Abstract
Water-specific aquaporins (AQP), such as the prototypical mammalian AQP1, stringently exclude the passage of solutes, ions, and even protons. Supposedly, this is accomplished by two conserved regions within the pore, a pair of canonical asparagine–proline–alanine (NPA) motifs, the central constriction, and an aromatic/arginine (ar/R) constriction, the outer constriction. Here, we analyzed the function of three residues in the ar/R constriction (Phe-56, His-180, and Arg-195) in rat AQP1. Individual or joint replacement of His-180 and Arg-195 by alanine and valine residues, respectively (AQP1-H180A, AQP1-R195V, and AQP1-H180A/R195V), did not affect water permeability. The double mutant AQP1-H180A/R195V allowed urea to pass. In line with the predicted solute discrimination by size, replacement of both Phe-56 and His-180 (AQP1-F56A/H180A) enlarged the maximal diameter of the ar/R constriction 3-fold and enabled glycerol and urea to pass. We further show that ammonia passes through all four AQP1 mutants, as determined (i) by growth complementation of yeast deletion strains with ammonia, (ii) by ammonia uptake from the external solution into oocytes, and (iii) by direct recordings of ammonia induced proton currents in oocytes. Unexpectedly, removal of the positive charge in the ar/R constriction in AQP1-R195V and AQP1-H180A/R195V appeared to allow the passage of protons through AQP1. The data indicate that the ar/R constriction is a major checkpoint for solute permeability, and that the exquisite electrostatic proton barrier in AQPs comprises both the NPA constriction as well as the ar/R constriction.
Keywords: mutational analysis, proton filter, solute selectivity
Orthodox aquaporins (AQPs) constitute one branch of water-conducting channels within the superfamily of major intrinsic proteins (1). In recent years, the protein structure of the prototypical mammalian AQP1 has been refined to 2.2-Å resolution (2–5). Two highly conserved structural features within the channel were proposed as filters that exclude the passage of solutes larger than water and of charged molecules, including protons. A central constriction is formed by the capping amino acids Asn-Pro-Ala (NPA constriction) at each positive end of two short α-helices, such that the two NPA motifs are pinched in the center of the pore. Proline and alanine are exchangeable to some extent, whereas asparagine is invariable (1). Extensive molecular dynamics/quantum mechanical simulations suggest that the free energy barrier located at the NPA constriction predominates in the exclusion of protons (reviewed in ref. 6). Depending on the computational approach, a second significant energy barrier, termed aromatic/arginine (ar/R) constriction, exists. It is located below the channel mouth and is even narrower than the central NPA constriction (6, 7). It is formed by four amino acids (Phe-56, His-180, Cys-189, and Arg-195 in rat AQP1). The ensemble of Phe, His, and Arg is highly conserved in orthodox AQPs. Their side chains directly flank the pore, whereas the less-conserved Cys-189 contributes with its backbone carbonyl oxygen to the ar/R constriction (Fig. 1A). Apart from the repulsion of positively charged ions by the arginine, pore selectivity appears to be based mainly on size exclusion. Indeed, the diameter of the ar/R constriction of orthodox AQPs perfectly fits the size of a water molecule (5). In solute channels, i.e., aquaglyceroporins, the ar/R constriction is wider and less polar (8, 9). This is due to replacement of histidine by a glycine, which makes room for an aromatic side chain from a neighboring tyrosine or phenylalanine (9). The current view holds that this architecture renders the ar/R constriction of the aquaglyceroporin permeable for glycerol and urea and concurrently impairs water permeability (5, 8).
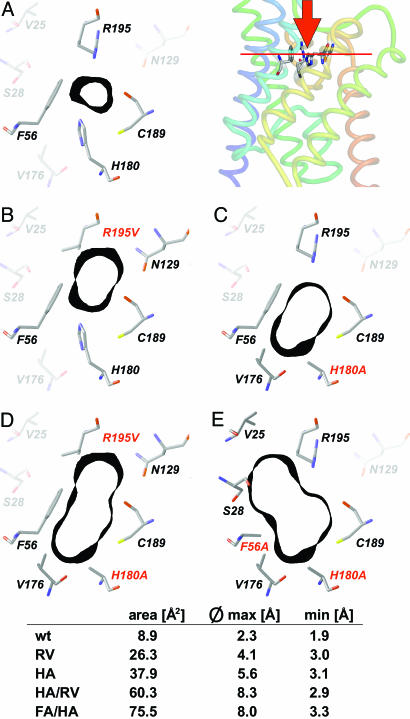
Fig. 1.
Shape of the ar/R constriction of AQP1 and mutants. For orientation, Top Right depicts a side view of the crystal structure of AQP1 (1J4N) with F56, H180, C189, and R195 as sticks (numbering according to the rat sequence; pymol software, DeLano Scientific, San Francisco). The red bar indicates the plane shown in the cross sections A–E.(A) Wild-type ar/R constriction. Amino acid residues that directly contribute to the constriction are drawn in full color. The black bands denote 1-Å-thick sections of the Connolly surface at this site (sybyl software, Tripos Associates, St. Louis). (B–E) Mutant outlines of ar/R constriction with individual mutations printed red. Calculations were based on the bovine AQP1 crystal structure (1J4N) and energy-optimized by using the sybyl threading algorithms. For additional description, see text.
Apart from these structure-based deliberations, an approach by targeted mutation has never been attempted to define the role of the ar/R constriction in the exquisite water specificity of AQPs and in the selection against other solutes including small ions. We asked whether solute permeability is defined by the diameter, the polarity, or both parameters of the ar/R constriction, and how these parameters affect passage of solutes. Therefore, we replaced Phe-56 and His-180 with the smaller alanine and Arg-195 with valine. Although the hydrophilicity around the ar/R constriction was considered instrumental for high water permeability (5, 8), the more hydrophobic environment did not affect water permeability but enabled passage of urea, glycerol, and even neutral ammonia. Surprisingly, removal of the arginine from the ar/R constriction (R195V) yielded what appeared to be proton-conducting AQP1 mutants. Obviously, mutants with a charge removal at a distance to the major electrostatic barrier, the NPA constriction, change proton conductivity by lowering this energy barrier, indicating an interaction and interdependence of both predicted energy barriers.
