Abstract
Neuropeptide FF (NPFF) has been proposed to play a role in pain modulation, opioid tolerance, and several other physiological processes. However, pharmacological agents that would help define physiological roles for this peptide are still missing. Here we report the discovery of a potent and selective NPFF receptor antagonist, RF9, that can be administered systemically. This compound does not show any effects by itself but can block efficiently the increase in blood pressure and heart rate evoked by NPFF. When chronically coinjected with heroin, RF9 completely blocks the delayed and long-lasting paradoxical opioid-induced hyperalgesia and prevents the development of associated tolerance. Our data indicate that NPFF receptors are part of a bona fide antiopioid system and that selective antagonists of these receptors could represent useful therapeutic agents for improving the efficacy of opioids in chronic pain treatment.
Keywords: cardiac function, analgesia, antiopioid, RFamide
The two mammalian peptides, neuropeptide FF (NPFF) and neuropeptide AF (NPAF) originate from the same gene and are members of the family of FMRFamide (Phe-Met-Arg-PheNH2)-like neuropeptides that all share an RFamide sequence at their C termini (1). Recently, two cDNAs encoding G protein-coupled receptors, for which NPFF and NPFF-related peptides display high affinity, have been cloned and are referred to as NPFF1R and NPFF2R (2, 3). Concomitantly, the characterization of a gene that encodes two neuropeptides highly similar to NPFF has been reported (4, 5). These two peptides, named NPSF and NPVF (alias RFRP-1 and RFRP-3, respectively), have been shown to activate preferentially NPFF1R, whereas NPFF displays a better activity for NPFF2R (5). These results, together with the distribution of NPSF/NPVF, NPFF1R, and NPFF2R mRNAs in rat brain (3-5) suggest that the physiologically relevant ligands for NPFF1R and NPFF2R receptor subtypes could be NPSF/NPVF and NPFF/NPAF, respectively. Although the role of NPSF/NPVF in vivo is still poorly documented, NPFF have been implicated in the regulation of several physiological processes, such as insulin release, food intake, memory, blood pressure, and electrolyte balance (6). Moreover, there is a large body of evidence suggesting that NPFF is involved in nociception and the modulation of opioid-induced analgesia (1, 7).
Opioid treatments are associated with several side effects, including the development of tolerance, which leads to increased doses of the drug to be used for relieving pain. It has been proposed that adaptative modifications in cellular responsiveness and, particularly, desensitization and down-regulation of opioid receptors are at the origin of this phenomenon (8). A challenging hypothesis is that stimulation of opioid receptors triggers activation of antiopioid systems that, in turn, produce hyperalgesia, thus diminishing the net analgesic effect of the opioid agonist (9-11). This phenomenon has been evidenced in vivo in rats, for which both acute and prolonged opioid treatments induce a long-lasting hyperalgesia that persists for several days after the last opioid administration (12-15). In man, several reports indicate that chronic opioid treatments can be associated with paradoxical hyperalgesia and/or allodynia (16-18), and enhancement in pain sensitivity has been reported in heroin addicts (19). According to this hypothesis, it can be predicted that drugs that oppose opioid-induced hyperalgesia may prevent the expression of tolerance to analgesic effect of opioids (9-11, 15, 20).
Several neuromodulator systems have been shown to display antiopioid properties, including the NPFF system (11, 20). Intracerebroventricular (i.c.v.) administration of NPFF can produce a transient hyperalgesia in rats (21, 22), whereas opioid administration triggers the release of NPFF-immunoreactive-like material from rat spinal cord both in vitro and in vivo (23, 24). Moreover, the administration of antibody against NPFF partly opposes tolerance to the analgesic effect of opioids (21, 25) and increases the density of μ-opioid-binding sites in several brain regions (26). However, the lack of NPFF receptor ligands (antagonists and agonists) showing metabolic stability combined with CNS bioavailability after systemic administration has severely limited our comprehension of the in vivo functions of this system. We describe here the identification of one compound, RF9, showing good affinity and potent antagonist activity at human NPFF (hNPFF) receptors. In rats, this compound blocks the increase in arterial blood pressure and heart rate evoked by NPFF. Moreover, it prevents the development of delayed and long-lasting paradoxical hyperalgesia induced by daily heroin administration and associated tolerance. Our findings point to NPFF antagonists as therapeutic agents that, coadministered with an opioid agonist, would improve its efficacy for relieving pain by limiting the development of tolerance.
