Abstract
Background: Influenza transmission in households is a subject of renewed interest, as the vaccination of children is currently under debate and antiviral treatments have been approved for prophylactic use.
Aims: To quantify the risk factors of influenza transmission in households.
Design of study: A prospective study conducted during the 1999 to 2000 winter season in France.
Setting: Nine hundred and forty-six households where a member, the index patient, had visited their general practitioner (GP) because of an influenza-like illness were enrolled in the study. Five hundred and ten of the index patients tested positive for influenza A (subtype H3N2). A standardised daily questionnaire allowed for identification of secondary cases of influenza among their household contacts, who were followed-up for 15 days. Of the 395 (77%) households that completed the questionnaire, we selected 279 where no additional cases had occurred on the day of the index patient's visit to the GP.
Methods: Secondary cases of influenza were those household contacts who had developed clinical influenza within 5 days of the disease onset in the index patient. Hazard ratios for individual clinical and demographic characteristics of the contact and their index patient were derived from a Cox regression model.
Results: Overall in the 279 households, 131 (24.1%) secondary cases occurred among the 543 household contacts. There was an increased risk of influenza transmission in preschool contacts (hazard ratio [HR] = 1.85, 95% confidence interval [CI] = 1.09 to 3.26) as compared with school-age and adult contacts. There was also an increased risk in contacts exposed to preschool index patients (HR = 1.93, 95% CI = 1.09 to 3.42) and school-age index patients (HR = 1.68, 95% CI = 1.07 to 2.65), compared with those exposed to adult index cases. No other factor was associated with transmission of the disease.
Conclusion: Our results support the major role of children in the dissemination of influenza in households. Vaccination of children or prophylaxis with neuraminidase inhibitors would prevent, respectively, 32–38% and 21–41% of secondary cases caused by exposure to a sick child in the household.
Keywords: antivirals, children, epidemiology, influenza, prospective studies, risk factors, vaccination
Introduction
THE epidemiology of influenza transmission in households has been the subject of great interest and investigation in the past, with the rationale that a better understanding of influenza transmission mechanisms could aid in the design of efficient control strategies.1 Several follow-up studies of families during one or several consecutive influenza seasons have described the occurrence and spread of infection in households in relation to age, family composition, crowding, circulating viral strains, exposure in the community, and prior immunity.2
The current thinking regarding influenza transmission is that children play a major role in the early stages of the epidemic, with the assumption that they are more susceptible than older age groups, and that they contribute more extensively to the spreading of the virus in the population.3 Furthermore, children spend a great deal of time in communities where daily contact with other people is extensive; for example, in schools, play groups, and daycare centres, and it is assumed that close contact favours transmission.3 However, the extent to which these mechanisms contribute to transmission has not been quantified.
Recently, there has been renewed interest in the study of influenza transmission in families, especially in light of the recent debates about whether large-scale vaccination of healthy children in daycare would be beneficial to other age groups,4,5 and whether contact prophylaxis with neuraminidase inhibitors could effectively prevent transmission.6-8 However, no data are available on the quantitative evaluation of the predictors of influenza transmission in households.
In this work, we analyse a prospective study of influenza transmission in families conducted in France during the 1999 to 2000 influenza season, where influenza-positive index patients were identified by virological tests.9 From this study we assess the risk factors for influenza transmission associated with the individual characteristics of index patients and their household contacts.
Methods
Study design
This study, which is described in detail elsewhere,9 was conducted within the framework of the French Sentinel network. The Sentinel network is a computerised public health surveillance system compiled with the voluntary and unpaid participation of 1790 general practitioners (GPs) located all over France. Since November 1984 it has been collecting weekly reports on 10 communicable diseases, including influenza-like illness. In addition to the continuous surveillance of disease activity, the network is a setting for thorough investigations conducted over limited time periods.10,11 One hundred and sixty-one of the GPs from the network volunteered to participate in this specific study of influenza transmission in households. They received training on the study protocol and virology sampling during a pilot phase in October 1999.
HOW THIS FITS IN
What do we know?
An important factor in the spread of influenza viruses is the transmission between household members. Children are more susceptible than older age groups, and they contribute more extensively to the spreading of the virus in the population.
What does this paper add?
This study quantifies the risk factors of influenza transmission in households and investigates the impact of two intervention strategies for controlling epidemics within them. Vaccination of children or prophylaxis with neuraminidase inhibitors would prevent, respectively, 32–38% and 21–41% of secondary cases caused by exposure to a sick child.