Results
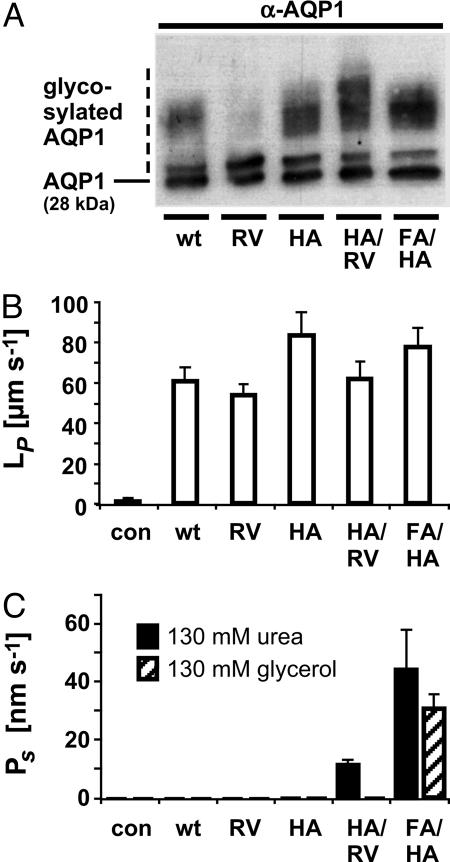
We generated the rat AQP1 mutants AQP1-H180A, AQP1-R195V, AQP1-H180A/R195V, and AQP1-F56A/H180A (red in Fig. 1 B–E). The effects of these mutations on the cross section of the ar/R constriction were calculated based on the AQP1 2.2-Å resolution structure (5). Mutation of Arg-195 to valine (R195V) removed a positive charge and expanded the pore area toward the side-chain amide of Asn-129 by 296% (Fig. 1 B and D), removal of the imidazole ring (H180A) enlarged the pore area by 426% toward Val-176 (Fig. 1 C and D), and replacement of Phe-56 (F56A) widened the outer constriction in direction to Val-25 and Ser-28 and increased the calculated area by 848% (Fig. 1E). In the oocyte system, expression levels of all mutants appeared to equal the wild-type AQP1, as shown by Western blotting using a specific antiserum (Fig. 2A). The slight differences observed in the degree of glycosylation (fuzzy upper bands in Fig. 2) do not affect permeability properties as reported earlier (10).
Fig. 2.
Water and solute permeability of AQP1 mutants. (A) Expression of AQP1 wild-type and mutants in Xenopus oocytes determined by Western blotting. (B) Water permeability of AQP1 wild-type and mutants. (C) Urea and glycerol permeability. (n = 3 for controls [con], n = 5–12 for AQP1 and mutants; error bars denote SEMs.)
Water and Solute Permeability. Water permeability was assessed by osmosis-based oocyte assays. Water permeated all AQP1 mutants similar to the wild-type AQP1 despite pronounced differences in polarity at the ar/R constriction (Fig. 2B). Notably, even the rather hydrophobic environment in AQP1-H180A/R195V did not affect water permeability (Fig. 2B). The Arrhenius activation energies for water permeation of 5 kcal/mol were identical for AQP1 and each mutant (not shown). The above findings allowed us to directly compare all primary data of oocyte measurements without prior normalization.
We then tested in isosmotic oocyte swelling assays whether the widened pore diameters in the AQP1 mutants would allow small uncharged solutes, such as urea and glycerol, to pass. The single mutants, AQP1-R195V and AQP1-H180A, were impermeable (Fig. 2C). The double mutants AQP1-F56A/H180A and AQP1-H180A/R195V, however, were permeated by urea (Fig. 2C). Particularly, AQP1-F56A/H180A turned out to be a robust solute channel with permeability for urea of 45 nm·s-1 and a lesser permeability for glycerol of 26 nm·s-1 (Fig. 2C). Larger polyols such as erythritol (four carbons) or pentitols did not pass.
Ammonia Permeability. AQP1 effectively excludes the passage of ammonia species (11), whereas other AQPs, such as tonoplast intrinsic protein (TIP)2;1 and AQP8, have been reported to conduct ammonia (11, 12). At the ar/R constriction of TIP2;1 and AQP8, the position of the histidine is switched to that of phenylalanine in AQP1 (see Fig. 1 A). We asked whether the wider diameter of the ar/R constriction and the reduced polarity of the AQP1 mutants would enable 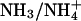 to pass.
to pass.
Initially, we used a yeast assay system based on the 31019bΔmep1-3 knock-out strain, which lacks all three endogenous ammonium transporters (13). AQP1 and its mutants were well expressed in this yeast strain (Fig. 3A). The cells were spotted on agar plates with 3 mM (NH4)2SO4 as the sole nitrogen source. Under these conditions, a slightly improved growth was observed in cells expressing AQP1-H180A or AQP1-F56A/H180A, potentially indicating uptake of NH3 or 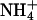 (Fig. 3B). The gain in proliferation was less pronounced at pH 5.5 than at pH 6.5 or pH 7.5 (Fig. 3B). In this pH range, the concentration of ammonia, NH3, increases from 0.53 μM at pH 5.5 to 5.3 μM at pH 6.5 and 53 μM at pH 7.5, whereas the relative ammonium,
(Fig. 3B). The gain in proliferation was less pronounced at pH 5.5 than at pH 6.5 or pH 7.5 (Fig. 3B). In this pH range, the concentration of ammonia, NH3, increases from 0.53 μM at pH 5.5 to 5.3 μM at pH 6.5 and 53 μM at pH 7.5, whereas the relative ammonium, 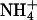 , concentration of 3 mM is barely affected. This indicated that ammonia permeated into the cells via AQP1-H180A or AQP1-F56A/H180A. The signal-to-noise ratio in this assay, however, was disappointingly small, because substantial growth of the 31019bΔmep1-3 strain as a control occurred possibly because of NH3 uptake through the endogenous yeast aquaglyceroporin Fps1 (12). This may have obscured the detection of NH3 permeability in the AQP1 mutants.
