Abstract
Background: High C-reactive protein (CRP) values are frequently found in patients with bacterial respiratory infection, and CRP testing has been shown to be useful in differentiating pneumonia from other respiratory infections. Raised CRP values may also be found in viral respiratory infection, and as a result there is a risk that antibiotics may be wrongly prescribed.
Aims: To describe the course of the CRP response during untreated upper respiratory tract infections and associations between the development of CRP values, erythrocyte sedimentation rate (ESR) and respiratory symptoms.
Design of study: Prospective study.
Setting: Seven general practices in northern Norway.
Method: Patients with upper respiratory tract infection aged 16 years or over, who were not treated with antibiotics and who had been ill for no more than 3 days, were recruited. Microbiological examinations were undertaken, together with measurements of CRP, ESR and recording of symptoms daily during the first week of illness and on days 10, 14 and 21.
Results: An aetiological agent was established in 23 of the 41 included subjects. These were: influenza A, influenza B, rhinovirus, and other agents. Among the 15 patients examined on both the second and the third day of illness, the median CRP value increased from 7–10 mg/l, and the mean value was from 19–24 mg/l between day 2 and day 3. Peak CRP values were reached on days 2 to 4. Higher CRP values were found in those infected with influenza A and B than in the other subjects (P<0.001). A CRP value >10 mg/l was found in 26 subjects during the first 7 days, compared to five subjects after 1 week. Evidence of a secondary infection with group A streptococci was found in two of these five subjects. The development of the symptoms of sore throat, fatigue, clamminess, and pain from muscles and joints followed a similar course as the CRP response, while stuffy nose, cough, sputum production, and dyspnoea tended to persist after the CRP values had approached the normal range.
Conclusion: A moderately elevated CRP value (10–60 mg/l) is a common finding in viral upper respiratory tract infection, with a peak during days 2–4 of illness. Moderately elevated CRP values cannot support a diagnosis of bacterial infection when the illness has lasted less than 7 days, but may indicate a complication of viral infection after a week.
Keywords: antibiotics, common cold, cough, C-reactive protein, erythrocyte sedimentation rate, influenza, upper respiratory infections
Introduction
RAPID tests for C-reactive protein (CRP), an acute-phase protein showing increased serum levels during infection and tissue damage, are widely used in primary care in the Nordic countries.1,2 In a Swedish survey in November 2000, CRP testing was performed in 28.7% of 2899 general practice patients with upper respiratory tract infections and in 48.1% of patients diagnosed as having the common cold.1 Substantially raised CRP values are usually found in pneumonia,3-6 and a high CRP value has been shown to be a strong predictor for this disease in general practice.7-9 However, raised CRP values may also be found in uncomplicated viral respiratory infections, particularly those caused by influenza virus and adenovirus.10 A study of repeated CRP measurements in patients with experimental rhinovirus and influenza virus infection showed peak values 3–5 days after viral challenge.11 Raised CRP values in viral infections during the first week may mislead the clinician in the decision to prescribe antibiotics, and may also explain why the CRP test has shown a higher specificity in predicting pneumonia after 1 week than in the first week of a respiratory tract infection.9 For valid interpretations of CRP results in patients with respiratory tract infections we need to know what range of CRP values can be expected when the infection is uncomplicated. The aim of this study is to describe the course of the CRP response during untreated respiratory tract infections and to analyse how CRP values are associated with patients' symptoms and with the erythrocyte sedimentation rate (ESR).
Method
Subjects
The subjects were recruited from seven general practice offices in northern Norway between March 2000 and May 2002. Adults aged 16 years or more with symptoms of respiratory tract infection, cough, sore throat or stuffy nose, were asked if they would enter the study. The duration of illness had to be 3 days or less. Subjects were excluded if a bacterial infection was suspected and antibiotics were prescribed. Ongoing treatment with oral or inhaled corticosteroids, or the presence of diseases known to be associated with raised CRP values, such as cancer, inflammatory bowel disease or arthritis, were also criteria for exclusion.
Examination
At inclusion the examining general practitioner (GP) registered chest and throat findings and made a tentative diagnosis of upper respiratory tract infection/common cold, influenza or acute bronchitis. Blood was drawn for CRP and ESR analysis. These blood tests were repeated on each of the following days until day 7 of the illness and then on days 10, 14 and 21. ESR was tested at the general practices using vacuum glasses (modified Westergren method). The patients reported the following eight symptoms in a diary: stuffy nose, sore throat, cough, dyspnoea, fatigue, clamm-iness, muscle/joint pain, and sputum production. The symptoms were classified as ‘very bothersome’, which was given a score of 2, ‘more bothersome than normal’, which scored 1, and ‘absent/as normal’, which scored 0. A symptom score was derived by adding up the scores of the eight symptoms. If increased sputum production was reported, the colour of the sputum was recorded.
