Abstract
The “false thumb” of pandas is a carpal bone, the radial sesamoid, which has been enlarged and functions as an opposable thumb. If the giant panda (Ailuropoda melanoleuca) and the red panda (Ailurus fulgens) are not closely related, their sharing of this adaptation implies a remarkable convergence. The discovery of previously unknown postcranial remains of a Miocene red panda relative, Simocyon batalleri, from the Spanish site of Batallones-1 (Madrid), now shows that this animal had a false thumb. The radial sesamoid of S. batalleri shows similarities with that of the red panda, which supports a sister-group relationship and indicates independent evolution in both pandas. The fossils from Batallones-1 reveal S. batalleri as a puma-sized, semiarboreal carnivore with a moderately hypercarnivore diet. These data suggest that the false thumbs of S. batalleri and Ailurus fulgens were probably inherited from a primitive member of the red panda family (Ailuridae), which lacked the red panda's specializations for herbivory but shared its arboreal adaptations. Thus, it seems that, whereas the false thumb of the giant panda probably evolved for manipulating bamboo, the false thumbs of the red panda and of S. batalleri more likely evolved as an aid for arboreal locomotion, with the red panda secondarily developing its ability for item manipulation and thus producing one of the most dramatic cases of convergence among vertebrates.
Keywords: convergence, Simocyon, Miocene, Batallones-1, Ailuridae
The fossil site of Batallones-1 (Madrid) is a Miocene carnivore trap, which is greatly improving our knowledge about the Late Miocene European carnivores (1, 2). With 98% of the remains corresponding to members of Carnivora and an unbiased representation of skeletal parts, the site is yielding unprecedented information about the anatomy of species that previously were poorly known. One example is Simocyon batalleri, traditionally known from cranial fragments and dentition, which is now the best-represented member of the genus, thanks to the Batallones-1 material. A study of the cranial fossils from Batallones-1 has provided further evidence of the systematic position of Simocyon as a member of the Ailuridae, has shown a trend in the evolution of this genus toward increased hypercarnivory and bone crushing, and has typified S. batalleri as a hunter of small prey and occasional scavenger (3). Now, the discovery that this animal had a “false thumb” throws new light on the evolution of this feature in both the giant panda (Ailuropoda melanoleuca) and the red panda (Ailurus fulgens) lineages.
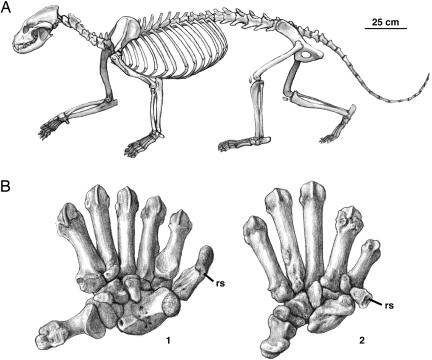
S. batalleri is the least abundant carnivore in the Batallones-1 association, and the fossils recovered correspond to two individuals. The fossils include crania and mandibles, cervical, thoracic, and lumbar vertebrae, nearly complete forelimbs, and partial hindlimbs (Fig. 1). The cervical vertebrae are unspecialized and similar to those of Ailurus fulgens, but the lumbar vertebrae are elongated, with pointed neural processes and elongated transverse processes, betraying a powerful musculature for flexing the back and the femur. These features are adaptations for a bounding gallop, a gait common in small musteloid carnivores, such as weasels (4), but energetically costly and thus uncommon in larger, terrestrial carnivores. Simocyon, about the size of a puma, is the largest known arctoid carnivore with such lumbar morphology, likely reflecting a scansorial habit, meaning that the animal would forage on the ground but would readily climb when necessary (5). Many features of the appendicular skeleton indicate climbing abilities and the lack of cursorial adaptations. The scapula has a large process for the teres major muscle, shared only with the arboreal kinkajou (Potos flavus) among the musteloidea and superficially similar to that of bears, which combines a large teres major process with the development of a postscapular fossa, absent in Simocyon (5). The morphology of the long bones of the forelimb indicates the presence of strong flexor muscles in addition to a great ability for lateral rotation and a crouched, semiplantigrade posture (5).