, concentration of 3 mM is barely affected. This indicated that ammonia permeated into the cells via AQP1-H180A or AQP1-F56A/H180A. The signal-to-noise ratio in this assay, however, was disappointingly small, because substantial growth of the 31019bΔmep1-3 strain as a control occurred possibly because of NH3 uptake through the endogenous yeast aquaglyceroporin Fps1 (12). This may have obscured the detection of NH3 permeability in the AQP1 mutants.
Fig. 3.
Yeast growth complementation on 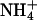 and methylammonium plates by AQP1 mutants. (A) Expression control of AQP1 and mutants in 31019bΔmep1-3 (endogenous
and methylammonium plates by AQP1 mutants. (A) Expression control of AQP1 and mutants in 31019bΔmep1-3 (endogenous 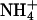 transporters deleted) by Western blotting. (B) 31019bΔmep1-3 expressing wild-type or mutant AQP1 were spotted at none, 1:102, and 1:104 dilutions (left to right) on agar plates containing 3 mM (NH4)2SO4 as a nitrogen source at the indicated pH values. Shown is the cell growth after 5 days. Here, cell growth indicates NH3 uptake. (B) BY4742Δfps1 yeast (deletion of the endogenous aquaglyceroporin Fps1) expressing wild-type or mutant AQP1 spotted at none, 1:10, and 1:100 dilutions (left to right) on selective medium containing 0.1% proline as the nitrogen source and 100 mM cytotoxic methylammonium at the indicated pH values. Cell growth was monitored after 5 days. The isogenic parent strain BY4742 (top row) as well as sham-transformed BY4742Δfps1 yeast (second row) were used as controls. Cell growth indicates passage of methylamine through AQP1 mutants (see text for further explanations).
transporters deleted) by Western blotting. (B) 31019bΔmep1-3 expressing wild-type or mutant AQP1 were spotted at none, 1:102, and 1:104 dilutions (left to right) on agar plates containing 3 mM (NH4)2SO4 as a nitrogen source at the indicated pH values. Shown is the cell growth after 5 days. Here, cell growth indicates NH3 uptake. (B) BY4742Δfps1 yeast (deletion of the endogenous aquaglyceroporin Fps1) expressing wild-type or mutant AQP1 spotted at none, 1:10, and 1:100 dilutions (left to right) on selective medium containing 0.1% proline as the nitrogen source and 100 mM cytotoxic methylammonium at the indicated pH values. Cell growth was monitored after 5 days. The isogenic parent strain BY4742 (top row) as well as sham-transformed BY4742Δfps1 yeast (second row) were used as controls. Cell growth indicates passage of methylamine through AQP1 mutants (see text for further explanations).
Ammonia passage through the yeast aquaglyceroporin Fps1 apparently has never been investigated before. Therefore, we used yeast strain BY4742Δfps1, which lacks the aquaglyceroporin Fps1, for growth complementation with proline as a nitrogen source. The medium was supplemented with 100 mM of the cytotoxic methylammonium, which is imported unidirectionally by the yeast ammonium transporters Mep1-3 (14, 15). Growth of the BY4742Δfps1 yeast deletion strain was minimal (Fig. 3C), indicating that efflux of the toxic methylamine was impossible, whereas the corresponding wild-type strain, BY4742, grew well in the presence of methylamine (Fig. 3C). The pH dependency of the phenotype unequivocally reflected the outward direction of methylamine release, because cell growth improved dramatically toward acidic pH values when the concentration gradient is increased due to protonation of methylamine (Fig. 3C). This finding on the side established that the yeast aquaglyceroporin Fps1 passes methylamine, and most likely ammonia, and readily explained why the 31019bΔmep1-3 yeast strain proliferated quite well despite the absence of all 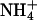 transporters. Thus, the use of the BY4742Δfps1 yeast deletion strain represents a significant technical advance to examine ammonia passage through AQPs.
transporters. Thus, the use of the BY4742Δfps1 yeast deletion strain represents a significant technical advance to examine ammonia passage through AQPs.
We exploited this technique in an attempt to rescue the BY4742Δfps1 aquaglyceroporin deletion strain by the concomitant expression of rat AQP1 and the set of mutants. Wild-type AQP1 did not abrogate the growth defect on methylammonium plates (Fig. 3C). However, expression of any of the AQP1 mutants of this study led to an excellent pH-dependent cell growth (Fig. 3C). As expected, lower pH values enhanced BY4742Δfps1 proliferation because of the increase of the methylamine gradient from the cytosol to the medium. This established that all rat AQP1 mutants readily passed the uncharged methylamine.
To confirm and quantify these findings, we used the Xenopus expression system and determined ammonia influx by measurements of acidification of the weakly buffered medium (11, 16). In case oocytes take up ammonia faster than ammonium ions, the pH in the medium drops. In control or AQP1-injected oocytes, the pH dropped only slightly during 10 min (Table 1). All mutants passed ammonia significantly faster, as seen by the more rapid decline of the external pH, yet quantitative differences between individual mutants existed (see statistics in Table 1). It appeared that the effects of the single mutants AQP1-H180A and AQP1-R195V were additive in the double-mutant AQP1-H180A/R195V, in which all potential positive charges of the ar/R constriction were removed.
