Google Scholar]
- Mur LA, Brown IR, Darby RM, Bestwick CS, Bi YM, Mansfield JW, Draper J (2000) A loss of resistance to avirulent bacterial pathogens in tobacco is associated with the attenuation of a salicylic acid-potentiated oxidative burst. Plant J 23: 609–621 [DOI] [PubMed] [Google Scholar]
- Mur LAJ, Bi YM, Darby RM, Firek S, Draper J (1997) Compromising early salicylic acid accumulation delays the hypersensitive response and increases viral dispersal during lesion establishment in TMV-infected tobacco. Plant J 12: 1113–1126 [DOI] [PubMed] [Google Scholar]
- Mur LAJ, Naylor G, Warner SAJ, Sugars JM, White RF, Draper J (1996) Salicylic acid potentiates defence gene expression in tissue exhibiting acquired resistance to pathogen attack. Plant J 9: 559–571 [Google Scholar]
- Mur LAJ, Sturgess FJ, Farrell GG, Draper J (2004) The AoPR10 promoter and certain homologous PR10 genes report oxidative stress in Arabidopsis. Mol Plant Pathol 5: 435–451 [DOI] [PubMed] [Google Scholar]
- Nibbe M, Hilpert B, Wasternack C, Miersch O, Apel K (2002) Cell death and salicylate- and jasmonate-dependent stress responses in Arabidopsis are controlled by single cet genes. Planta 216: 120–128 [DOI] [PubMed] [Google Scholar]
- Niki T, Mitsuhara I, Seo S, Ohtsubo N, Ohashi Y (1998) Antagonistic effect of salicylic acid and jasmonic acid on the expression of pathogenesis-related (PR) protein genes in wounded mature tobacco leaves. Plant Cell Physiol 39: 500–507 [Google Scholar]
- O'Donnell PJ, Schmelz E, Block A, Miersch O, Wasternack C, Jones JB, Klee HJ (2003) Multiple hormones act sequentially to mediate a susceptible tomato pathogen defense response. Plant Physiol 133: 1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cardeñas ML, Narvaez-Vasquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13: 179–191 [PMC free article] [PubMed] [Google Scholar]
- Peña-Cortes H, Albrecht T, Prat S, Weiler EW, Willmitzer L (1993) Aspirin prevents wound-induced gene expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta 191: 123–128 [Google Scholar]
- Penninckx IA, Eggermont K, Terras FR, Thomma BP, De Samblanx GW, Buchala A, Metraux JP, Manners JM, Broekaert WF (1996) Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell 8: 2309–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx IA, Thomma BP, Buchala A, Metraux JP, Broekaert WF (1998) Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 10: 2103–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, Nielsen HB, Lacy M, Austin MJ, Parker JE, et al (2000) Arabidopsis MAP kinase 4 negatively regulates systemic acquired resistance. Cell 103: 1111–1120 [DOI] [PubMed] [Google Scholar]
- Pieterse CM, van Wees SC, Hoffland E, van Pelt JA, van Loon LC (1996) Systemic resistance in Arabidopsis induced by biocontrol bacteria is independent of salicylic acid accumulation and pathogenesis-related gene expression. Plant Cell 8: 1225–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston CA, Lewandowski C, Enyedi AJ, Baldwin IT (1999) Tobacco mosaic virus inoculation inhibits wound-induced jasmonic acid-mediated responses within but not between plants. Planta 209: 87–95 [DOI] [PubMed] [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8: 1809–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CA (1990) Protease inhibitors in plants: genes for improving defenses against insects and pathogens. Annu Rev Phytopathol 28: 425–449 [Google Scholar]
- Sano H, Seo S, Orudgev E, Youssefian S, Ishizuka K (1994) Expression of the gene for a small GTP binding protein in transgenic tobacco elevates endogenous cytokinin levels, abnormally induces salicylic acid in response to wounding, and increases resistance to tobacco mosaic virus infection. Proc Natl Acad Sci USA 91: 10556–10560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller F, Schaller A, Stintz A (2005) Biosynthesis and metabolism of jasmonates. J Plant Growth Regul 23: 179–199 [Google Scholar]
- Shah J, Tsui F, Klessig DF (1997) Characterization of a salicylic acid-insensitive mutant (sai1) of Arabidopsis thaliana, identified in a selective screen utilizing the SA-inducible expression of the tms2 gene. Mol Plant Microbe Interact 10: 69–78 [DOI] [PubMed] [Google Scholar]