Fig. 1.
Skeletal anatomy of S. batalleri.(A) Skeletal reconstruction of S. batalleri. The pelvis, femora, tibiae, fibulae, sacrum, and caudal vertebrae are not known and have been reconstructed on the basis of related taxa. (B) Articulated right carpus and metacarpus in palmar view, showing the position of the radial sesamoid (rs) in Ailuropoda melanoleuca (Left; 1) and S. batalleri (Right; 2) (not at scale). Art by M. Antón; manus of giant panda modified from ref. 6.
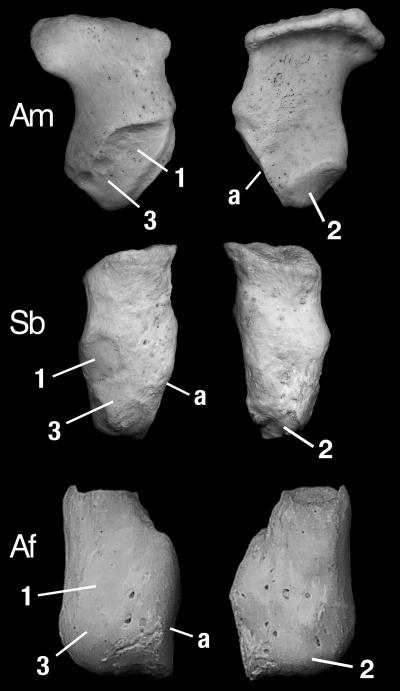
The radial sesamoid of Simocyon (Fig. 2) is the only known instance of a well developed false thumb in any carnivore, living or fossil, other than the pandas. It is elongated, with an articulation facet for the posterior tubercle of the scapholunar; this facet is posterolaterally placed in the giant panda, whereas, in Simocyon and the red panda, it faces laterally. The medial margin of the bone in the three species shows three small facets for the attachment of several ligaments and the abductor pollicis brevis and opponens pollicis muscles, whereas, in the lateral surface, there is a large attachment area for the abductor pollicis longus muscle. Although both pandas have hypertrophied radial sesamoids, their morphologies are different: in the giant panda, it is relatively much larger and flattened, with a rough and massive tip, whereas, in the red panda and Simocyon, it is relatively smaller and not flattened, with a concave tip. An additional difference in the anatomy of the manus is that, in the red panda (and all procyonids), there is a flexor brevis digitorum manus muscle attaching on the fifth metacarpal that flexes the hand and reinforces the grasping action of the radial sesamoid, whereas the giant panda lacks this muscle (6, 7). As in modern pandas, the false thumb of Simocyon would be associated with a more or less differentiated section of the palmar pad, and the pulling action of the abductor-adductor musculature on the bone would fold the pad, allowing it to close around objects. This mechanism provides gripping abilities to a hand otherwise constrained by the lack of opposability of the true thumb or pollex, which is aligned with the other digits. Pandas employ this gripping action for grasping and manipulating bamboo stems and leaves (6-10), but this gripping action would also improve the grip of the forepaws of Simocyon during arboreal locomotion, especially on thin branches. Independently of the evolutionary causes of the origin of the false thumb, such arboreal adaptations would be important for a medium-sized carnivore, with small canines and without cursorial abilities like Simocyon, which coexisted with larger, dangerous competitors like the saber-toothed cats, Machairodus and Paramachairodus, and the bear-dog Amphicyon. Both Machairodus and Amphicyon were much larger than Simocyon, but their large size limited their climbing abilities, so it would be relatively simple for Simocyon to escape from them by using short bursts of speed that would take it to the nearest tree and then climbing to safety. Confrontation with Paramachairodus would pose a different problem because this pumasized saber-toothed cat was both dangerously armed and a capable climber. An ability to move along thin branches would have allowed Simocyon both to access carcasses cached by Paramachairodus on the trees and to retreat beyond the reach of even this agile cat.
Fig. 2.