A household was enrolled when a member visited the GP and met the following inclusion criteria: the patient had had a fever >38°C within 48 hours of the visit, together with respiratory signs; there was at least one other member in the household; the consulting patient was the first case in the household; the patient was not hospitalised as a result of this visit. If the inclusion criteria were met, the patient was considered to be the index case of the household. Following discussion of this observational follow-up by the study scientific committee and jurists from the institutional sponsor, oral consent was obtained from the index patient (or the index patient's parents if the index patient was a child). All studies conducted within the framework of the Sentinel network are approved by the French Commission Nationale de l'Informatique et des Libertés (approval no. 471 393).
Information concerning social and demographic characteristics of the household was collected upon enrollment in the study. Daily details about 13 symptoms (fever >38°C; feverishness; cough; sore throat; nasal congestion, rhinorrhea or sneezing; dysphonia; fatigue; headache; stiffness or myalgias; otalgias; ocular symptoms; loss of appetite; sleep disturbances), medication, visits to physicians, and missed days of work of each household member, were reported in a standardised questionnaire for the 15 days following the initial visit of the index patient to their GP. The initial visit was counted as day 0 of the follow-up. A daily severity score was calculated as the proportion of the 13 symptoms reported on a given day (ranging from 0 to 1) as described elsewhere.9 All participants who completed the questionnaire were included in the study.
Demographic characteristics
Between January 2000 and March 2000, 946 index patients and their household contacts were enrolled in the study. Nasal swabs were obtained from all index patients. Respiratory syncytial virus, parainfluenzae virus and adenovirus infections were diagnosed with the immunofluorescence test.9 Influenza was diagnosed where one or more results with the immunofluorescence test, viral culture and the polymerase chain reaction (PCR) test were positive. Of the 946 index patients, 510 were influenza A (subtype H3N2)-positive and, of these, 395 (77%) completed the follow-up with their household contacts (Figure 1). The 510 influenza A-positive patients were located in 21 of the 21 administrative regions of France. The median number of index cases per region was 16, with a range of 2–61. At inclusion, the only difference between households that completed the study (n = 395) and those lost to follow-up (n = 115) was in the sex of the index patient: 19% of the households with a female index patient and 26% of those with a male index patient were lost to follow-up (P = 0.04). In particular, there was no difference regarding variables such as the age of the index patient, severity of the disease on the first day of illness, body temperature, and inclusion date.
Figure 1.
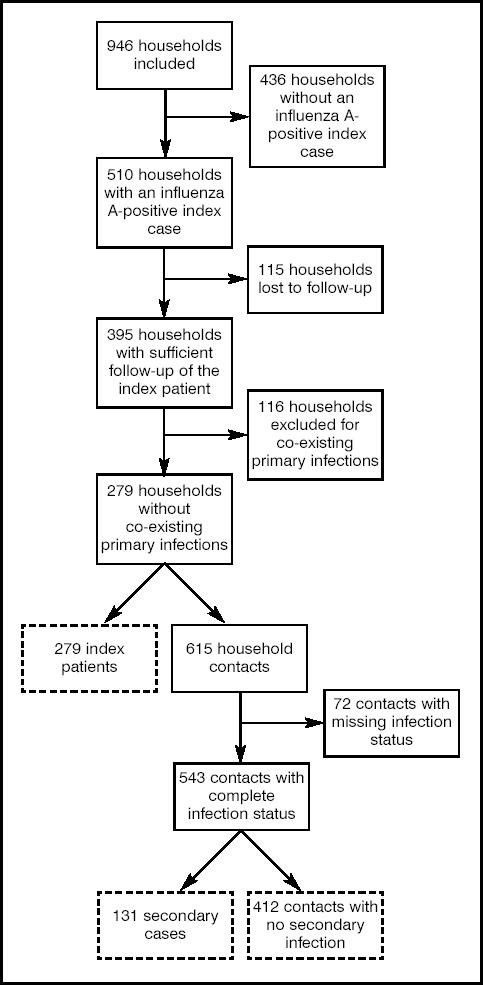
Flow diagram of the study. Dotted boxes denote subjects included in the main analysis.