Results
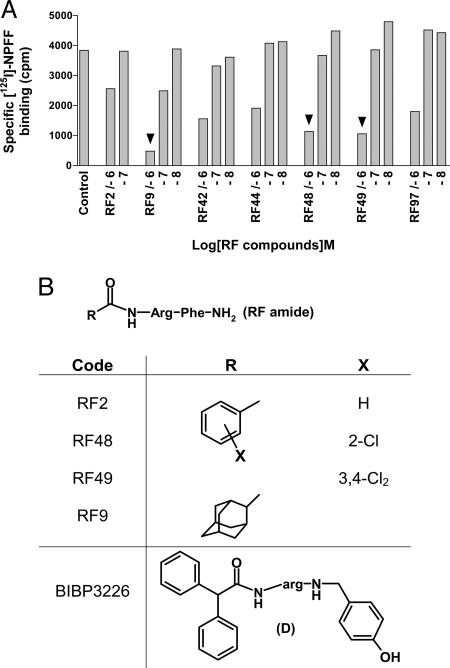
Identification of NPFF Receptor Ligands by Screening of RFamide Derivatives. To identify NPFF receptor ligands, we focused our interest in the search of small peptides (dipeptides). In a preliminary experiment, we had shown that benzoyl-RFamide dipeptide RF2 (Fig. 1B) displayed reasonable affinity for NPFF receptors from rat spinal cord (data not shown) and recombinant hNPFF2R (Table 1). We therefore screened derivatives from the RFamide dipeptide by competition on recombinant hNPFF2R (Fig. 1). We prepared ≈100 derivatives by substitution of the phenyl ring of N-benzoyl RFamide or replacement by other heterocycles (e.g., indole or quinoline), or other nonaromatic lipophilic groups (tertiobutyl, cyclohexyl, or adamantane). Three concentrations of each compound were tested (0.01, 0.1, and 1 μM) for their capacity to inhibit the binding of [125I]Tyr-NFF to membrane homogenates of COS-1 cells expressing recombinant hNPFF2R (data not shown). As expected, our reference, RF2, showed significant competition activity at 1 μM, whereas six compounds displayed a better competition activity at hNPFF2R than RF2 (Fig. 1 A). We selected RF9, RF48, and RF49 (Fig. 1B) for further pharmacological characterization.
Fig. 1.
Screening of RFamide derivatives on hNPFF2R. (A) hNPFF2R membranes were labeled with [125I]Tyr-NPFF, and three concentrations of RFamide derivatives were tested in competition experiments. Each concentration was tested in duplicate. Results for the reference and the six most active compounds are shown. Arrowheads indicate compounds that were selected for further characterization. (B) Structures of RF2, RF9, RF48, RF49, and BIBP3226.
Table 1. Binding affinities for hNPFF2R and hNPFF1R of ligands selected from the screening.
|
Ki, nM
|
||
|---|---|---|
| Ligands | hNPFF2R | hNPFF1R |
| NPFF | 0.2* ± 0.05 | 9.8 ± 0.8 |
| BIBP 3226 | 461 ± 107 | 12† ± 1 |
| RF2 | 526 ± 51 | 756 ± 91 |
| RF9 | 75 ± 9 | 58 ± 5 |
| RF48 | 27 ± 3 | 169 ± 11 |
| RF49 | 38 ± 2 | 153 ± 6 |
Values are mean ± SEM from three or more separate experiments performed in duplicate. Ki values were determined by using [125I]Tyr-NPFF for hNPFF2R and [125I]YVP for hNPFF1R.
Represents the Kd value for [125I]Tyr-NPFF
Value from ref. 42
Pharmacological Characterization of NPFF Receptor Ligands in Vitro. In a first step, we determined the Ki values of the selected compounds and of reference ligands RF2, NPFF, and BIBP3226 (Table 1) for recombinant hNPFF1R and for hNPFF2R. BIBP3226, the prototypical neuropeptide Y receptor subtype Y1 antagonist, displays structural similarities to RFamide derivatives (Fig. 1B) and has recently been shown to bind to the two NPFF receptor subtypes (27, 28). RF2 displayed submicromolar affinity for both receptors (526 and 756 nM, respectively). As expected, the three ligands selected from the screening exhibited better affinity for hNPFF2R and hNPFF1R than did RF2. RF48 and RF49 displayed the best affinity for hNPFF2R (27 and 38 nM) and a slight selectivity for this receptor [Ki (hNPFF2R)/Ki (hNPFF1R) ratio of 1:6 and 1:4, respectively], whereas RF9 displayed an equally good affinity for both receptor subtypes. Because these compounds are structurally related to BIBP3226, we investigated further the affinity of RF9 for human neuropeptide Y receptor subtype Y1 and for a subset of related G protein-coupled receptors, including the three other RFamide receptors (GPR10, GPR54, and GPR103), opioid (μ, δ, κ) and ORL-1 receptors. No competition activity was observed for RF9 on these receptors at doses up to 10 μM, except on μ and κ, for which we observed a slight competition at this concentration (see Fig. 5, which is published as supporting information on the PNAS web site). Altogether, these results indicate a good selectivity of RF9 for NPFF receptors.