Comparisons of left radial sesamoids of the three species of carnivores with false thumbs, represented at the same size. (Left) Internal face. (Right) External face. Am, Ailuropoda melanoleuca; Sb, Simocyon batalleri; Af, Ailurus fulgens; 1, abductor pollicis brevis muscle; 2, abductor pollicis longus muscle; 3, opponens pollicis muscle; a, articulation facet with scapholunar.
This evidence has implications for the relationship between the giant and red pandas and for the evolution of their false thumbs. Although most specialists now agree about the ursid affinities of the giant panda (11-14), the position of the red panda has remained controversial. Molecular and morphological evidence has been interpreted by some to support a sister-group relationship with the Musteloidea (Procyonidae + Mustelidae) as the family Ailuridae (13-17), whereas others include it in the Procyonidae, as the subfamily Ailurinae (18, 19). Still others have proposed a close relationship between the red panda and the ursids, or ursids + pinnipeds (20-22).
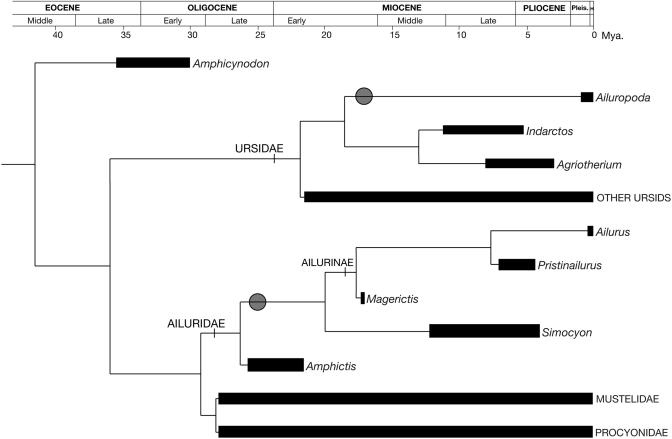
The similarities in the radial sesamoids of Simocyon and Ailurus reinforce the notion of a sister-group relationship between the two genera, to the exclusion of procyonids and other musteloids to which the ailurids have been previously associated (Fig. 3). This fact agrees with views that postulate an independent family Ailuridae as a basal sister-group of Musteloidea (13-17). In all known carnivores, other than the pandas and Simocyon, the radial sesamoid retains its primitive state as a small, lens-shaped bone that develops on the tendon of the abductor pollicis longus muscle where it glides over the scapholunar. In turn, the false thumb of the giant panda has been seen as an exaggeration of a trend toward hypertrophy of the radial sesamoid already present in ursids, which also have the same distinctive pattern of muscle insertions (6). In summary, the retention of the primitive pattern in all other arctoids and the morphological differences between ailurids and the giant panda suggest that the false thumb was developed independently by each group from a common ancestor with primitively shaped radial sesamoids; this basal arctoid could be placed near the split between Ursidae, Ailuridae, and Musteloidea, which has been dated as early as the Oligocene (30-40 million years ago) (13, 23). The absence of a radial sesamoid in the Pliocene fossil ursid Agriotherium (24), and its very small size in the Miocene Indarctos (24), considered as the closest relatives of the giant panda (7), strongly supports this convergence and indicates how closely tied the secondary herbivorous adaptation of the giant panda was to the development of the false thumb.
Fig. 3.
Schematic tree summarizing the phylogenetic relationships and temporal ranges of the members of Arctoidea referred to in this work, highlighting the independent appearance of the false thumb (gray circle). Data on sister-group relationships and divergence dates are from refs. 11-17, 28, 30, and 32.