HOW THIS FITS IN
What do we know?
The C-reactive protein (CRP) test is widely used in the Nordic countries and has been shown to be a useful tool in differentiating pneumonia from other respiratory tract infections. High CRP values are frequently seen in bacterial infections, but elevated values are also seen in viral respiratory infections, and peak values have been demonstrated 3–5 days after viral challenge.
What does this paper add?
A moderately elevated CRP value (10–60 mg/l) cannot support a diagnosis of bacterial infection when the illness has lasted less than 7 days, but may indicate a complication of viral infection after a week.
Microbiological examinations
Venous blood was drawn for serological examinations at inclusion and on days 10 and 21 of the illness. The sera were assayed for the following: IgM, IgA and IgG with enzyme immunoassay (enterovirus, Mycoplasma pneumoniae); IgM and IgG (Chlamydia pneumoniae, Epstein Barr virus); IgA and IgG (Bordetella pertussis); or complement fixation test (adenovirus, influenza A and B, respiratory syncytial virus [RSV] and parainfluenza I and III). Samples with optical density counts above the cut-off for IgM, or a fourfold rise of IgG, IgA, or complement fixations test titres were considered positive.
At inclusion a specimen from the nasopharynx was collected with a rayon swab. This was examined for the following: adenovirus (using culture, immunofluorescence and polymerase chain reaction [PCR]); influenzas A and B; RSV A and B; parainfluenza I, II, III, using a multiplex PCR for analysis; and for the bacterias M. pneumoniae, C. pneumoniae and B. pertussis using in-house PCR tests. The specimens were also analysed by in-house PCR tests for rhinovirus and metapneumovirus. At inclusion and on day 10, swabs both from the throat and the nose were cultured and examined by a medical laboratory technician or a microbiologist. All examinations were carried out at the department of medical microbiology at the University Hospital of North Norway, except the rhinovirus and the metapneumovirus PCR, which were analysed at St Olav's University Hospital in Trondheim. The aetiology of infection was determined after assessment of all microbiological results for each patient.
CRP analysis
All sera were stored at −20°C until tested. The CRP was measured by the immunoturbidimetric method. The analytical sensitivity (lower detection limit) of the assay is 3 mg/l, and the coefficient of variation is 4.0%. The samples with a CRP less <5 mg/l were analysed using the high (ultra) sensitive CRP method (particle-enhanced immunoturbidimetric assay). The analytical sensitivity (lower detection limit) of this assay is 0.03 mg/l, and the measuring range is 0.1–20 mg/l. The analytical coefficient of variation is 3.6%.
Statistical analysis
The patients were divided into groups according to aetiological agents, and the statistical significance of differences between groups were examined using the Mann–Whitney test, Student's t-test, χ2 test, and multiple regression for repeated measurements (general linear models). The correlation between measures was examined by calculating Pearson's coefficient. SPSS statistical software was used.
The patients gave written consent to participate, and the study was approved by the Regional Committee of Medical Research Ethics.
Results
Forty-four subjects were recruited. One male patient dropped out after 4 days due to discomfort from the blood testing. Two female patients were excluded due to starting antibiotic treatment within the first week of illness; both reported increasing symptoms in the throat and tested positive for a group A streptococcus antigen.
Among the 41 subjects included in the analysis, there were 20 males and 21 females, with a mean age of 37 years (range = 16–73 years). One entered the study on the first day of illness, 16 on the second day, and 24 on the third day. In 32 of the 41 patients, complete data were obtained on all eight of the scheduled examination days between day 3 and day 21, while data were obtained from seven of the scheduled days in seven subjects. One patient dropped out after day 7, and in one patient the CRP results from days 2–6 were not obtained, due to insufficient amounts of serum.
Microbiological findings
An aetiological agent was established in 23 of the 41 subjects. Influenza virus and rhinovirus were the agents most frequently detected (Table 1). Twenty-one of the diagnoses were based on the PCR results, one was based on bacterial culture (group A streptococcus), and one on a fourfold rise in antibody titre against adenovirus found by parallel testing. Four of the five influenza A and six of the seven influenza B diagnoses were confirmed by a fourfold rise in antibody titre, while the PCR diagnosis of parainfluenza virus, RSV and adenovirus could not be confirmed serologically. Four of the patients with influenza B were included within 1 month at the same general practice in a small rural community, and the same was the case for two of the influenza A patients, but in another region. Two of the patients with rhinovirus infection were living together.