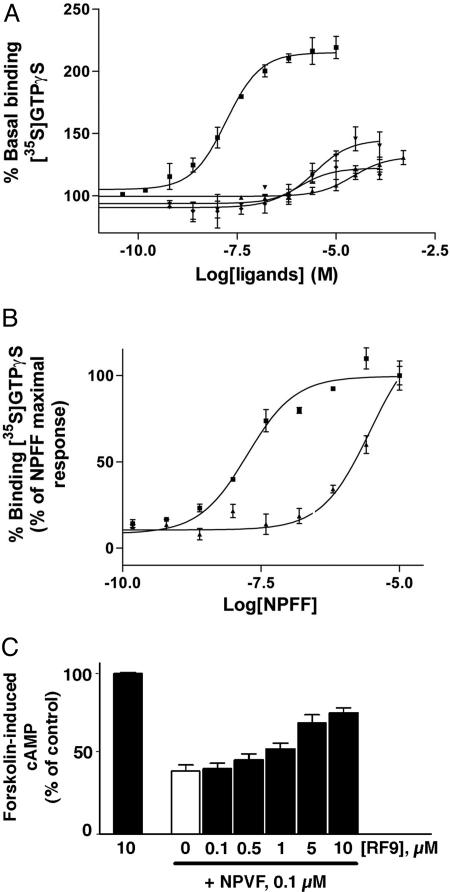
We further characterized these compounds in a functional assay consisting of agonist-promoted stimulation of the [35S]GTPγS binding to hNPFF2R cell membranes. To this purpose, COS cells were transiently transfected with a construct encoding hNPPF2R fused to a signal sequence to increase its expression level (29). NPFF stimulated the [35S]GTPγS binding to COS-signal peptide/hNPFF2R membranes with an EC50 of 22.5 ± 1 nM and a maximal activity corresponding to 205 ± 5% that of the basal level of [35S]GTPγS binding (Fig. 2A and Table 2). From the four tested compounds, RF2, RF48, and RF49 slightly stimulated GTPγS binding, with EC50 values of >10,000, 1,950 ± 450 and 694 ± 103 nM, respectively, and maximal activity ranging from 122% to 145% (Table 2). These results indicate that these compounds had partial agonist activity at high concentrations. In contrast, RF9 had no effect on [35S]GTPγS binding at concentrations up to 100 μM. To further confirm the antagonist activity of this compound, we performed concentration-effect curves of NPFF in the presence of a high concentration of RF9 corresponding to 100× Ki of this compound for hNPFF2R (Fig. 2B). RF9 (7.5 μM) shifted the concentration-effect curve of NPFF to the right by ≈160-fold (Ke = 45 ± 5 nM). This result confirmed that RF9 displays potent antagonist activity at hNPFF2R in vitro.
Fig. 2.
In vitro characterization of compounds selected from the screening. (A) Stimulation of [35S]GTPγS binding to hNPFF2R by NPFF (▪), RF2 (▴), RF48 (▾) and RF49 (♦). (B) Stimulation of [35S]GTPγS binding to hNPFF2R by NPFF alone (▪) or NPFF in presence of 7.5 μM RF9 (▴). RF9 shifted the concentration-effect curve of NPFF to the right by ≈160-fold. Data are expressed as percentage of basal [35S]GTPγS binding and represent mean ± SE from at least two separate experiments in triplicate. (C) RF9 (black bars) reversed the inhibition of forskolin-induced cAMP by NPVF in CHO-hNPFF1R cells. RF9 alone was inactive, whereas NPVF (white bar) inhibited ≈60% of the stimulated cAMP. Error bars represent the mean ± SEM of data from three experiments performed in duplicate.
Table 2. Stimulation of [35S]GTPγS binding to hNPFF2R membranes by NPFF and ligands selected from the screening.
| hNPFF2
|
||
|---|---|---|
| Ligands | EC50, nM | Maximal activation, % |
| NPFF | 22.5 ± 1 | 205 ± 5 |
| RF2 | >10,000 | 133 ± 7 |
| RF9 | >100,000 | — |
| RF 48 | 1950 ± 450 | 145 ± 10.5 |
| RF 49 | 694 ± 103 | 122 ± 6 |
Maximal activation is expressed as the percentage of basal [35S]GTPγS binding, and values represent mean ± SEM from at least two separate experiments performed in triplicate. —, not determined.
We next examined the capacity of compounds selected from the screening to inhibit forskolin-stimulated cAMP accumulation in Chinese hamster ovary (CHO) cells expressing hNPFF1R (28). In this test, NPVF (0.1 μM), the endogenous ligand of NPFF1R, strongly inhibited forskolin-stimulated cAMP accumulation, whereas RF2, RF9, RF48, and RF49 were inactive up to 10 μM (Fig. 2C and data not shown). We then showed that RF9 dose-dependently reverses the inhibitory effect of NPVF with an EC50 of 4.7 ± 1.2 μM (Fig. 2C), thus confirming that this compound displays antagonist activity at hNPFF1R as well.
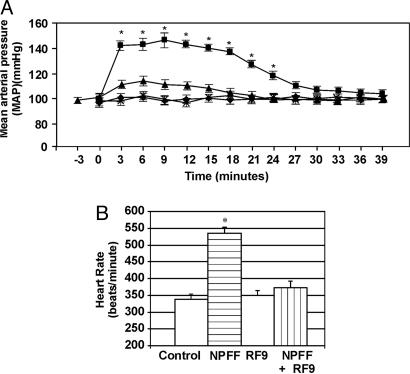
RF9 Prevents Increase in Blood Pressure and Heart Rate Elicited by NPFF in Vivo. We next wanted to investigate whether RF9 can directly antagonize NPFF effects in vivo. Because i.c.v. administration of NPFF in rats has been shown to cause a rise in arterial blood pressure (30, 31), we decided to examine the effect of RF9 in this paradigm. As expected, administration of 10 μg of NPFF i.c.v. into the lateral ventricles resulted in an elevation in mean arterial blood pressure (MAP) that occurred within 1 min of onset of i.c.v. infusion and was sustained for ≈25 min before returning to normal levels (Fig. 3A). The increase in MAP was also accompanied by a significant increase in heart rate over saline-infused rats (Fig. 3B). RF9 (10 μg) infused alone did not result in a significant alteration of MAP or heart rate. Conversely, MAP and heart rate increases evoked by NPFF were significantly blocked when NPFF was applied in conjunction with RF9 (Fig. 3). This result demonstrates the capacity of RF9 for antagonizing NPFF action in vivo and confirmed that this activity is mediated by NPFF receptors.