The false thumbs of the two extant panda species thus appear to be an example of convergent evolution through the selection of an unlikely trait under the pressure of similarly specialized herbivorous habits. But the presence of a false thumb in a carnivore with such a different lifestyle as that inferred for Simocyon complicates this view. The absence in Simocyon of the masticatory specializations of Ailurus for herbivory clearly indicates that their common ancestor, which first evolved the false thumb, would have been a generalized carnivore, not a bamboo feeder. The dentition of Amphictis, a possible basal ailurid from the Late Oligocene and Early Miocene of Eurasia (25), is very similar to that of Simocyon, supporting the notion that the modified dentition of Ailurus is a secondary specialization already present in the fossil ailurine Magerictis imperialensis from the Middle Miocene of Spain (17 million years ago) (26), in other more recent forms from France ≈12 million years ago (27), and in the more advanced Pristinailurus bristoli from the Late Miocene of North America (28). Thus, the separation between Simocyon and the Ailurus lineage would have occurred very early in the evolution of the family Ailuridae but later than the appearance of the false thumb. In this respect, the striking similarities between the postcranial skeletons of Simocyon and Ailurus point to an arboreal common ancestor, and it is known that carnivores, unlike primates, are handicapped for efficient arboreal locomotion by the lack of opposable thumbs. The presence of a false thumb would have allowed the ancestral ailurid to move in the trees with almost primate-like ability. The presence of the flexor brevis digitorum manus muscle attaching on the fifth metacarpal of ailurids and procyonids facilitates in some taxa, like the very arboreal kinkajous, the convergence of the digits toward the center of the palm during flexion, in a fashion termed “pseudoopposition,” which provides a better grip for holding thin branches (29). The lack of this muscle in the giant panda is likely a consequence of the long history of the ursids as terrestrial carnivores. It follows that the hypertrophy of the radial sesamoid in Ailuridae was not originally selected because of its advantages for food manipulation, but rather for improved grasp during arboreal locomotion. This hypothesis is further supported by the fact that some basal arctoids, such as Amphicynodon of the Early Oligocene of Europe, display arboreal adaptations in the skeleton (30).
Over a quarter of a century ago, Stephen Jay Gould celebrated the concept of contingency in evolution as illustrated by the development of the giant panda's false thumb (31). The evidence presented in this article, indicating that the false thumb of the red panda did not originally evolve for its present function of grasping bamboo, but rather shifted from its earlier function as an aid in arboreal locomotion, provides an even more striking example of how evolution works opportunistically from the available materials.
Acknowledgments
We thank J. F. Pastor, from the Museo Anatómico (Valladolid, Spain), for access to osteological specimens. We also thank the Comunidad Autónoma de Madrid (Dirección General de Patrimonio Histórico) for the continuous funding support and research permissions. This study is part of Research Projects BTE2002-00410 (to J.M.) (Dirección General de Investigación; Ministerio de Ciencia y Tecnología) and EX2003-0243 (to M.J.S.) (Secretaría de Estado de Educación y Universidades and European Social Funding). National Geographic Society Grant 6964-01 (to J.M.) and the Fondation Singer-Polignac (Paris) provided additional support.
Author contributions: M.J.S., M.A., S.P., and J.M. designed research, performed research, and wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Pozo, M., Calvo, J. P., Silva, P., Morales, J., Peláez-Campomanes, P. & Nieto, M. (2004) Geogaceta 35, 143-146. [Google Scholar]
- 2.Morales, J., Alcalá, L., Alvárez-Sierra, M. A., Antón, M., Azanza, B., Calvo, J. P., Carrasco, P., Fraile, S., García-Paredes, I., Gómez, E., et al. (2004) Geogaceta 35, 139-142. [Google Scholar]
- 3.Peigné, S., Salesa, M. J., Antón, M. & Morales, J. (2005) Acta Palaeontol. Pol. 50, 219-238. [Google Scholar]
- 4.Gambaryan, P. P. (1974) How Mammals Run: Anatomical Adaptations (Wiley, New York).