Table 1.
Microbiological findings in 41 patients with unrelated respiratory infections.
| Aetiological agent | Number of subjects |
|---|---|
| Definite findings (total = 23) | |
| Influenza A | 5 |
| Influenza B | 7 |
| Rhinovirus | 5 |
| Parainfluenza 1 | 1 |
| Parainfluenza 3 | 1 |
| Respiratory syncytial virus | 1 |
| Adenovirus | 2 |
| Group A streptococcus | 1 |
| Uncertain findings | 7 |
| No findings | 11 |
| Total patients | 41 |
In addition to these 23 patients, microbiological findings of more uncertain importance were obtained in seven patients. Two patients had a fourfold rise in titre against influenza A, but we did not have enough serum to be able to confirm these results by parallel testing. One patient from the same community, and recruited in the same month, had a high titre against influenza A at inclusion. Two patients from other communities had a high titre against adenovirus at inc-lusion. C. pneumoniae IgM was found in 2 patients, but both had a high C. pneumoniae IgG titre as well at inclusion. These seven patients with uncertain results were classified as having ‘unknown aetiology’ in the analyses.
The patient with primary group A streptococcal infection had a positive culture on both day 3 and day 10. The diagnosis was confirmed by a fourfold rise in anti-streptolysine titre. Pneumococci, Haemophilus influenzae, and Staphylococcus aureus were found in the noses of some patients, but these findings were not emphasised in the aetiological diagnoses.
CRP analysis
Among the 15 patients examined on both the second and third days of illness, the median CRP value increased from 7–10 mg/l (the mean value was from 19–24 mg/l) between day 2 and day 3. The peak CRP values (means and med-ians) were reached within 4 days (Figure 1). The CRP values were significantly higher among the 23 subjects with an established aetiological agent than in the 18 with no definite aetiological agent detected (P<0.001, tested by a repeated measurements procedure). This was the case on days 3–7 (P<0.005 on each day), while no difference was found on days 10–21. Higher CRP values were found in the subjects with influenza A or B than in the others (P<0.001), and on day 3 the median values were 25 and 4.4 mg/l in those with and without influenza, respectively (P<0.01). CRP values >10 mg/l were found in 26 patients during the first week and in five patients after day 7. The changes in CRP values in subgroups of patients are shown in detail in Figures 2–6. Continuously declining values after the third or fourth day of illness were found in all but three patients. In one patient with influenza A infection an increasing CRP value was found between days 14 and 21 (Figure 2), and group A streptococcal pharyngitis was diagnosed with a ‘near-patient’ antigen test on day 21 in this patient. Increasing CRP values were found after day 6 in one patient with influenza B infection (Figure 3). Her symptom score increased from 9 to 10 between days 6 and 7, due to increased soreness of the throat. A fourfold rise in anti-DNase B titre was found. A third patient, who had serological signs of recent infection with adenovirus (titre 160 on day 3), had a stable CRP value between 9 and 15 mg/l throughout all the examinations (Figure 6). She reported having a very uncomfortable stuffy nose and cough until day 10. There was also also one other patient who had a CRP value >10 mg/l after 10 days of illness (Figure 2). This was the patient with influenza A who did not have CRP measurements from days 2–6. The development of her symptom score did not indicate a secondary infection.
Figure 1.
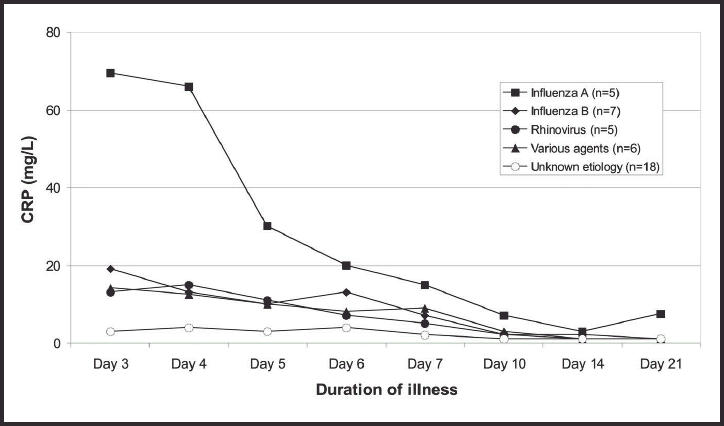
Median C-reactive protein (CRP) values in 41 subjects with untreated respiratory tract infection, showing duration of illness and aetiological agent.