- Shirasu K, Nakajima H, Rajasekhar VK, Dixon RA, Lamb C (1997) Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signals in the activation of defense mechanisms. Plant Cell 9: 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasankar S, Sheldrick B, Rothstein SJ (2000) Expression of allene oxide synthase determines defense gene activation in tomato. Plant Physiol 122: 1335–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel SH, Koornneef A, Claessens SM, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Metraux JP, Brown R, Kazan K, et al (2003) NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15: 760–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel I, Hause B, Miersch O, Kurz T, Maucher H, Weichert H, Ziegler J, Feussner I, Wasternack C (2003) Jasmonate biosynthesis and the allene oxide cyclase family of Arabidopsis thaliana. Plant Mol Biol 51: 895–911 [DOI] [PubMed] [Google Scholar]
- Stintzi A, Weber H, Reymond P, Browse J, Farmer EE (2001) Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proc Natl Acad Sci USA 98: 12837–12842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout MJ, Fidantsef AL, Duffey SS, Bostock RM (1999) Signal interactions in pathogen and insect attack: systemic plant-mediated interactions between pathogens and herbivores of the tomato, Lycopersicon esculentum. Physiol Mol Plant Pathol 54: 115–130 [Google Scholar]
- Thaler JS, Fidantsef AL, Bostock RM (2002) Antagonism between jasmonate- and salicylate-mediated induced plant resistance: effects of concentration and timing of elicitation on defense-related proteins, herbivore, and pathogen performance in tomato. J Chem Ecol 28: 1131–1159 [DOI] [PubMed] [Google Scholar]
- Thaler JS, Fidantsef AL, Duffey SS, Bostock RM (1999) Tradeoffs in plant defense against pathogens and herbivores? A field demonstration using chemical elicitors of induced resistance. J Chem Ecol 25: 1597–1609 [Google Scholar]
- Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF (1998) Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95: 15107–15111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen H, Overmyer K, Keinanen M, Kollist H, Kangasjarvi J (2004) Mutual antagonism of ethylene and jasmonic acid regulates ozone-induced spreading cell death in Arabidopsis. Plant J 39: 59–69 [DOI] [PubMed] [Google Scholar]
- Turner JG, Ellis C, Devoto A (2002) The jasmonate signal pathway. Plant Cell (Suppl) 14: S153–S164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LC, Bakker PA, Pieterse CM (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36: 453–483 [DOI] [PubMed] [Google Scholar]
- van Poecke RM, Dicke M (2002) Induced parasitoid attraction by Arabidopsis thaliana: involvement of the octadecanoid and the salicylic acid pathway. J Exp Bot 53: 1793–1799 [DOI] [PubMed] [Google Scholar]
- van Wees SC, de Swart EA, van Pelt JA, van Loon LC, Pieterse CM (2000) Enhancement of induced disease resistance by simultaneous activation of salicylate- and jasmonate-dependent defense pathways in Arabidopsis thaliana. Proc Natl Acad Sci USA 97: 8711–8716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane JR (1971) Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature 231: 232–235 [DOI] [PubMed] [Google Scholar]
- Vignutelli A, Wasternack C, Apel K, Bohlmann H (1998) Systemic and local induction of an Arabidopsis thionin gene by wounding and pathogens. Plant J 14: 285–295 [DOI] [PubMed] [Google Scholar]
- Walters D (2003) Resistance to plant pathogens: possible roles for free polyamines and polyamine catabolism. New Phytol 159: 109–115 [DOI] [PubMed] [Google Scholar]
- Wasternack C, Hause B (2002) Jasmonates and octadecanoids: signals in plant stress responses and development. Prog Nucleic Acid Res Mol Biol 72: 165–221 [DOI] [PubMed] [Google Scholar]
- Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280: 1091–1094 [DOI] [PubMed] [Google Scholar]
- Xu Y, Chang P, Liu D, Narasimhan ML, Raghothama KG, Hasegawa PM, Bressan RA (1994) Plant defense genes are synergistically induced by ethylene and methyl jasmonate. Plant Cell 6: 1077–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K, Kachroo P, Tsui F, Sharma SB, Shah J, Klessig DF (2001) Environmentally sensitive, SA-dependent defense responses in the cpr22 mutant of Arabidopsis. Plant J 26: 447–459 [DOI] [PubMed] [Google Scholar]