We used a clinical definition of influenza, without laboratory testing, to identify secondary patients. Clinical influenza was defined as the presence of a fever >38°C, or feverishness when the temperature was not taken, or at least two of the following symptoms: cough; sore throat; nasal congestion, rhinorrhoea, or sneezing; fatigue; headache; stiffness; myalgias. This definition is based on the criteria that define influenza-like illness in clinical trials of neuraminidase inhibitors.7,8,13 Household contacts who developed clinical influenza within 5 days of the initial visit of the index patient were classified as secondary patients. To avoid ambiguity about the true introducer of infection in the household, we excluded households in which one or more contacts had developed clinical influenza on the day of the initial GP visit of the index patient (day 0) from the main analysis. The incubation period of influenza is around 1–1.5 days,14,15 therefore this procedure minimises the probability that the household members (index patient and contacts) were infected from a common source from outside the household and not by transmission within the household. Among the 395 households that completed the study, 116 reported potential co-existing primary patients and were excluded from the main analysis. We subsequently performed sensitivity analyses with an extended dataset that included those households where co-existing primary cases were reported on the day of the initial visit, and also a more specific definition of clinical influenza based on the combined presence of fever >38°C and cough.16
In the remaining 279 of households, the mean number of children per household under 15 years of age was 0.71. This is in line with the national figure of 0.68 for French households of two or more members.12 The mean age of index patients was 38.4 years (standard deviation [SD] = 19.4), 241 (86.4%) of them were adults (mean age = 43.1 years, SD = 16.6) and 38 (13.6%) were children aged ≥15 years (mean age = 9.1 years, SD = 4.7) of whom 10 (3.6%) were <5 years of age. Ten per cent of the index patients were vaccinated against influenza, and 14% had experienced clinical influenza in the preceding year.
In the 279 households there were 615 contact members. We disregarded 72 contacts with insufficient information on clinical follow-up, and in the final analysis included 543 contacts, with a mean age of 32.1 years (SD = 19.9), comprising 401 (73.8%) adults (mean age = 40.4 years, SD = 16.3) and 142 (26.2%) children (mean age = 8.7 years, SD = 4.1) of whom 36 (6.6%) were below 5 years of age. The proportion of children among the 72 contacts who did not fully complete the questionnaire was 26%. Seven per cent of the household contacts were vaccinated against influenza, and 9% had experienced clinical influenza in the preceding year.
Statistical analysis
Although occurrence of secondary transmission is the ult-imate outcome of interest in this work, time to transmission is important as well, because the level of exposure to influenza in the household is not constant over time. Household members became sick and recovered during the study follow-up. Adjustment for factors that varied between households; for example, exposure to influenza and household structure, was needed to assess the true role of individual predictors of transmission. The Cox model is a popular regression model used to assess the relation of explanatory covariates to the time of occurrence of events, which in this study is the onset of clinical influenza in contacts. This model allows adjustment for time-dependent covariates; for example, the level of exposure to influenza in the household.
Extensions to the Cox model exist that deal with the dependence between observations, in particular for correlated household members. In this study, dependence between household contacts is due to shared household structure and exposure to the same index patient.17 Household contacts who had not developed clinical influenza within 5 days (412/543 [75.9%]) were considered as censored (for these contacts, transmission events had not occurred within 5 days but may occur later).
Similarly to previous studies of influenza transmission in families,18 we distinguished between preschool children (0–5 years old), school-age children (6–15 years old), and adults (>15 years) to quantify the effect of age. Size limitation did not allow for more refined subcategories, such as infants or adolescents. We included the following covariates separately in the model: age of the household contact (0–5, 6–15, >15 years); influenza vaccination of the contact; influenza-like illness of the contact in the previous year; history of chronic disease and tobacco consumption of the contact; duration of illness of the index patient (above or below the median); and severity of disease of the index patient on the first day of symptoms (above or below the median). We also adjusted for three household-specific parameters that could confound individual characteristics: the number of children ≥15 years in the household, the number of adults, and the level of exposure to influenza infection in the household. We used a daily index, calculated as the sum of the severity scores of the household members (the daily severity score was the proportion of symptoms reported on a given day among the 13 listed in the questionnaire and ranged from 0 to 1) as a proxy for the level of exposure to influenza.9
Upon completion of the study and data entry by trained personnel, less than 4% of the information was missing. Because of the low rate of missing values, these values were not replaced. All statistical analyses were carried out by statisticians.