Figure 2.
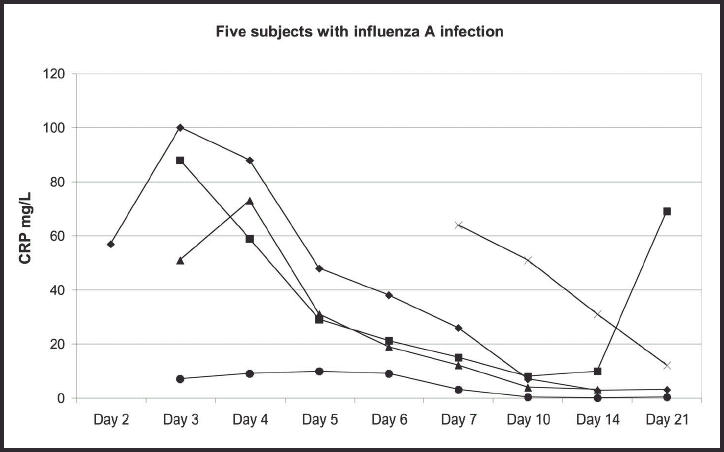
The development of C-reactive protein (CRP) values in five subjects with influenza A infection, showing duration of illness.
Figure 6.
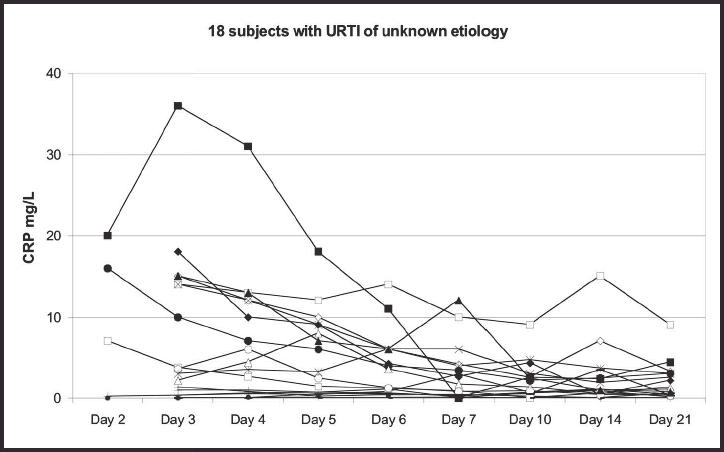
The development of C-reactive protein (CRP) values in 18 subjects with infections of unknown aetiology, showing duration of illness.
Figure 3.
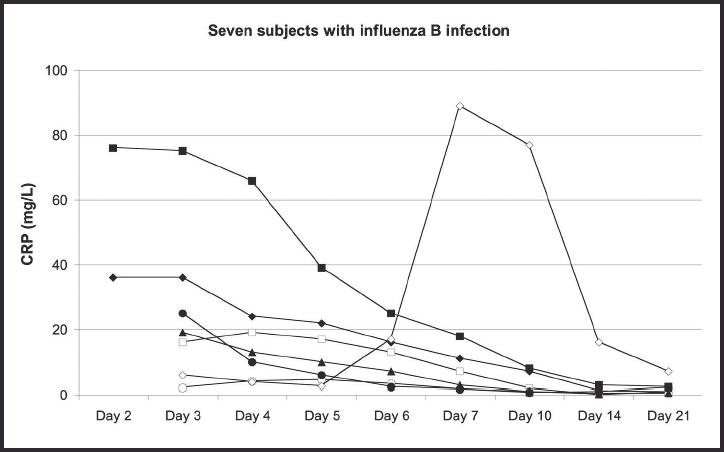
The development of C-reactive protein (CRP) values in seven subjects with influenza B infection, showing duration of illness.
The highest CRP value, of 125 mg/l, was found on day 4 in the female patient who had a primary group A streptococcal infection. The CRP value declined as rapidly in this untreated patient as in the patients with viral infections (Figure 5). The two patients with possible, but not established, influenza A infection had CRP values of 15 and 18 mg/l, respectively, on day 3 (Figure 6).
Figure 5.