Fig. 3.
RF9 blocks blood pressure effects of NPFF. (A) Changes in MAP [expressed in mmHg (1 mmHg = 133 Pa)] in rats receiving either i.c.v. saline (♦), NPFF (▪), RF9 (×), or NPFF and RF9 applied together (▴). Time 0 indicates the injection point for i.c.v. saline or drug applications. (B) Heart rate changes that accompany MAP alterations in A. Pooled MAP and heart rate data are from five animals. *, significant difference in MAP or heart rate compared with control (P < 0.05).
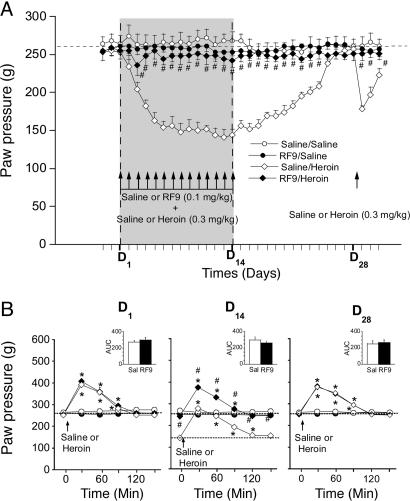
Coadministration of RF9 with Heroin Prevents Delayed Heroin-Induced Hyperalgesia and Associated Tolerance. Because NPFF had been shown previously to display antiopioid and pronociceptive properties, we decided to investigate whether RF9 could oppose to delayed heroin-induced hyperalgesia and associated tolerance. Therefore, we used a model of discontinuous opioid administration at low doses in rats because it better mimics opioid use in chronic pain patients or heroin addicts. We measured the basal nociceptive threshold of animals after coadministration of RF9 with heroin by using the paw-pressure vocalization test. The basal nociceptive threshold values were measured every day 1 h before drug administration (see Materials and Methods). As described in ref. 13, when injected alone for 2 weeks, once-daily doses of 0.3 mg/kg heroin induced a gradual lowering of the basal nociceptive threshold value (P < 0.05, Dunnett's test) (Fig. 4A) that returned to the predrug value on day 25 (D25), i.e., 11 days after cessation of heroin treatment (P > 0.05, Dunnett's test). In this paradigm, 0.1 mg/kg saline or RF9 (s.c.) alone did not alter the basal nociceptive threshold of the animals (P > 0.05, one-way ANOVA). When injected 30 min before each daily heroin administration, RF9 completely prevented the progressive decrease of the nociceptive threshold (P > 0.05, one-way ANOVA). As expected, when we measured the analgesic effect of the first (D1) and the last (D14) heroin administrations (Fig. 4B), we observed a strong decrease of the maximum analgesic effect of heroin in rats that were treated by the opioid for 2 weeks [peak at 279 g corresponding to 29,6% maximal possible effect (MPE)] compared with the first herion injection on D1 (peak at 392 g corresponding to 40% MPE). These data demonstrate that tolerance did develop in these animals. However, we observed no change in both the time course and area under the curve (AUC) related to the analgesic effect of heroin between D1 and D14 (Fig. 4B), suggesting that this change in MPE reflects a shift of basal nociceptive threshold rather than a lack of pharmacological effect of the opioid. When RF9 was coadministered with heroin, both the basal nociceptive threshold and AUC were identical at D1 and D14 (P > 0.05, two-way ANOVA), indicating that neither tolerance nor hyperalgesia developed in these animals. When injected in animals that had recovered their predrug nociceptive threshold after 14 once-daily heroin administrations, a 0.3 mg/kg heroin injection (D28) produced the same analgesic effect than at D1 (P > 0.05, two-way ANOVA) (Fig. 4B). As we described in ref. 13, analgesia was followed by an exaggerated decrease of the nociceptive threshold for several days (P < 0.05, Dunnett's test) (Fig. 4A) compared with the initial heroin administration on D1. Such a hyperalgesia was not observed in rats that received prior 14 once-daily coadministrations of RF9 and heroin (P > 0.05, one-way ANOVA). Altogether, these results demonstrate that blocking NPFF receptor can prevent efficiently heroin-induced hyperalgesia and associated tolerance.
Fig. 4.
Coadministration of RF9 with heroin prevents heroin-induced delayed hyperalgesia and tolerance. (A) Delayed effects of 14 once-daily coadministrations of saline or RF9 (0.1 mg/kg, s.c.) 30 min before 0.3 mg/kg heroin or saline on basal nociceptive threshold in rats (n = 8 rats per group). The basal nociceptive threshold was determined once daily 60 min before each heroin or saline administration and after the heroin treatment was stopped. When rats in the saline/heroin group had recovered their predrug nociceptive threshold (D28), changes in nociceptive threshold induced by 0.3 mg/kg heroin in the saline/heroin and RF9/heroin groups were estimated for several days. Mean paw-pressure values for triggering vocalization (±SEM) are expressed in grams. #, P < 0.05 with a Newman-Keuls test when the RF9/heroin and saline/heroin groups were compared. (B) Analgesic effect induced by 0.3 mg/kg heroin (or saline) in the saline/saline (○), RF9/saline (•), saline/heroin (⋄), and RF9/heroin (♦) groups on D1,D14, and D28 (n = 8 rats per group). The nociceptive threshold of animals was measured 30 min after heroin injection and then every 30 min until the end of the pharmacological effect. (Inset) Comparison of AUC. Mean paw-pressure values for triggering vocalization (±SEM) are expressed in grams. *, P < 0.05 with Dunnett's test as compared with basal nociceptive value; #, P < 0.05 with a Newman-Keuls test comparing the RF9/heroin group with the saline/heroin group.