Results
Descriptive analysis
Overall, 131 (24.1%) of the 543 contacts developed symptoms of influenza within 5 days of the onset of disease in the index patient, and hence were considered as secondary cases. Influenza transmission was observed in 97 (35%) households. Of the 97 households, 67 (69.1%) reported one secondary case, 26 (26.8%) reported two, and four (4.1%) reported three. The median time lag between the onset of influenza in the index patient and the onset of symptoms in the secondary patient was 2 days (range = 1–5 days) (Figure 2). The demographic and medical data for the secondary patients and non-case household contacts are presented in Table 1. We found no significant differences in the individual characteristics of these two groups of contacts with regard to age, sex, smoking status, history of chronic disease, influenza vaccination, or previous influenza-like illness. However, clinical influenza was reported in 38.5% (10/26) of contacts in households where the index patient belonged to the 0–5 years age group, in 33.7% (28/83) of the 6–15 years age group and in 21.4% (93/434) of adults, (Cochran-Armitage trend test, P = 0.004). Note that these estimates are not adjusted on household structure. Households in which the index patient was a child had more children than those where the index patient was an adult (respective median number of children = 2 versus 1, P<0.001).
Figure 2.
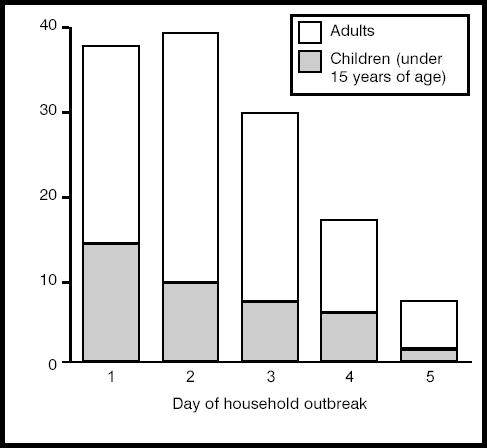
Number of secondary cases of influenza by day of household outbreak. Overall there were 131 secondary cases. Day 0 is the day of onset of influenza A (H3N2) illness in the index case = day of the visit to the general practitioner.
Table 1.
Comparison of demographic data for household contacts (n = 543), grouped by secondary cases and non-case contacts.
| Demographic data | Non-cases (n = 412) | Secondary cases (n = 131) | P-value |
|---|---|---|---|
| Mean age (SD) | 32.1 (19.4) | 32.0 (21.3) | 0.95 |
| Age (n [%]) | |||
| 0–5 years | 22 (5.3) | 14 (11.5) | 0.09a |
| 6–15 years | 80 (19.4) | 26 (19.9) | |
| >15 years | 310 (75.2) | 91 (68.9) | |
| Male sex (n [%]) | 191 (46.4) | 65 (49.6) | 0.51 |
| Current smoker (n [%]) | 75 (18.2) | 18 (13.7) | 0.24 |
| Chronic diseases (n [%]) | 45 (10.9) | 20 (15.3) | 0.14 |
| Influenza vaccination (n [%]) | 28 (6.8) | 6 (4.6) | 0.36 |
| Influenza-like illness in the previous year (n [%]) | 32 (7.8) | 14 (10.7) | 0.33 |
aP for trend = 0.06. SD = standard deviation.
Risk factors of influenza transmission in households
The Cox statistical analysis showed that transmission of influenza was clearly associated with the age of both the index patient and the contact. We found an increased risk of clinical influenza in preschool contacts compared with adults, with a hazard ratio (HR) of 1.85, 95% confidence interval (CI) = 1.09 to 3.26. There was no increased risk in school-age contacts (HR = 1.12, 95% CI = 0.73 to 1.71). There was also an increased risk of clinical influenza in contacts exposed to preschool index patients (HR = 1.93, 95% CI = 1.09 to 3.42) and school-age index patients (HR = 1.68, 95% CI = 1.07 to 2.65), compared with those exposed to adult index patients. No other factor related either to the contact or to the index patient was associated with influenza transmission (Table 2).
Table 2.