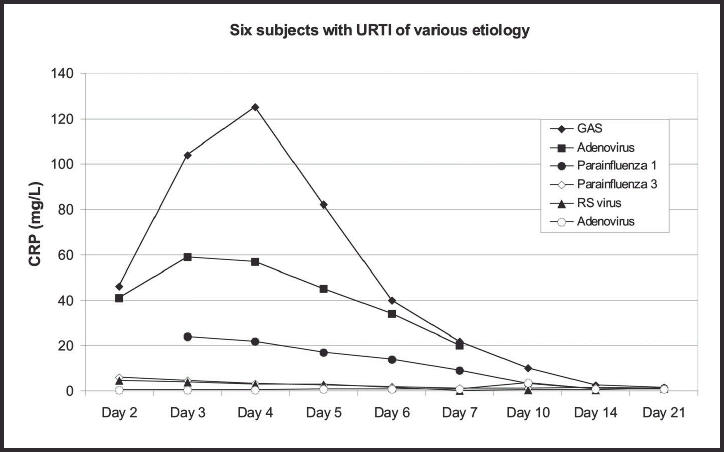
The development of C-reactive protein (CRP) values in seven subjects with infections of various aetiology, showing duration of illness. GAS=group A streptococcal infection; URTI = upper respiratory tract infection; RS = respiratory syncytial.
ESR
The highest median ESR values were found after 4–5 days (Figure 7), and occurred 1–2 days later than the peak CRP values. Higher ESR values were found in the patients with influenza A than in the other patients and the difference between this group and the others was statistically significant (P<0.001) when tested by a repeated measurements proc-edure. The ESR strongly correlated with the CRP value on days 3–6 (Pearson coefficient = 0.55–0.71, P<0.001), but there was no correlation on days 7 and 10. A statistically significant correlation was also found on days 14 and 21.
Figure 7.
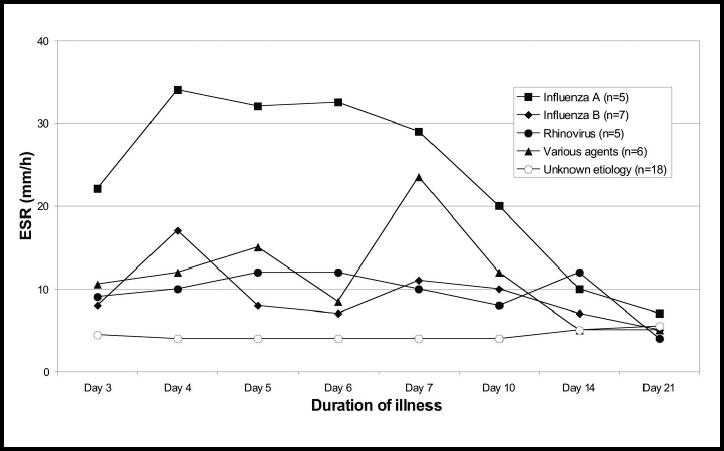
Median erythrocyte sedimentation rate in 41 subjects with untreated respiratory tract infection showing duration of illness and aetiological agent.
Signs and symptoms
There was no statistically significant difference in symptom score between the aetiological groups (Figure 8). The patients with influenza A infection reported more pain in their muscles and joints on day 3 than the other patients (a mean degree of 1.4 versus 0.68, P<0.05). Looking at all the 41 patients, there was a decline in the mean degree of pain from muscles and joints, as well as of sore throat, fatigue and clamminess between days 3 and 4, and these symptoms were more than halved on day 7. A different trend was found for stuffy nose, cough, sputum production and dyspnoea. The mean degree of these symptoms increased from days 3–4 and declined more slowly until day 7 (Figure 9). Fewer than 50% of those who reported sputum production had coloured sputum on day 3, but on days 6 and 7 the frequency was 67%. Coloured sputum was more frequently reported in the patients with influenza A and B than in the other patients (P<0.05) (Figure 10).
Figure 8.
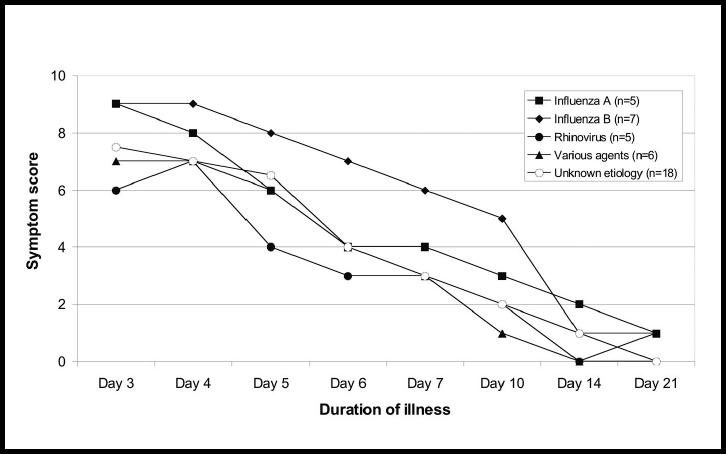
Median symptom score (on a scale of 0 to 16) in 41 subjects with untreated respiratory tract infection showing duration of illness and aetiological agent.