Table 1. Medium acidification by ammonia uptake of oocytes expressing AQP1 wild-type and mutants.
| Acidification rate | AQP1 | HA | RV | HA/RV | HA/FA |
|---|---|---|---|---|---|
| JH+ [10-10 mol H+ s-1 oocyte-1] | 0.41 ± 0.035 (5) | 0.70 ± 0.03 (4) | 0.58 ± 0.08 (5) | 1.6 ± 0.07 (4) | 0.56 ± 0.03 (5) |
| P < 0.003 | P < 0.003 | P < 0.0005 | P < 0.008 |
Oocytes were transferred into weakly buffered medium containing 20 mM NH4Cl. The initial rate of acidification of the bathing solution (JH+) is indicated. (±SEM; n is given in parentheses). Uninjected controls were as AQP1-expressing oocytes.
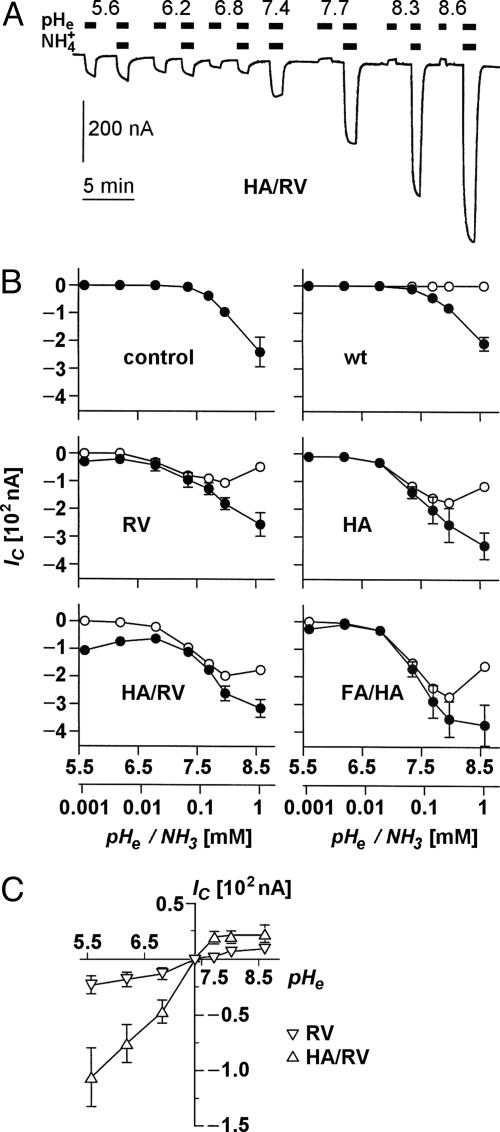
Ammonia-Induced Clamp Currents. In voltage-clamped oocytes, ammonia uptake is accompanied by an inwardly directed proton current to mitigate intracellular shifts toward alkaline pH (11, 16). We carried out clamp current recordings (-50 mV) with oocytes expressing wild-type or mutant AQP1 after an abrupt change from an ammonium-free medium to an isosmotic buffer containing 5 mM ammonium (Fig. 4A). By working in a range from pH 5.6 to pH 8.6, ammonia concentrations were varied over 3 orders of magnitude from 1.12 to 1,120 μM. Ammonia-induced currents for AQP1 and our set of mutants are shown in Fig. 4B (filled symbols). In oocytes expressing wild-type AQP1 as well as in uninjected controls, ammonia induced currents were absent at pH values <7.4; in the alkaline range, currents increased equally (Fig. 4 A and B Top), indicating an AQP1-independent ammonia uptake by diffusion across the cell membrane. Expression of our AQP1 mutants, however, induced significant currents already in the acidic range that increased sharply around pH 7.0 and approached a plateau around pH 8.0 (Fig. 4B, RV, HA, HA/RV, and FA/HA). With the mutants AQP1-R195V and AQP1-R195V/H180A, we unexpectedly noticed a substantial increase in the recorded currents toward lower pH values (Fig. 4B). This led us to test for proton passage through the AQP1 mutants in the same pH range as above but without addition of ammonium to the buffer.
Fig. 4.
Ammonia- and pH-induced proton currents in Xenopus oocytes expressing AQP1 wild-type and mutants. (A) Sample trace of clamp current (-50 mV) induced in an AQP1-H180A/R195V-expressing oocyte by changes in pHe and 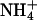 of the bathing solution. The baseline current was -100 nA. pHe was intermittently changed from 7.4 (control) to a range of values from 5.6 to 8.6 ± a concurrent change in
of the bathing solution. The baseline current was -100 nA. pHe was intermittently changed from 7.4 (control) to a range of values from 5.6 to 8.6 ± a concurrent change in 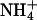 (5 mM Na+ was replaced by 5 mM
(5 mM Na+ was replaced by 5 mM 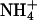 ), as indicated by the bars on top. In the acidic range, pHe changes resulted in inward currents that were independent of the presence of
), as indicated by the bars on top. In the acidic range, pHe changes resulted in inward currents that were independent of the presence of 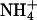 . In the alkaline range, changes in pHe resulted in small outward currents, whereas simultaneous addition of
. In the alkaline range, changes in pHe resulted in small outward currents, whereas simultaneous addition of 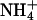 induced large inward currents. The LP of the oocyte was 9.6·10-3 cm·s-1. (B) Filled symbols: total clamp currents (-50 mV) induced by 5 mM
induced large inward currents. The LP of the oocyte was 9.6·10-3 cm·s-1. (B) Filled symbols: total clamp currents (-50 mV) induced by 5 mM 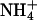 at various pHe values in uninjected oocytes (control) and oocytes expressing AQP1 wild-type and mutants. The open symbols represent the currents induced by AQP-facilitated ammonia passage, i.e., we subtracted the background current induced by ammonia diffusion (control, Top Left) and the current produced by ammonia-independent proton permeation of certain AQP1 mutants (depicted in C). (C) Clamp currents elicited exclusively by changes in pHe. For clarity, we show only the currents for AQP1-R195V and AQP1-H180A/R195V. The currents for AQP1 wild-type and the AQP1-H180A mutant were not significantly different from the miniscule currents already observed in uninjected control oocytes. The current for AQP1-F56A/H180A (not shown) was only significantly different at pHe 5.6, i.e., 17 ± 2 nA (n > 5 for AQPs and n = 3 for uninjected controls).