Hazard ratios for individual predictors of influenza household transmission, adjusted on a daily score for exposure to influenza infection, the number of children under 15 years old and the number of adults in the household.
| Individual predictor | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Household contact | ||
| Age | ||
| >15 years | 1 | |
| 6–15 years | 1.12 (0.73 to 1.71) | 0.60 |
| 0–5 years | 1.85 (1.09 to 3.26) | 0.02 |
| Influenza-like illness | 1.54 (0.89 to 2.64) | 0.12 |
| Influenza vaccination | 0.83 (0.40 to 1.70) | 0.61 |
| Chronic diseases | 1.31 (0.72 to 2.36) | 0.38 |
| Current smoker | 1.20 (0.93 to 1.55) | 0.15 |
| Index patient | ||
| Age | ||
| >15 years | 1 | |
| 6–15 years | 1.68 (1.07 to 2.65) | 0.02 |
| 0–5 years | 1.93 (1.09 to 3.42) | 0.02 |
| Severity of disease on day 1 (≥ mediana) | 1.35 (0.91 to 1.99) | 0.13 |
| Duration of disease (≥ medianb) | 1.40 (0.96 to 2.03) | 0.08 |
aThe median severity of disease on day 0 (proportion of 13 clinical symptoms reported on the day of the visit to the general practitioner) was 0.65 for index cases (range = 0–1).
bThe median duration of disease was 8 days in index cases (range = 0–15). CI = confidence interval.
We tested the effect of discarding the households where co-existing primary cases were reported on the day of the initial visit to the GP from the analysis. We repeated the initial statistical analysis with an extended dataset comprising all households (n = 395), in which 313 secondary cases were reported (secondary attack rate in contacts = 38.3%). We retrieved similar results, but all hazard ratio estimates were somewhat closer to one than those of the main analysis. There was an increased risk of clinical influenza in preschool contacts (HR = 1.90, 95% CI = 1.35 to 2.67) but not in school-age contacts (HR = 0.94, 95% CI = 0.71 to 1.26). There was also an increased risk of clinical influenza in contacts exposed to young index patients, with a hazard ratio of 1.62, 95% CI = 1.31 to 2.00 for preschool index patients and a hazard ratio of 1.27, 95% CI = 1.03 to 1.57 for school-age index patients.
The additional sensitivity analysis using a more specific definition of clinical influenza (based on fever and cough) gave results in line with those from the main analysis. The secondary attack rate in contacts was 18.1%. There was an increased risk of clinical influenza in preschool contacts (HR = 2.28, 95% CI = 1.46 to 3.59) but not in school-age contacts (HR = 0.61, 95% CI = 0.37 to 1.01). Although no more significant, the magnitude of risk and confidence intervals associated with the age of the index patient was consistent with the previous estimates (HR = 1.03, 95% CI = 0.42 to 2.54) for preschool index patients and HR = 1.44, 95% CI = 0.83 to 2.51 for school-age index patients.
Discussion
The present study identifies age of index patients and age of contacts as the main predictors of influenza transmission in families. These factors appear to be more important than other individual variables, whether they are related to the contact person or to the index patient. Based on our risk estimates, 40–48% of the secondary cases exposed to a child sick with influenza in the household are attributable to transmission from the child.
Strengths and limitations
Two factors may have harmed the validity of our results. The first is that the household contacts were not tested for influenza infection to limit intervention bias.9 It is therefore possible that some of the clinical infections detected here may be due to respiratory viruses other than influenza. However, there was little circulation of other respiratory viruses in the community during the study period: only 25 of the 946 (2.6%) index patients tested positive for respiratory syncytial virus and none were found to be positive for parainfluenzae virus or adenovirus. Furthermore, a recent investigation of the genetic sequences of influenza viruses recovered in families suggested that transmission from community sources was rare in families where an index patient had tested positive for influenza A.19
Instead of laboratory tests, we used a broad clinical definition based on fever or respiratory signs to identify secondary cases. Indeed, 38% of contacts classified as secondary patients did not report a fever. In patients consulting physicians for a respiratory illness during an influenza epidemic period, the relative risk that fever >37.8°C is associated with an influenza diagnosis was 2.5 in one study (4.6 for influenza A [H3N2] specifically),20 and 3.3 in another.16 However, syndromes associated with true influenza infection do not necessarily always include fever. In the latter studies, 30–40% of patients with respiratory syndromes caused by influenza were afebrile. Furthermore, by applying a specific combination of cough and fever as case definition,16 we found risk estimates consistent with those derived from our original broader definition, although some of our risk est-imates were no longer significant due to lack of statistical power.