Discussion
Although in recent years great advances have been made in the understanding of mechanisms that underlie pain, opioids are still the most powerful analgesics. However, their use is limited by tolerance that accrues after repeated exposure. Our discovery of a potent selective antagonist of NPFF receptors that can be administered systemically has enabled us to establish that blockade of NPFF receptors prevents the development of sustained hyperalgesia and consequently opposes the associated decrease in maximum heroin analgesic effect. This result strengthens the hypothesis that development of tolerance to opioids is not only due to a decrease in cellular responsiveness but may also originate from the secondary up-regulation of antiopioid systems with pronociceptive properties leading to long-lasting enhancement in pain sensitivity (9-11, 15, 20).
In previous reports, several substances, including N-methyl-d-aspartate receptor antagonists have been shown to prolong opioid analgesic effect and prevent long-lasting hyperalgesia (9, 10, 13, 15). However, these substances act on neurotransmitter systems that are critical to normal brain function; therefore, their use is limited by serious side effects (32). Antagonists of antiopioid peptide receptors could be a new and safer strategy because their side effects are often more limited because of lower receptor density in the CNS. Although we have not yet investigated all of the functions that are considered modulated by the NPFF system, we show here that RF9 can be systemically administered and does not display any effects on the nociceptive threshold and on the blood pressure or the heart rate when administered alone. These results suggest that the basal level of activation of this system is low under normal conditions. In keeping with this notion, it has been shown that NPFF levels in the spinal fluid of morphine-treated rats are elevated significantly (33) and that antisense oligonucleotides to human SQA-NPFF attenuate tolerance to analgesic effect of morphine in mice (34). Our data showing that coadministration of the NPFF receptor antagonist RF9 with heroin prevents the development of tolerance are in good agreement with these results and suggest that the NPFF system is triggered by activation of the opioid system as observed in in vitro studies (23). Overall, our results and data from the literature indicate that RF9 should display limited adverse side effects.
A large body of evidence already suggests that NPFF plays important roles in the control of pain and analgesia through its interaction with the opioid system (1, 6). However, it has been shown that NPFF displays both antiopioid and proopioid actions in animal models of pain, depending on the route of administration. Thus, i.c.v. administration of NPFF reverses morphine-induced analgesia in rats (33), whereas intrathecal administration induces a long-lasting opioid-induced analgesia and prolongs morphine-induced analgesia (35). Recently, a novel family of G protein-coupled receptors specifically expressed in neurons of trigeminal and dorsal root ganglia was identified (36). Some of these receptors display a good affinity for NPFF and are most likely involved in pain processing (37). In addition to the NPFF2R receptor subtype that is expressed in the dorsal horn of spinal cord in rodents (38), these receptors may also be activated after local intrathecal injection of a high concentration of NPFF, thus mediating spinal analgesic activity of this peptide. Our results of systemic administration of RF9 on opioid-induced hyperalgesia clearly define NPFF and its receptors as a bona fide antiopioid system.
In rats that have been chronically treated with heroin, we observed that a single heroin challenge, administered once the animals had recovered their initial basal nociceptive threshold, induced analgesia followed by a marked hyperalgesia. This finding indicates that these animals have developed a latent process of pain sensitization, which has been proposed to be due to a long-lasting up-regulation of pronociceptive systems (9). The fact that we did not observe the development of hyperalgesia in RF9-treated rats both during the chronic heroin treatment and after the single heroin challenge strongly suggests that the NPFF system is involved in pain sensitization. In the same heroin model, we have previously reported that naloxone precipitates a marked hyperalgesia 2 months after stopping heroin administrations (13). Collectively, these data suggest that return to predrug nociceptive thresholds after cessation of heroin administrations is not due to a deactivation of pronociceptive systems but would rather result from a progressive opposition by an active opioid-dependent, counter-adaptation system leading to a new equilibrium (allostasis) associated with a high-level balance between antinociceptive and pronociceptive systems.
The precise role of both NPFF receptor subtypes has not been clarified yet. Distribution of NPFF1R and NPFF2R in several mammalian species (3, 38, 39) indicates that NPFF2R is localized to pain-processing regions, whereas NPFF1R would most likely participate in neuroendocrine function. A notable exception is human spinal cord, where NPFF1R expression has been observed while NPFF2R is present in the rodent, rabbit, and monkey (3, 38, 39). This exception suggests that in humans NPFF1R could play the same role in pain processing as does NPFF2R in other mammals. We show here that RF9 compound displays both the same affinity and antagonist activity at NPFF1R and NPFF2R subtypes. Therefore, it is likely that RF9 will display comparable activity in man and in rats independently of the NPFF receptor subtype involved in pain processing.
In conclusion, although the precise mechanism by which NPFF acts to block opioid-induced hyperalgesia and associated tolerance is currently unknown, we provide convincing evidence that NPFF system acts as an antiopioid system with pronociceptive properties. We describe the discovery of a potent and selective NPFF receptor antagonist and demonstrate that blocking this system in vivo represents an innovative strategy for improving the efficacy of opioids as therapy in chronic pain.