Figure 9.
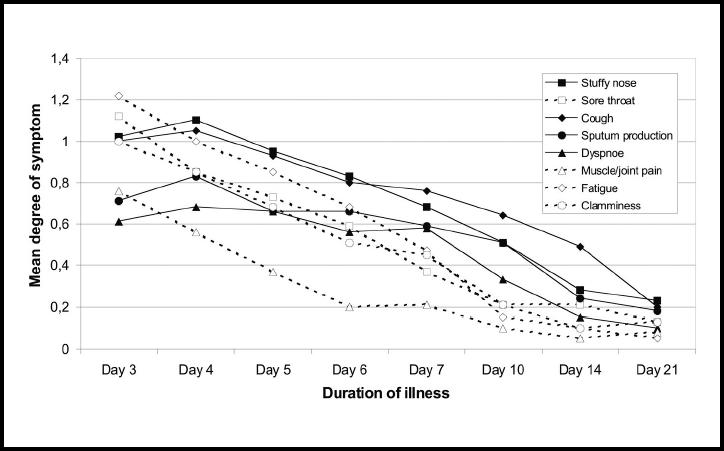
Mean degree of eight symptoms (scored as 0, 1 or 2, with 2 being most severe) in 41 subjects with untreated respiratory tract infection, showing duration of illness.
Figure 10.
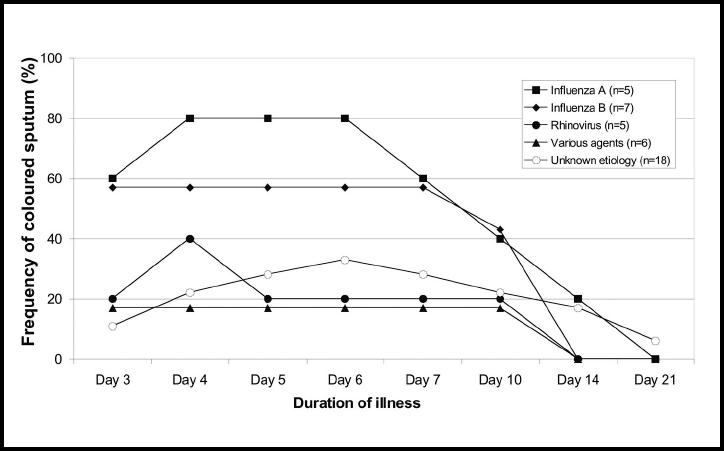
Frequency (%) of coloured sputum in 41 subjects with untreated respiratory tract infection, showing duration of illness and aetiological agent.
There was no statistically significant correlation between the symptom score and the CRP value on the third day of illness. On days 4–10 a significant correlation was found (Pearson correlation = 0.39–0.59) with the highest correlation on day 7 (P<0.001). There was no significant correlation on day 14, and a correlation found on day 21 was not significant when the patient who had a group A streptococcal infection between days 14 and day 21 was excluded from the analysis.
A statistically significant correlation between the symptom score and the ESR was found on days 3 and 4 (Pearson correlation = 0.41–0.34, P<0.01 and P<0.05, respectively), but not on days 5–14. A correlation found on day 21 was not significant when the patient who had a group A streptococcal infection between days 14 and day 21 was excluded from the analysis.
GPs recorded redness of the throat in 35 patients and pathological chest findings in four of the patients at inclusion. The occurrence of the signs did not differ between the aetiological groups.
GPs' diagnosis
A diagnosis was recorded by the GP in 39 of the 41 patients. These were: upper respiratory tract infection (n = 25), influenza (n = 13) and acute bronchitis (n = 1). Eight of the 13 patients with influenza A or B were diagnosed as having influenza. Five patients without proven influenza (including two patients with possible influenza) were also given the diagnosis. Accordingly, the sensitivity of the GPs' diagnosis of influenza was 62%, while the specificity was 81%.
Discussion
Summary of main findings
Raised CRP values were found in the majority of the patients with viral infection and the highest values were found in those with influenza A and B infections. The CRP values reached a peak during days 2–4 in our study, and then fell rapidly over the following days. Values above 10 mg/l were only found in five of the 41 patients after a week of illness, and among two of these evidence of bact-erial superinfection was found. Higher CRP values were found in the patients with an established aetiological agent than in those without. The CRP response followed the presence of pain in muscles and joints, soreness of the throat, fatigue and clamminess quite closely. Cough and expect-oration, on the other hand, tended to persist while the CRP values approached the normal range.