at various pHe values in uninjected oocytes (control) and oocytes expressing AQP1 wild-type and mutants. The open symbols represent the currents induced by AQP-facilitated ammonia passage, i.e., we subtracted the background current induced by ammonia diffusion (control, Top Left) and the current produced by ammonia-independent proton permeation of certain AQP1 mutants (depicted in C). (C) Clamp currents elicited exclusively by changes in pHe. For clarity, we show only the currents for AQP1-R195V and AQP1-H180A/R195V. The currents for AQP1 wild-type and the AQP1-H180A mutant were not significantly different from the miniscule currents already observed in uninjected control oocytes. The current for AQP1-F56A/H180A (not shown) was only significantly different at pHe 5.6, i.e., 17 ± 2 nA (n > 5 for AQPs and n = 3 for uninjected controls).
Proton Permeability. A graph showing pH-dependent proton currents is depicted in Fig. 4C. This clearly identified those AQP1 mutants with replacement of Arg-195, i.e., AQP1-R195V and more so AQP1-R195V/H180A, as proton passing pores. Proton flux occurred in both directions according to the established pH gradient. The clamp current was not affected if Na+ in the external solution was replaced by choline (0.95 ± 0.03, n = 3), as tested at external pH 5.6 in paired experiments on oocytes expressing AQP1-R195V/H180A. Accordingly, any effects of pH changes on Na+ leakage currents were ruled out.
To determine whether the effect of acidity on clamp currents was according to the Grotthuss mechanism, i.e., along a proton wire, we compared the currents at different pH values in H2O or D2O, because the mobility of D+ is 1.5 times lower than that of H+, whereas it is similar for H3O+ and D3O+ (17). In paired experiments, the clamp currents induced by changing bath acidity of D2O solution (pD) from 7.0 to 5.2 were equal to those induced by changing pH from 7.4 to 5.6 (0.99 ± 0.07, n = 5). Thus, the absence of an isotope effect allows us to conclude that protons did not permeate according to the Grotthuss mechanism (17). Accordingly, charge permeation through AQPs is most likely restricted by the electrostatic barrier repulsion within the pore as predicted by molecular dynamics/quantum mechanical simulations (6).
Correction of Ammonia-Induced Currents. Taken together, the above results indicated that the clamp current recordings in the presence of ammonium were composed of three individual components: (i) the background current induced by ammonia diffusion (Fig. 4B, control, Top Left), (ii) the current produced by ammonia-independent proton permeation of certain AQP1 mutants (Fig. 4C), and (iii) the current induced by aquaporin-facilitated ammonia passage. The latter component was isolated by subtraction of i and ii from the total current (represented by the filled symbols in Fig. 4B). Respectively, corrected curves are depicted by open symbols in Fig. 4B. From this it is evident that the AQP1 double mutants had the highest ammonia permeability (Fig. 4 D and E) followed by AQP1-H180A and AQP1-R195V.
Discussion
AQP1 certainly is the prototypical water channel in terms of its comprehensive functional characterization (2–5, 7, 18–21). It is thought that the polarity at the ar/R constriction of water-specific AQPs, which is due to Arg/His residues, is responsible for the isolation of single water molecules from its net of hydrogen bonds in water (5). Accordingly, loss of hydrogen donor/acceptor sites, such as removal of the histidine or arginine side chains, should reduce water permeability. Yet, our data show this is not the case, and water permeability and Arrhenius activation energies remained unaffected even in the double mutant AQP1-H180A/R195V. Actually, TgAQP from Toxoplasma gondii (22), TbAQP2 from Trypanosoma brucei (23), and a subfamily of plant tonoplast intrinsic proteins (24) have a valine or leucine instead of the arginine, and our data now indicate that His-180 or Arg-195 in AQP1 are dispensable for water sequestration. Perhaps, in the AQP1-R195V and AQP1-H180A/R195V mutants, Asn-129 can replace Arg-195 as an alternative hydrogen bond donor and contribute to the isolation of water molecules (Fig. 1 B and D).
The pore layout of AQP1-H180A (Fig. 1C) is dominated by Arg-195 as a polar residue and is reminiscent of aquaglyceroporin constriction sites (9). The AQP1-H180A water permeability is at wild-type level. Additional elimination of the phenyl ring (AQP1-F56A/H180A) enhances polarity by providing access to the backbone and side-chain oxygens of Ser-28, yet it has no effect on water flux. The data imply, then, that AQP1 water permeability is independent of the polarity at the ar/R constriction.
The constriction diameter expectedly determined solute permeability. The planar urea molecules match the predicted pore aperture of AQP1-H180A/R195V (Fig. 1D). Glycerol permeability was observed only after removal of the obstructive F56 side chain. This enabled entry of three consecutive carbon hydroxyls, yet chain elongation abruptly became prohibitive. Thus, the 20-Å-long channel interior probably contains additional constraints, e.g., the bulky Phe-24 opposite to the NPA constriction (7).