Materials and Methods
Synthesis of RFamide Derivatives. Boc-Arg(Pmc)-OH and H-Phe-NH2 (Bachem, Germany) were reacted overnight in dimethylformamide in presence of hexafluorophosphate of N-oxy tris[(dimethylamino)phosphonium]benzotriazole and N-methylmorpholine at room temperature yielding the dipeptide (86%), which was submitted to quantitative acid hydrolysis (2.4 M HCl/AcOEt) at 20°C overnight. The free NH2 intermediate was then N-acylated in the presence of the corresponding carboxylic acid, affording the N-acyl Arg(Pmc)-Phe-NH2. After a last step of deprotection in the presence of trifluoroacetic acid/dichloromethane (50:50), the trifluoroacetate of RF-amide was obtained and purified by HPLC (218TP C18 column; i.d., 22 mm; length, 250 mm; pore size, 10- to 15-μm gradient, H2O plus 0.1% trifluoroacetic acid/MeOH: (100/0-0/100), 40 mn, 10 ml/mn; Dionex).
Cloning of hNPFF2R. The cDNA encoding hNPFF2R was subcloned into the pcDNA3 expression vector (Invitrogen) (see Supporting Materials and Methods, which is published as supporting information on the PNAS web site). In a second construct, a sequence encoding a signal sequence and a Flag epitope was fused to the 5′ coding sequence of hNPFF2R cDNA, resulting in a SF-hNPFF2R construct.
Cell Membrane Preparations and Receptor Binding Assays. COS-1 cells were grown and electroporated with pcDNA3/hNPFF2R or SF-hNPFF2R, and membranes were prepared as described in ref. 40. Membranes from CHO cells stably expressing hNPFF1R were prepared as described in ref. 28. hNPFF2R membranes were incubated in a final volume of 0.2 ml containing 50 mM Hepes (pH 7.4), 10 mM CaCl2, 10 mM MgCl2, 0.1% BSA, 0.1 mM phenylmethylsulfonyl fluoride, 0.2 nM [125I]Tyr-NPFF [specific activity, 2,000 Ci/mmol (1 Ci = 37 GBq); Amersham Biosciences, which is now GE Healthcare], and the ligands to be tested as described in ref. 41. Nonspecific binding was determined in the presence of 1 μM NPFF. Typical total and nonspecific binding were 2500 and 500 cpm, respectively. hNPFF1R membranes were incubated in a final volume of 0.5 ml containing 50 mM Tris·HCl (pH 7.4), 60 mM NaCl, 25 μM bestatin (Sigma-Aldrich), 0.1% BSA, 0.05 nM [125I]YVP (specific activity, 2,175 Ci/mmol), and the ligands to be tested as described in ref. 42. Nonspecific binding was determined in the presence of 1 μM YVPNLPQRFa. Typical total and nonspecific binding were 2,800 and 300 cpm, respectively. Competition experiments with neuropeptide Y subtype Y1, opioid, and RFamide receptors are described in Supporting Materials and Methods.
Cellular Assays. For [35S]GTPγS binding experiments, SF-hNPFF2R membrane proteins (5 μg) were incubated for 30 min at 30°C in 20 mM Hepes, pH 7.4/100 mM NaCl/3 mM MgCl2/3 μM GDP/10 mg/ml saponin/0.1 nM [35S]GTPγS and ligands. Nonspecific binding was determined in the presence of 10 μM GTPγS. Incubation mixtures were rapidly filtered and washed with 20 mM Hepes, pH 7.4/100 mM NaCl/3 mM MgCl2 on Whatman GF/B filters. Bound radioactivity was determined by scintillation counting. Antagonist Ke value was calculated by using the following formula: Ke = [antagonist]/(dose ratio) - 1. For intracellular cAMP experiments, CHO cells stably expressing hNPFF1R were assayed as described in ref. 28.
Blood Pressure and Heart Rate Measurements. Experimental details are given in Supporting Materials and Methods. Rats (n = 5) were catheterized for measurement of arterial blood pressure and received an in-dwelling cannula into the lateral cerebral ventricle as described in ref. 31. Arterial blood pressure and heart rate were continuously monitored. NPFF (10 μg in 10 μl of saline) was injected into the lateral ventricle over 15-20 sec. Control injections of saline (10 μl) were carried out in the same animals. Upon return of blood pressure to baseline, RF9 (10 μg) was injected i.c.v. followed by another i.c.v. injection of NPFF with RF9. After 1.5 h., another i.c.v. infusion of NPFF was repeated. Data from each animal were pooled to obtain blood pressure and heart rate changes with i.c.v. saline, NPFF, RF9, and RF9 with NPFF.
Measurement of Nociceptive Mechanical Threshold. Experimental details are given in Supporting Materials and Methods. Nociceptive mechanical thresholds in rats (n = 8) were determined by using the paw-pressure vocalization test. Heroin (0.3 mg/kg; Francopia, Gentilly, France) was injected once-daily for 14 days. Basal nociceptive threshold was measured once-daily 60 min before heroin administration and after the cessation of heroin injections until the end of the experiment. Changes in nociceptive threshold were also measured every 30 min after heroin injection on D1, D14, and D28 until the end of the pharmacological effect. RF9 (0.1 mg/kg) or saline was injected 30 min before heroin or saline injection. Four experimental groups were constituted: saline/saline, RF9/saline, saline/heroin, and RF9/heroin.