Strengths and limitations of this study
A uniform pattern of the CRP response could be observed due to the daily recordings and measurements. Our use of the hypersensitive CRP test in the study made it possible to observe minor elevations and also the fact that the CRP values decreased continuously after day 10 of the illness. The investigation took place over a 2-year period and it is certain that many eligible patients have not been included. The GPs were probably particularly aware of the study during influenza outbreaks, and influenza infections may have been over-represented in the study. The close follow-up of patients made it possible for GPs to include subjects who otherwise might have been treated with antibiotics. CRP testing might have been clinically considered in at least some of the patients.
Comparison with the existing literature
The CRP peak after 2–4 days is in accordance with the findings of Whicher and his colleagues, who also found that the peak CRP value was reached earlier in influenza than in rhinovirus infection.11 The demonstrated decline in CRP values between the fourth and seventh days helps to explain why the specificity for pneumonia of a CRP value >50 mg/l has been found to be higher after 1 week than during the first week of illness (0.95 and 0.85, respectively).9
The persistence of cough after the CRP values had become lower than 10 mg/l is in accordance with previous findings in patients with acute bronchitis. CRP values lower than 10 mg/l are usually found in this disease, for which patients usually consult after 1 week of illness.12,13 The persistence of cough and expectoration may in many cases not reflect the seriousness of the infection, but rather a post-infectious host response.
Stuffy nose was also a symptom that tended to persist after 1 week. Puhakka and colleagues have showed that viral sinusitis frequently develops in the common cold.14 In their study a median CRP value of <10 mg/l (range = <10–37 mg/l) was found on day 7 in patients with viral sinusitis. Sinusitis may also have developed in some of the patients in our study, and they recovered without antibacterial treatment.
Production of coloured sputum was common in the patients with influenza. Influenza viruses damage the epith-elium of the airways and bring about a considerable inflammatory response with involvement of white blood cells.15 Purulent sputum has also previously been found to be a common clinical feature in uncomplicated influenza.16 Our findings remind us not to take this symptom as a sign of bacterial infection.
Implications for future research or clinical practice
This study shows that moderately elevated CRP values of 10–60 mg/l in patients with respiratory tract infection cannot support a diagnosis of bacterial infection when the illness has lasted less than 7 days. After a week, however, such values may indicate a complication of a viral infection. The use of the PCR test has made it possible to detect an aet-iological agent in 56% of cases. The striking difference in CRP values being dependent on whether an aetiological agent could be detected or not, may represent blind spots in our understanding of upper respiratory tract infections. We did not look at whether some viruses, such as coronaviruses, tend to cause low CRP values, but this may explain some of the differences observed.
Conflict of interest
Hasse Melbye has previously conducted a study evaluating the CRP test, which was funded by Axis Shield.
Figure 4.
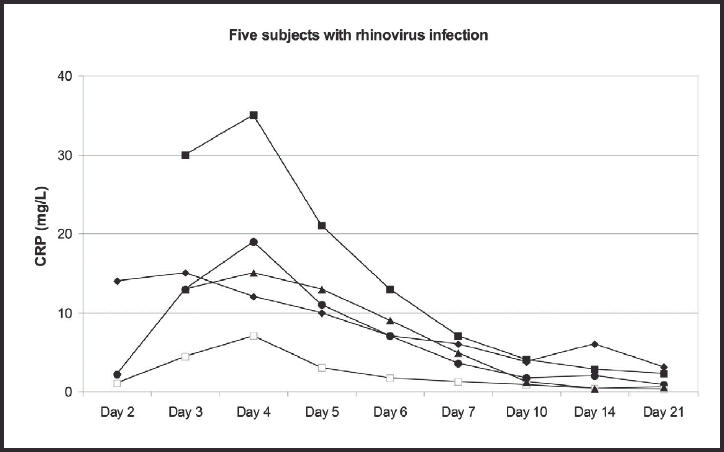
The development of C-reactive protein (CRP) values in five subjects with rhinovirus infection, showing duration of illness.
Acknowledgments
The study was funded by the Programme for Research and Development in Primary Health Care of Northern Norway. We would like to thank the staff of the general practices in the following municipalities in the north of Norway: Bardu, Brønnøysund, Hattfjelldal, Nordreisa, Nordkapp, Kvenangen, and Tromsø (Skansen Medical Office), for recruiting and examining the patients.