- 5.Taylor, M. E. (1989) in Carnivore Behavior, Ecology, and Evolution, ed. Gittleman, J. L. (Cornell Univ. Press, Ithaca, New York), Vol. 1, pp. 382-409. [Google Scholar]
- 6.Davis, D. D. (1964) Fieldiana Zool. 3, 1-339. [Google Scholar]
- 7.Chorn, J. & Hoffmann, R. S. (1978) Mamm. Species 110, 1-6. [Google Scholar]
- 8.Roberts, M. S. & Gittleman, J. L. (1984) Mamm. Species 222, 1-8. [Google Scholar]
- 9.Endo, H., Hayashi, Y., Yamagiwa, D., Kurohmaru, M., Koie, H., Yamaya, Y. & Kimura, J. (1999) J. Anat. 195, 295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endo, H., Yamagiwa, D., Hayashi, Y., Koie, H., Yamaya, Y. & Kimura, J. (1999) Nature 397, 309-310. [DOI] [PubMed] [Google Scholar]
- 11.Ledje, C. & Arnason, U. (1996) J. Mol. Evol. 42, 135-144. [DOI] [PubMed] [Google Scholar]
- 12.Goldman, D., Rathna Giri, P. & O'Brien, S. J. (1989) Evolution 43, 282-295. [DOI] [PubMed] [Google Scholar]
- 13.Bininda-Emonds, O. R., Gittleman, J. L. & Purvis, A. (1999) Biol. Rev. Camb. Philos. Soc. 74, 143-175. [DOI] [PubMed] [Google Scholar]
- 14.Flynn, J. J., Finarelli, J. A., Zehr, S., Hsu, J. & Nedbal, M. A. (2005) Syst. Biol. 54, 317-337. [DOI] [PubMed] [Google Scholar]
- 15.Flynn, J. J., Nedbal, M. A., Dragoo, J. W. & Honeycutt, R. L. (2000) Mol. Phylogenet. Evol. 17, 190-199. [DOI] [PubMed] [Google Scholar]
- 16.Flynn, J. J. & Nedbal, M. A. (1998) Mol. Phylogenet. Evol. 9, 414-426. [DOI] [PubMed] [Google Scholar]
- 17.Ginsburg, L. (1999) in The Miocene Land Mammals of Europe, eds. Rössner, G.-E. & Heissig, K. (Pfeil, Munich), pp. 109-148.
- 18.O'Brien, S. J., Nash, W. G., Wildt, D. E., Bush, M. E. & Benveniste, R. E. (1985) Nature 317, 140-144. [DOI] [PubMed] [Google Scholar]
- 19.Wang, X. (1997) J. Vert. Paleontol. 17, 184-198. [Google Scholar]
- 20.Wozencraft, W. C. (1989) in Carnivore Behavior, Ecology, and Evolution, ed. Gittleman, J.-L. (Cornell Univ. Press, Ithaca, New York),Vol. 1, pp. 495-535. [Google Scholar]
- 21.Wyss, A. R. & Flynn, J. J. (1993) in Placentals, Mammal Phylogeny, eds. Szalay, F., Novacek, M. & McKenna, M. (Springer, New York), pp. 32-52.
- 22.Vrana, P. B., Milinkovitch, M. C., Powell, J. R. & Wheeler, W. C. (1994) Mol. Phylogenet. Evol. 3, 47-58. [DOI] [PubMed] [Google Scholar]
- 23.Wayne, R. K., Benveniste, R. E., Janczewski, D. N. & O'Brien, S. J. (1989) in Carnivore Behavior, Ecology, and Evolution, ed. Gittleman, J. L. (Cornell Univ. Press, Ithaca, New York), Vol. 1, pp. 465-494. [Google Scholar]
- 24.Roussakis, S. J. (2001) Senckenb. Lethaea 81, 347-358. [Google Scholar]
- 25.Baskin, J. (1998) in Evolution of Tertiary Mammals of North America, eds. Janis, C. M., Scott, K. M. & Jacobs, L. L. (Cambridge Univ. Press, Cambridge, U.K.), Vol. 1, pp. 144-151. [Google Scholar]
- 26.Ginsburg, L., Morales, J., Soria, D. & Herráez, E. (1997) C. R. Acad. Sci. 325, 447-451. [Google Scholar]
- 27.Ginsburg, L., Maridet, O. & Mein, P. (2001) Geodiversitas 23, 81-85. [Google Scholar]
- 28.Wallace, S. C. & Wang, X. (2004) Nature 431, 556-559. [DOI] [PubMed] [Google Scholar]
- 29.McClearn, D. (1992) J. Mamm. 73, 245-261. [Google Scholar]
- 30.Cirot, E. (1992) Ph.D. thesis (Université de Poitiers, Poitiers, France).
- 31.Gould, S. J. (1980) The Panda's Thumb (Norton, New York).
- 32.de Beaumont, G. (1982) Arch. Sci. Geneve 35, 143-152. [Google Scholar]