Size exclusion alone cannot account for selectivity against ammonia, because it has the same dimension as a water molecule and a dipole moment close to that of water (1.5 vs. 1.8 debye). In the conjugated acid, 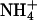 , the positive charge is distributed over the entire molecule, and the volume of
, the positive charge is distributed over the entire molecule, and the volume of 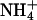 closely resembles that of K+.
closely resembles that of K+. 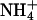 channels, such as AmtB from Escherichia coli, use a two-step process to discriminate between
channels, such as AmtB from Escherichia coli, use a two-step process to discriminate between 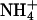 and K+ (25). First, a proton is abstracted, and then NH3 is passed through the lipophilic tube and a proton is reacquired. Because of the deprotonation step, this mechanism is pH-independent. The similarity of the hydrophobic pore lining between AmtB, and essentially all AQPs, suggests that the AQPs should pass NH3, yet this is not the case. Our data indicate that NH3 permeability depends on the makeup of the ar/R constriction. Comparing different classes of AQPs that pass or do not pass NH3, it appears that hydrophobic niches at the entry site are needed to permit NH3 passage. Indeed, NH3 permeability correlated well with the lipophilicity of the ar/R constriction present in the different AQP1 mutants (Fig. 4B). However, because AQPs lack the ability to abstract a proton from an
and K+ (25). First, a proton is abstracted, and then NH3 is passed through the lipophilic tube and a proton is reacquired. Because of the deprotonation step, this mechanism is pH-independent. The similarity of the hydrophobic pore lining between AmtB, and essentially all AQPs, suggests that the AQPs should pass NH3, yet this is not the case. Our data indicate that NH3 permeability depends on the makeup of the ar/R constriction. Comparing different classes of AQPs that pass or do not pass NH3, it appears that hydrophobic niches at the entry site are needed to permit NH3 passage. Indeed, NH3 permeability correlated well with the lipophilicity of the ar/R constriction present in the different AQP1 mutants (Fig. 4B). However, because AQPs lack the ability to abstract a proton from an 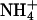 ion, ammonia passage relies on the pH-dependent concentration of uncharged NH3.
ion, ammonia passage relies on the pH-dependent concentration of uncharged NH3.
The most unexpected finding was the proton passage through those AQP1 mutants that lack the arginine of the ar/R constriction. How does this transit of protons and ammonia compare quantitatively to the passage of water? The experimentally determined water conductance, Lp, of a single oocyte that expresses wild-type or mutant AQP1, was ≈7·10-3 cm·sec-1 (Fig. 2B). Earlier, the unit conductance, Lp, for a single wild-type AQP1 was determined to be ≈3·10-14 cm3·sec-1 (26). Thus, it can be calculated that the oocytes express ≈1011 AQP1 copies per cm2 membrane. The rates at which the oocytes acidify the weakly buffered medium (Table 1) yield an ammonia permeability (PNH3) of ≈10-4 cm·sec-1 for AQP1-R195V, AQP1-H180A, and AQP1-F56A/H180A and ≈2·10-4 cm·sec-1 for the AQP1-H180A/R195V mutant. At an expression level of 1011·cm-2, this translates into a unit ammonia conductance of ≈10-15 cm3·sec-1, i.e., ammonia permeability is one-tenth of the water permeability. These values of PNH3 are similar to those reported for the mammalian AQPs AQP3, -7, -8, -9, and the plant AQP tonoplast intrinsic protein 2;1 (11).
The ability of the mutants to pass current under clamp conditions was by far smaller than their ability to support permeation of uncharged molecules. With a PNH3 of 10-4 cm·sec-1, the electroneutral flux of ammonia amounts to 10-10 M·cm-2·sec-1 at 1 mM ammonia (i.e., 5 mM of extracellular NH4Cl at pH 8.6). This is 100-fold higher than the fluxes that are derived from the clamp currents obtained under identical conditions (Fig. 4). At a clamp potential of -50 mV, the AQP-expressing oocytes have a conductance of up to 1 μA cm-2 upon stimulation by ammonia and of up to 160 nA·cm-2 upon stimulation by low pH, equivalent to conductances in the order of 10-5 S·cm-2. Relating these values to the expression density of AQP1, this translates to an AQP1 unit conductance of 10-16 S, i.e., by far less than that of canonical ion channels, which have conductances of 10-12 S or higher. Accordingly, the permeation rate of charged molecules is miniscule compared with the rate of water permeation. At a transmembrane osmotic gradient of 1 mOsm, it is calculated that each AQP passes 105 water molecules per second, whereas the charge transfer by the AQP1 mutants as determined here is equivalent to the passage of ≈50 ions per second.
Our data not only provide experimental evidence that the ar/R constriction constitutes a major selectivity filter in orthodox AQPs, but they also show that the structural makeup of AQP1 ensures that transit of protons is completely blocked. Considering the high copy number of AQPs in the membrane, e.g., almost 200,000 AQP1 molecules per red cell (27), this seems to be a physiologically meaningful precaution to maintain cellular homeostasis. Finally, the set of AQP1 mutants has initiated work to adapt and improve theoretical models on two core functions of a water-specific channel protein, i.e., isolation of a molecule from bulk water and exclusion of protons.
Materials and Methods
Cloning of AQP1 Mutants. Point mutations were introduced into rat AQP1 (GenBank accession no. NM_012778) by PCR by using the corresponding mutation primers and nearby restriction sites (primer list is available from E.B.). In this way, the mutants AQP1-R195V, AQP1-H180A, AQP1-R195V/H180A, and AQP1-F56A/H180A were generated. Fidelity of all constructs was verified by DNA sequencing. They were subcloned into the HindIII/XbaI sites of pOG1 for cRNA transcription and into the SmaI site of pDR196 for expression in yeast.
Expression in Xenopus Oocytes and Swelling Assays. After linearization of the AQP1 constructs in the pOG1 vector with NotI, T7 RNA polymerase was used for reverse transcription (mMessage mMachine kit, Ambion, Austin, TX). Five nanograms of cRNA was injected into defolliculated Xenopus laevis oocytes. The oocytes were incubated for 3–4 days in ND96 buffer (96 mM NaCl/2 mM KCl/1.8 mM CaCl2/1 mM MgCl2/5 mM Hepes, pH 7.4) at 15°C. AQP1 expression was checked by Western blotting of the membrane proteins of a single oocyte by using a specific AQP1 antiserum (gift of L. King, Johns Hopkins University, Baltimore). Osmotic water permeability, Lp [μm/s], was derived from the rate of initial cell shrinkage (10 s) observed upon addition of 20 mOsm of mannitol and is given per true oocyte surface area (0.53 cm2; ref. 28). Isosmotic permeability for urea and glycerol was tested by transferring oocytes that were injected with both wild-type AQP1 and the test mutant into a medium in which 65 mM NaCl was replaced by 130 mM solute (29). The coefficient of solute permeability, Ps [μm/s], was calculated from Ps = [osmtotal·V0·d(V/V0)/dt]/[S· (solout - solin)] (30) with osmtotal = 300 mOsm (total osmolarity of the system), and (solout - solin) = 130 mOsm (osmotic solute gradient), V0 = 9·10-4 cm3 (initial oocyte volume), d(V/V0)/dt (relative volume increase in s-1), S = 0.53 cm2 (true oocyte surface area; ref. 28).