The choice of a time period of 1–5 days from the inclusion of index patients to the onset of symptoms in secondary patients (mean delay = 2.4 days) minimised the risk of infections from non-influenza pathogens and from extra-household sources. Indeed, in the present study, we found influenza transmission in 24.1% of the household contacts and in 34.8% of the households. These figures are within the range of previously published estimates in comparable placebo groups of clinical trials.5,7,8
Overall, although we do not know the exact proportion of patients with influenza among the contacts showing symptoms of clinical influenza, we can provide an estimate. It has been reported that 75–80% of household transmissions occur directly from the influenza-positive index patient or from the same source of infection as the index patient.7,8 In this group, all of the clinical secondary cases have an influenza aetiology. The remaining 20–25% are due to transmission from the community at large.7,8 In this second group, the probability of infection by influenza equals the prevalence of influenza in the community. From the proportion of influenza infections in index patients at inclusion we estimate the prevalence of influenza in the community at 54% in this study. A plausible range estimate of the proportion of influenza infection among secondary patients is therefore 88.5–90.8%.
Reasons for increased transmission from children
The role of children in the dissemination of influenza is commonly accepted,2,3 and can be explained by three different and possibly complementary mechanisms. First, children are believed to experience a large number of extra-household contacts with their peers in schools or daycare centres, although very little quantitative information is available on the subject. Our study was not designed to test this mechanism. Second, children are assumed to be more susceptible to influenza infection because of lower immunity, although it depends on virus (sub)types and setting.3,21 Accordingly, we found evidence of increased susceptibility to clinical influenza in preschool children. We have no clear explanation as to why there was no increased susceptibility in school-age children, but influenza A (H3N2) infections usually have a wider distribution of age-specific attack rates than influenza A (H1N1) or influenza B infections.18 It is also possible that few differences in susceptibility between adults and school-age children occurred in this particular year, due to the circulation of the same influenza viruses (A/Sydney/5/97-like viruses, A/H3N2 subtype) for the third consecutive winter. Third, children could also be more infectious both because of an increased amount of virus shedding and an increased duration of the infectious period, as reported in recent clinical studies.22,7 Our results are in line with these findings.
Strategies for limiting secondary transmission of influenza in households
This work provides a quantification of the major role of children, and particularly younger children, in the transmission of influenza in families. Based on attributable fractions of exposure to sick children in the household of around 40–48% we can assess the potential impact of two intervention strategies. The first is the vaccination of children in advance of the epidemic season. The efficacy of influenza vaccine has been estimated to be around 80% in preventing the disease in children.23 Thus, 32–38% of the secondary household cases from exposure to a sick child could be averted by vaccinating children. The second is the prophylactic treatment of household contacts with neuraminidase inhibitors after exposure to a child sick with influenza. Parents usually consult a GP or a paediatrician if their child has symptoms of influenza-like illness, so the diagnostic of influenza needs to be established. Using rapid influenza tests during the visit would allow identification of 72–95% of children truly sick with influenza.24 If the time since onset of symptoms in the child diagnosed with influenza is less than 48 hours, then prophylaxis of contacts can be initiated with an efficacy of around 74–89% in preventing the disease.23 This strategy would prevent 21–41% of cases in exposed household contacts. These figures should help clinicians choose adequate strategies for controlling the size of influenza epidemics within households.
Acknowledgments
We thank the patients, their families and the 161 GPs of the French Sentinelles network who participated in the study. This study was partly supported by a grant from GlaxoSmithKline, Marly-Le-Roi, France. Cécile Viboud was supported by a grant from the French Ministry of Education and Research and the Fondation pour la Recherche Médicale at the time of this study.