Analysis of the Data. All data are expressed as means ± SEM. For in vitro experiments, nonlinear regression analysis of the data were performed with prism 2.0 (GraphPad, San Diego). For blood pressure and heart rate data, Student's paired t test was used for determining the significance of effect between the different treatment groups. For nociceptive threshold data, one-way and two-way ANOVA with repeated measurements followed by Dunnett's test were performed. AUC for analgesic effects were calculated by using the trapezoidal method. The MPE was calculated as 100 × (paw pressure value after injection - baseline paw pressure value)/600 - baseline paw pressure value). A Newman-Keuls test was used for multiple comparisons between groups. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank the Institut de Génétique et de Biologie Moléculaire et Cellulaire core facilities for technical assistance. This work was supported by the Centre National de la Recherche Scientifique, the Hôpital Universitaire de Strasbourg, the Institut Nationale de la Santé et de la Recherche Médicale, the Université Louis Pasteur, the Université Victor Ségalen Bordeaux 2, the Université Bordeaux 1, the Ministère de l'Education Nationale de Enseignement Supérieur et de la Recherche Français, the Association pour la Recherche Sur le Cancer, and the Heart and Stroke Foundation of Canada.
Author contributions: F.S., J.H.J., J.-J.B., and G.S. designed research; F.S., M.S., J.-P.L., E.L., D.M., A.M., C.M., and P.L. performed research; M.P. contributed new reagents/analytic tools; F.S., M.S., J.-P.L., E.L., D.M., C.M., J.-J.B., and G.S. analyzed data; and F.S., J.H.J., B.L.K., J.-J.B., and G.S. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AUC, area under the curve; CHO, Chinese hamster ovary; Dn, day n; i.c.v., intracerebroventricular; MAP, mean arterial blood pressure; MPE, maximal possible effect; NPFF, neuropeptide FF; hNPFF, human NPFF.
References
- 1.Roumy, M. & Zajac, J. M. (1998) Eur. J. Pharmacol. 345, 1-11. [DOI] [PubMed] [Google Scholar]
- 2.Zajac, J. M. (2001) Trends Pharmacol. Sci. 22, 63. [DOI] [PubMed] [Google Scholar]
- 3.Bonini, J. A., Jones, K. A., Adham, N., Forray, C., Artymyshyn, R., Durkin, M. M., Smith, K. E., Tamm, J. A., Boteju, L. W., Lakhlani, P. P., et al. (2000) J. Biol. Chem. 275, 39324-39331. [DOI] [PubMed] [Google Scholar]
- 4.Hinuma, S., Shintani, Y., Fukusumi, S., Iijima, N., Matsumoto, Y., Hosoya, M., Fujii, R., Watanabe, T., Kikuchi, K., Terao, Y., et al. (2000) Nat. Cell Biol. 2, 703-708. [DOI] [PubMed] [Google Scholar]
- 5.Liu, Q., Guan, X. M., Martin, W. J., McDonald, T. P., Clements, M. K., Jiang, Q., Zeng, Z., Jacobson, M., Williams, D. L., Jr., Yu, H., et al. (2001) J. Biol. Chem. 276, 36961-36969. [DOI] [PubMed] [Google Scholar]
- 6.Panula, P., Aarnisalo, A. A. & Wasowicz, K. (1996) Prog. Neurobiol. 48, 461-487. [DOI] [PubMed] [Google Scholar]
- 7.Panula, P., Kalso, E., Nieminen, M., Kontinen, V. K., Brandt, A. & Pertovaara, A. (1999) Brain Res. 848, 191-196. [DOI] [PubMed] [Google Scholar]
- 8.Taylor, D. A. & Fleming, W. W. (2001) J. Pharmacol. Exp. Ther. 297, 11-18. [PubMed] [Google Scholar]
- 9.Simonnet, G. & Rivat, C. (2003) NeuroReport 14, 1-7. [DOI] [PubMed] [Google Scholar]
- 10.Ossipov, M. H., Lai, J., Vanderah, T. W. & Porreca, F. (2003) Life Sci. 73, 783-800. [DOI] [PubMed] [Google Scholar]
- 11.Rothman, R. B. (1992) Synapse 12, 129-138. [DOI] [PubMed] [Google Scholar]
- 12.Celerier, E., Rivat, C., Jun, Y., Laulin, J. P., Larcher, A., Reynier, P. & Simonnet, G. (2000) Anesthesiology 92, 465-472. [DOI] [PubMed] [Google Scholar]
- 13.Celerier, E., Laulin, J. P., Corcuff, J. B., Le Moal, M. & Simonnet, G. (2001) J. Neurosci. 21, 4074-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larcher, A., Laulin, J. P., Celerier, E., Le Moal, M. & Simonnet, G. (1998) Neuroscience 84, 583-589. [DOI] [PubMed] [Google Scholar]