Expression in Yeast and Growth Assays. To analyze ammonia transport, we used yeast strain 31019bΔmep1-3 (MATa ura3 mep1Δ mep2Δ::LEU2 mep3Δ::KanMX), which lacks all endogenous ammonium transporters (13). Yeast cells expressing wild-type or mutant AQP1 from the respective pDR196 plasmids (31) were grown overnight in liquid minimum YNB-glucose medium (3%; Difco) supplemented with 20 mM (NH4)2SO4. The cultures were diluted to an OD600 of 1, and 10 μl of a series of three dilutions (1:10 or 1:100 steps) were spotted on agar plates of Mes- or 4-morpholinepropanesulfonic (Mops) acid-buffered (20 mM, pH 5.5, pH 6.5, and pH 7.5) YNB-glucose medium containing 3 mM (NH4)2SO4 as the sole nitrogen source. For growth complementation with methylammonium, yeast strain BY4742Δfps1 (MATα his3-1 leu2Δ0 lys2Δ0 ura3Δ0 fps1::LEU2) lacking the endogenous aquaglyceroporin Fps1 and its isogenic parent strain BY4742, both from Euroscarf (www.unifrankfurt.de/fb15/mikro/euroscarf), were used. Growth complementation assay was carried out on agar plates of Mes- or Mops acid-buffered (20 mM, pH 5.5, pH 6.5, and pH 7.5) YNB-glucose medium supplemented with 0.1% proline as a nitrogen source and 100 mM methylammonium (Sigma). After 5 days of incubation, differences in growth were judged by eye.
Medium Acidification. Measurements of ammonia uptake (32) were adapted to oocytes (11). In brief, 20 oocytes were washed twice in nonbuffered saline and placed in a 8- by 2-mm steel net. The net was transferred into 2 ml of a well-stirred test solution of low buffer capacity (0.625 mM·pH-1) containing 70 mM NaCl, 20 mM mannitol, 20 mM NH4Cl, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 0.1 mM Hepes, pH 7.4, totaling 214 mOsm. pH changes were measured by using a small pH-electrode (XC 161 microelectrode, Radiometer, Copenhagen) inserted through a tight-sealing Teflon lid. Student's t test was used for comparison.
Electrophysiology. Measurements of clamp currents were performed as described (11, 33). Briefly, oocytes were placed in a continuously perfused 30-μl chamber in which the bathing solution could be changed within 5 s (90% complete). The oocytes were impaled with two microelectrodes to clamp the membrane potential to -50 mV and to record currents. The control bathing solution contained 90 mM NaCl, 20 mM mannitol, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 10 mM Hepes or Tris, pH 7.4, totaling 214 mOsm. For recording ammonia-induced proton currents, 5 mM NaCl was isosmotically replaced by NH4Cl, and the pH was adjusted to 5.6, 6.4, 6.8, 7.4, 7.7, 8.0, or 8.6. Na+-free solutions were obtained by replacement with choline chloride. In some experiments, H2O was replaced by deuterium oxide, D2O. The reading of acidity of such solutions by glass pH electrodes (pD) deviates from the true pD by 0.40 units (17, 34). To obtain an acidity equivalent to 7.4, for example, D2O solutions were adjusted to a nominal pH of 7.0. Resulting currents were recorded for 60 s.
Acknowledgments
T. Soland, B. Lynderup, and S. Christoffersen are thanked for expert technical assistance. We are grateful to L. King for providing the anti-AQP1 antibody. We thank D. Domeyer for generating the AQP1 structure models and P. Krippeit-Drews for help with the Xenopus frogs. This work was funded by the Deutsche Forschungsgemeinschaft (Be2253/2) and the European Union (LSHP-CT-2004-012189). T.Z. and L.M.H. were supported by the Nordic Centre of Excellence Program in Molecular Medicine, the Danish Research Council, the Lundbeck Foundation, the Augustinus Foundation, and the Ib Henriksens Foundation.
Author contributions: E.B., B.W., and T.Z. designed research; E.B., B.W., L.M.H., and T.Z. performed research; E.B., B.W., L.M.H., J.E.S., and T.Z. analyzed data; and E.B., B.W., J.E.S., and T.Z. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AQPn, aquaporin n; NPA, asparagine–proline–alanine sequence; ar/R, aromatic/arginine.