References
- 1.Longini IM, Jr, Koopman JS, Monto AS, Fox JP. Estimating household and community transmission parameters for influenza. Am J Epidemiol. 1982;115:736–751. doi: 10.1093/oxfordjournals.aje.a113356. [DOI] [PubMed] [Google Scholar]
- 2.Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16:351–373. doi: 10.1093/oxfordjournals.epirev.a036158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monto AS. Interrupting the transmission of respiratory tract infections: theory and practice. Clin Infect Dis. 1999;28:200–204. doi: 10.1086/515113. [DOI] [PubMed] [Google Scholar]
- 4.McIntosh K, Lieu T. Is it time to give influenza vaccine to healthy infants? N Engl J Med. 2000;342:275–276. doi: 10.1056/NEJM200001273420409. [DOI] [PubMed] [Google Scholar]
- 5.Hurwitz ES, Haber M, Chang A, et al. Effectiveness of influenza vaccination of daycare children in reducing influenza-related morbidity among household contacts. JAMA. 2000;284:1677–1682. doi: 10.1001/jama.284.13.1677. [DOI] [PubMed] [Google Scholar]
- 6.Wright P. Influenza in the family. N Engl J Med. 2000;343:1331–1332. doi: 10.1056/NEJM200011023431808. [DOI] [PubMed] [Google Scholar]
- 7.Welliver R, Monto AS, Carewicz O, et al. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. JAMA. 2001;285:748–754. doi: 10.1001/jama.285.6.748. [DOI] [PubMed] [Google Scholar]
- 8.Hayden FG, Gubareva LV, Monto AS, et al. Inhaled zanamivir for the prevention of influenza in families. Zanamivir Family Study Group. N Engl J Med. 2000;343:1282–1289. doi: 10.1056/NEJM200011023431801. [DOI] [PubMed] [Google Scholar]
- 9.Carrat F, Sahler C, Rogez S, et al. Influenza burden-of-illness: estimates from a national prospective survey of household contacts in France. Arch Intern Med. 2002;162:1842–1848. doi: 10.1001/archinte.162.16.1842. [DOI] [PubMed] [Google Scholar]
- 10.Valleron AJ, Bouvet E, Garnerin P, et al. A computer network for the surveillance of communicable diseases: the French experiment. Am J Public Health. 1986;76:1289–1292. doi: 10.2105/ajph.76.11.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chauvin P, Valleron AJ. Attitude of French general practitioners to the public health surveillance of communicable diseases. Int J Epidemiol. 1995;24:435–440. doi: 10.1093/ije/24.2.435. [DOI] [PubMed] [Google Scholar]
- 12.Cristofari M, Labarthe G. Des ménages de plus en plus petits. [Smaller and smaller households] INSEE Première. 2001;789:1–4. [Google Scholar]
- 13.Monto AS, Pichichero ME, Blanckenberg SJ, et al. Zanamivir prophylaxis: an effective strategy for the prevention of influenza types A and B within households. J Infect Dis. 2002;186:1582–1588. doi: 10.1086/345722. [DOI] [PubMed] [Google Scholar]
- 14.Fritz RS, Hayden FG, Calfee DP, et al. Nasal cytokine and chemokine responses in experimental influenza A virus infection: results of a placebo-controlled trial of intravenous zanamivir treatment. J Infect Dis. 1999;180:586–593. doi: 10.1086/314938. [DOI] [PubMed] [Google Scholar]
- 15.Hayden FG, Treanor JJ, Fritz RS, et al. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA. 1999;282:1240–1246. doi: 10.1001/jama.282.13.1240. [DOI] [PubMed] [Google Scholar]
- 16.Monto AS, Gravenstein S, Elliott M, et al. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160:3243–3247. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- 17.Therneau TM. Extending the Cox model. New York: Springer-Verlag; 2000. [Google Scholar]
- 18.Monto AS, Sullivan KM. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiol Infect. 1993;110:145–160. doi: 10.1017/s0950268800050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gubareva LV, Novikov DV, Hayden FG. Assessment of hemagglutinin sequence heterogeneity during influenza virus transmission in families. J Infect Dis. 2002;186:1575–1581. doi: 10.1086/345372. [DOI] [PubMed] [Google Scholar]
- 20.Carrat F, Tachet A, Rouzioux C, et al. Evaluation of clinical case definitions of influenza: detailed investigation of patients during the 1995-1996 epidemic in France. Clin Infect Dis. 1999;28:283–290. doi: 10.1086/515117. [DOI] [PubMed] [Google Scholar]
- 21.Longini IM, Jr, Koopman JS, Haber M, Cotsonis GA. Statistical inference for infectious diseases. Risk-specific household and community transmission parameters. Am J Epidemiol. 1988;128:845–859. doi: 10.1093/oxfordjournals.aje.a115038. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson K, Webster RG, Hay A. Textbook on influenza. Oxford: Blackwell Science Ltd; 1998. [Google Scholar]
- 23.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet. 2003;362:1733–1745. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uyeki TM, Fukuda K, Cox NJ. Influenza surveillance with rapid diagnostic tests. Clin Infect Dis. 2002;34:1422. doi: 10.1086/340268. [DOI] [PubMed] [Google Scholar]