References
- 1.André M, Odenholt I, Schwan AM, et al. Upper respiratory tract infections in general practice: diagnosis, antibiotic prescribing, duration of symptoms and use of diagnostic tests. Scand J Infect Dis. 2002;34:880–886. doi: 10.1080/0036554021000026952. [DOI] [PubMed] [Google Scholar]
- 2.Honkanen PO, Rautakorpi UM, Huovinen P, et al. Diagnostic tools in respiratory tract infections: use and comparison with Finnish guidelines. Scand J Infect Dis. 2002;34:827–830. doi: 10.1080/0036554021000026939. [DOI] [PubMed] [Google Scholar]
- 3.Hansson LO, Hedlund JU, Ortqvist AB. Sequential changes of inflammatory and nutritional markers in patients with community-acquired pneumonia. Scand J Clin Lab Invest. 1997;57:111–118. doi: 10.1080/00365519709056378. [DOI] [PubMed] [Google Scholar]
- 4.Smith RP, Lipworth BJ, Cree IA, et al. C-reactive protein. A clinical marker in community-acquired pneumonia. Chest. 1995;108:1288–1291. doi: 10.1378/chest.108.5.1288. [DOI] [PubMed] [Google Scholar]
- 5.Smith RP, Lipworth BJ. C-reactive protein in simple community-acquired pneumonia. Chest. 1995;107:1028–1031. doi: 10.1378/chest.107.4.1028. [DOI] [PubMed] [Google Scholar]
- 6.Ritland N, Melbye H. [C-reactive protein, SR and white blood cell count in acute lower respiratory tract diseases. The usefulness of blood tests in diagnosis of pneumonia] Tidsskr Nor Laegeforen. 1991;111:2249–2252. [PubMed] [Google Scholar]
- 7.Hopstaken RM, Muris JW, Knottnerus JA, et al. Contributions of symptoms, signs, erythrocyte sedimentation rate, and C-reactive protein to a diagnosis of pneumonia in acute lower respiratory tract infection. Br J Gen Pract. 2003;53:358–364. [PMC free article] [PubMed] [Google Scholar]
- 8.Melbye H, Straume B, Aasebo U, Brox J. The diagnosis of adult pneumonia in general practice. The diagnostic value of history, physical examination and some blood tests. Scand J Prim Health Care. 1988;6:111–117. doi: 10.3109/02813438809009300. [DOI] [PubMed] [Google Scholar]
- 9.Melbye H, Straume B, Brox J. Laboratory tests for pneumonia in general practice: the diagnostic values depend on the duration of illness. Scand J Prim Health Care. 1992;10:234–240. doi: 10.3109/02813439209014067. [DOI] [PubMed] [Google Scholar]
- 10.Ruuskanen O, Putto A, Sarkkinen H, et al. C-reactive protein in respiratory virus infections. J Pediatr. 1985;107:97–100. doi: 10.1016/s0022-3476(85)80624-7. [DOI] [PubMed] [Google Scholar]
- 11.Whicher JT, Chambers RE, Higginson J, et al. Acute phase response of serum amyloid A protein and C reactive protein to the common cold and influenza. J Clin Pathol. 1985;38:312–316. doi: 10.1136/jcp.38.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melbye H, Berdal BP, Straume B, et al. Pneumonia — a clinical or radiographic diagnosis? Etiology and clinical features of lower respiratory tract infection in adults in general practice. Scand J Infect Dis. 1992;24:647–655. doi: 10.3109/00365549209054652. [DOI] [PubMed] [Google Scholar]
- 13.Jonsson JS, Sigurdsson JA, Kristinsson KG, et al. Acute bronchitis in adults. How close do we come to its aetiology in general practice? Scand J Prim Health Care. 1997;15:156–160. doi: 10.3109/02813439709018507. [DOI] [PubMed] [Google Scholar]
- 14.Puhakka T, Makela MJ, Alanen A, et al. Sinusitis in the common cold. J Allergy Clin Immunol. 1998;102:403–408. doi: 10.1016/S0091-6749(98)70127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julkunen I, Melen K, Nyqvist M, et al. Inflammatory responses in influenza A virus infection. Vaccine. 2000;19(Suppl 1):S32–S37. doi: 10.1016/s0264-410x(00)00275-9. [DOI] [PubMed] [Google Scholar]
- 16.Cox NJ, Subbarao K. Influenza. Lancet. 1999;354:1277–1282. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]