References
- 1.Zardoya, R. (2005) Biol. Cell 97, 397-414. [DOI] [PubMed] [Google Scholar]
- 2.Walz, T., Hirai, T., Murata, K., Heymann, J. B., Mitsuoka, K., Fujiyoshi, Y., Smith, B. L., Agre, P. & Engel, A. (1997) Nature 387, 624-627. [DOI] [PubMed] [Google Scholar]
- 3.Cheng, A., van Hoek, A. N., Yeager, M., Verkman, A. S. & Mitra, A. K. (1997) Nature 387, 627-630. [DOI] [PubMed] [Google Scholar]
- 4.Murata, K., Mitsuoka, K., Hirai, T., Walz, T., Agre, P., Heymann, J. B., Engel, A. & Fujiyoshi, Y. (2001) Nature 407, 599-605. [DOI] [PubMed] [Google Scholar]
- 5.Sui, H., Han, B. G., Lee, J. K., Walian, P. & Jap, B. K. (2001) Nature 414, 872-878. [DOI] [PubMed] [Google Scholar]
- 6.de Groot, B. L. & Grubmüller, H. (2005) Curr. Opin. Struct. Biol. 15, 176-183. [DOI] [PubMed] [Google Scholar]
- 7.de Groot, B. L. & Grubmüller, H. (2001) Science 294, 2353-2357. [DOI] [PubMed] [Google Scholar]
- 8.Tajkhorshid, E., Nollert, P., Jensen, M. O., Miercke, L. J., O'Connell, J., Stroud, R. M. & Schulten, K. (2002) Science 296, 525-530. [DOI] [PubMed] [Google Scholar]
- 9.Fu, D., Libson, A., Miercke, L. J., Weitzman, C., Nollert, P., Krucinski, J. & Stroud, R. M. (2000) Science 290, 481-486. [DOI] [PubMed] [Google Scholar]
- 10.van Hoek, A. N, Wiener, M. C., Verbavatz, J. M., Brown, D., Lipniunas, P. H., Townsend, R. R. & Verkman, A. S. (1995) Biochemistry 34, 2212-2219. [DOI] [PubMed] [Google Scholar]
- 11.Holm, L. M., Jahn, T. P., Møller, A. L. B., Schjoerring, J. K., Ferri, D., Klærke, D. A. & Zeuthen, T. (2005) Pflügers Arch. 450, 415-428. [DOI] [PubMed] [Google Scholar]
- 12.Luyten, K., Albertyn, J., Skibbe, W. F., Prior, B. A., Ramos, J., Thevelein, J. M. & Hohmann, S. (1995) EMBO J. 14, 1360-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marini, A. M., Soussi-Boudekou, S., Vissers, S. & Andre, B. (1997) Mol. Cell Biol. 17, 4282-4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marini, A. M., Matassi, G., Raynal, V., André, B., Cartron, J.-P. & Chérif-Zahar, B. (2000) Nat. Genet. 26, 341-344. [DOI] [PubMed] [Google Scholar]
- 15.Marini, A. M., Vissers, S., Urrestarazu, A. & André, B. (1994) EMBO J. 13, 3456-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jahn, T. P., Møller, A. L. B., Zeuthen, T., Holm, L. M., Klærke, D. A., Mohsin, B., Kühlbrandt, W. & Schjoerring, J. K. (2004) FEBS Lett. 574, 31-36. [DOI] [PubMed] [Google Scholar]
- 17.DeCoursey, T. E. & Cherney, V. V. (1997) J. Gen. Physiol. 109, 415-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agre, P., Borgnia, M. J., Yasui, M., Neely, J. D., Carbrey, J., Kozono, D., Beitz, E., Hoffert, J., Leitch, V. & King, L. S. (2001) Curr. Top. Membr. 51, 1-38. [Google Scholar]
- 19.de Groot, B. L., Frigato, T., Helms, V. & Grubmüller, H. (2003) J. Mol. Biol. 333, 279-293. [DOI] [PubMed] [Google Scholar]
- 20.Zhu, F., Tajkhorshid, E. & Schulten, K. (2004) Biophys. J. 86, 50-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vidossich, P., Cascella, M., Carloni, P. (2004) Proteins 55, 924-931. [DOI] [PubMed] [Google Scholar]
- 22.Pavlovic-Djuranovic, S., Schultz, J. E. & Beitz, E. (2003) FEBS Lett. 555, 500-504. [DOI] [PubMed] [Google Scholar]
- 23.Uzcategui, N., Szialles, A., Pavlovic-Djuranovic, S., Palmada, M., Figarella, K., Boehmer, C., Lang, F., Beitz, E. & Duszenko, M. (2004) J. Biol. Chem. 279, 42669-42676. [DOI] [PubMed] [Google Scholar]
- 24.Maurel, C., Reizer, J., Schroeder, J. J. & Chrispeels, M. J. (1993) EMBO J. 12, 2241-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khademi, S., O'Connell, J., Remis, J., Robles-Colmenares, Y., Miercke, L. J., Stroud, R. M. (2004) Science 305, 1587-1594. [DOI] [PubMed] [Google Scholar]
- 26.Engel, A. & Stahlberg, H. (2002) Int. Rev. Cytol. 215, 75-104. [DOI] [PubMed] [Google Scholar]
- 27.Denker, B. M., Smith, B. L., Kuhajda, F. P. & Agre, P. (1988) J. Biol. Chem. 263, 15634-15642. [PubMed] [Google Scholar]
- 28.Zampighi, G. A., Kreman, M., Boorer, K. J., Loo, D. D., Bezanilla, F., Chandy, G., Hall, J. E. & Wright, E. M. (1995) J. Membr. Biol. 148, 65-78. [DOI] [PubMed] [Google Scholar]
- 29.Hansen, M., Kun, J. F. J., Schultz, J. E. & Beitz, E. (2002) J. Biol. Chem. 277, 4874-4882. [DOI] [PubMed] [Google Scholar]
- 30.Zeidel, M. L., Suresh, V. A., Smith, B. L. & Agre, P. (1992) Biochemistry 31, 7436-7440. [DOI] [PubMed] [Google Scholar]
- 31.Rentsch, D., Laloi, M., Rouhara, I., Schmelzer, E., Delrot, S. & Frommer, W. B. (1995) FEBS Lett. 370, 264-268. [DOI] [PubMed] [Google Scholar]
- 32.Klocke, R. A., Anderson, K. K., Rotman, H. H. & Forster, R. E. (1972) Am. J. Physiol. 222, 1004-1013. [DOI] [PubMed] [Google Scholar]
- 33.Zeuthen, T., Meinild, A. K., Klærke, D. A., Loo, D. D., Wright, E. M., Belhage, B. & Litman, T. (1997) Biol. Cell 89, 307-312. [DOI] [PubMed] [Google Scholar]
- 34.Glasoe, P. K. & Long, F. A. (1960) J. Phys. Chem. 164, 189-190. [Google Scholar]