- 15.Mao, J., Price, D. D. & Mayer, D. J. (1995) Pain 62, 259-274. [DOI] [PubMed] [Google Scholar]
- 16.Arner, S., Rawal, N. & Gustafsson, L. L. (1988) Acta Anaesthesiol. Scand. 32, 253-259. [DOI] [PubMed] [Google Scholar]
- 17.De Conno, F., Caraceni, A., Martini, C., Spoldi, E., Salvetti, M. & Ventafridda, V. (1991) Pain 47, 337-339. [DOI] [PubMed] [Google Scholar]
- 18.Devulder, J. (1997) J. Neurosurg. Anesthesiol. 9, 146-148. [DOI] [PubMed] [Google Scholar]
- 19.White, J. M. (2004) Addict. Behav. 29, 1311-1324. [DOI] [PubMed] [Google Scholar]
- 20.McNally, G. P. (1999) Neurosci. Biobehav. Rev. 23, 1059-1078. [DOI] [PubMed] [Google Scholar]
- 21.Yang, H. Y., Fratta, W., Majane, E. A. & Costa, E. (1985) Proc. Natl. Acad. Sci. USA 82, 7757-7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberling, P., Stinus, L., Le Moal, M. & Simonnet, G. (1993) Peptides 14, 919-924. [DOI] [PubMed] [Google Scholar]
- 23.Devillers, J. P., Boisserie, F., Laulin, J. P., Larcher, A. & Simonnet, G. (1995) Brain Res. 700, 173-181. [DOI] [PubMed] [Google Scholar]
- 24.Stinus, L., Allard, M., Gold, L. & Simonnet, G. (1995) Peptides 16, 1235-1241. [DOI] [PubMed] [Google Scholar]
- 25.Lake, J. R., Hammond, M. V., Shaddox, R. C., Hunsicker, L. M., Yang, H. Y. & Malin, D. H. (1991) Neurosci. Lett. 132, 29-32. [DOI] [PubMed] [Google Scholar]
- 26.Goodman, C. B., Heyliger, S., Emilien, B., Partilla, J. S., Yang, H. Y., Lee, C. H., Cadet, J. L. & Rothman, R. B. (1998) Peptides 19, 1703-1709. [DOI] [PubMed] [Google Scholar]
- 27.Mollereau, C., Gouarderes, C., Dumont, Y., Kotani, M., Detheux, M., Doods, H., Parmentier, M., Quirion, R. & Zajac, J. M. (2001) Br. J. Pharmacol. 133, 1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mollereau, C., Mazarguil, H., Marcus, D., Quelven, I., Kotani, M., Lannoy, V., Dumont, Y., Quirion, R., Detheux, M., Parmentier, M. & Zajac, J. M. (2002) Eur. J. Pharmacol. 451, 245-256. [DOI] [PubMed] [Google Scholar]
- 29.Guan, X. M., Kobilka, T. S. & Kobilka, B. K. (1992) J. Biol. Chem. 267, 21995-21998. [PubMed] [Google Scholar]
- 30.Thiemermann, C., al-Damluji, S., Hecker, M. & Vane, J. R. (1991) Biochem. Biophys. Res. Commun. 175, 318-324. [DOI] [PubMed] [Google Scholar]
- 31.Jhamandas, J. H. & MacTavish, D. (2003) J. Neuroendocrinol. 15, 24-32. [DOI] [PubMed] [Google Scholar]
- 32.Chizh, B. A., Headley, P. M. & Tzschentke, T. M. (2001) Trends Pharmacol. Sci. 22, 636-642. [DOI] [PubMed] [Google Scholar]
- 33.Malin, D. H., Lake, J. R., Hammond, M. V., Fowler, D. E., Rogillio, R. B., Brown, S. L., Sims, J. L., Leecraft, B. M. & Yang, H. Y. (1990) Peptides 11, 969-972. [DOI] [PubMed] [Google Scholar]
- 34.Gelot, A., Frances, B., Gicquel, S. & Zajac, J. M. (1998) Eur. J. Pharmacol. 358, 203-206. [DOI] [PubMed] [Google Scholar]
- 35.Gouardères, C., Tellez, S., Tafani, J.-A. M. & Zajac, J.-M. (1993) Synapse 13, 231-240. [DOI] [PubMed] [Google Scholar]
- 36.Simonin, F. & Kieffer, B. L. (2002) Nat. Neurosci. 5, 185-186. [DOI] [PubMed] [Google Scholar]
- 37.Dong, X., Han, S., Zylka, M. J., Simon, M. I. & Anderson, D. J. (2001) Cell 106, 619-632. [DOI] [PubMed] [Google Scholar]
- 38.Gouarderes, C., Puget, A. & Zajac, J. M. (2004) Synapse 51, 249-269. [DOI] [PubMed] [Google Scholar]
- 39.Zeng, Z., McDonald, T. P., Wang, R., Liu, Q. & Austin, C. P. (2003) J. Chem. Neuroanat. 25, 269-278. [DOI] [PubMed] [Google Scholar]
- 40.Becker, J. A., Wallace, A., Garzon, A., Ingallinella, P., Bianchi, E., Cortese, R., Simonin, F., Kieffer, B. L. & Pessi, A. (1999) J. Biol. Chem. 274, 27513-27522. [DOI] [PubMed] [Google Scholar]
- 41.Elshourbagy, N. A., Ames, R. S., Fitzgerald, L. R., Foley, J. J., Chambers, J. K., Szekeres, P. G., Evans, N. A., Schmidt, D. B., Buckley, P. T., Dytko, G. M., et al. (2000) J. Biol. Chem. 275, 25965-25971. [DOI] [PubMed] [Google Scholar]
- 42.Gouarderes, C., Quelven, I., Mollereau, C., Mazarguil, H., Rice, S. Q. & Zajac, J. M. (2002) Neuroscience 115, 349-361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.