Abstract
Receptor-operated Ca2+ entry (ROCE) and store-operated Ca2+ entry (SOCE) are known to be inhibited by tyrosine kinase inhibitors and activation of C-type transient receptor potential channel (TRPC) isoform 3 (TRPC3), a cation channel thought to be involved in SOCE and/or ROCE, was recently shown to depend on src tyrosine kinase activity. What is not known is the step at which src acts on TRPC3 and whether the role for tyrosine kinases in ROCE or SOCE is a general phenomenon. Using in vitro and in cell protein-protein interaction assays we now report that src phosphorylates TRPC3 at Y226 and that formation of phospho-Y226 is essential for TRPC3 activation. This requirement is unique for TRPC3 because (i) mutation of the cognate tyrosines of the closely related TRPC6 and TRPC7 had no effect; (ii) TRPC6 and TRPC7 were activated in src-, yes-, and fyn-deficient cells; and (iii) src, but not yes or fyn, rescued TRPC3 activation in src-, yes-, and fyn-deficient cells. The Src homology 2 domain of src was found to interact with either the N or the C termini of all TRPCs, suggesting that other tyrosine kinases may play a role in ion fluxes mediated by TRPCs other than TRPC3. A side-by-side comparison of the effects of genistein (a general tyrosine kinase inhibitor) on endogenous ROCE and SOCE in mouse fibroblasts, HEK and COS-7 cells, and ROCE in HEK cells mediated by TRPC3, TRPC6, TRPC7, and TRPC5 showed differences that argue for ROCE and SOCE channels to be heterogeneous.
Keywords: G protein-coupled receptors, signal transduction, store depletion
Changes in cytosolic Ca2+ in nonexcitable cells in response to hormones and growth factors that activate C-type phospholipases (PLCs) are biphasic: An initial transient rise is followed by a less pronounced but sustained elevation (reviewed in refs. 1-3). This sustained phase of elevated Ca2+ persists until the hormones and growth factors that activate PLCs cease to act (4). The initial phase originates from the inositol trisphosphate-induced release of Ca2+ from cellular stores and is transient because of the action of membrane ATPases that pump Ca2+ back into the stores and out of the cells. The sustained phase of the increase in cytosolic Ca2+ requires the continued activation of a Ca2+ influx pathway to maintain sustained responses.
Although agonist-induced Ca2+ influx has been the object of many studies over the last 20 years, neither its molecular basis nor the mechanism of its activation are well understood. This type of Ca2+ influx is referred to as receptor-operated Ca2+ entry (ROCE). Store-operated Ca2+ entry (SOCE) is a related form of Ca2+ entry that may or may not involve the same molecular entities that are responsible for ROCE. SOCE can be triggered independently of PLC activation by mere depletion of the same stores that occurs upon inactivation of endogenous sarcoplasmic reticulum-endoplasmic reticulum Ca2+ ATPases or by simple chelation of Ca2+ that leaks from the stores into the cytoplasm. SOCE is also referred to as capacitative Ca2+ entry. Thus, capacitative Ca2+ entry and SOCE are operational terms used to indicate Ca2+ entry triggered by the depletion of the stores in the absence of PLC activation. As is the case for ROCE, the molecular basis for and mechanism(s) of activation of SOCE channels are poorly understood.
The C-type transient receptor potential ion channels (TRPCs) have been postulated as candidates for forming ROCE and/or capacitative Ca2+ entry channels (5-7). There are seven TRPC channels (TRPC1-TRPC7) (8, 9), which, when expressed in model cells, form Ca2+-permeable, largely nonselective cation channels. Expression and assembly studies have shown that TRPCs can form heteromeric channels (10), and all but TRPC6 can be activated by store depletion (reviewed in ref. 11). The main attribute not yet recapitulated by expressing TRPCs alone or in combination in model cells is the high Ca2+ selectivity seen for CCE channels in normal cells (ref. 12; reviewed in ref. 13).
A likely scenario by which SOCE occurs is one in which SOCE channels coexist with Ca2+-permeable nonselective ROCE channels (14). As is the case with SOCE channels, the activation mechanism(s) operating on ROCE channels also is largely a matter for speculation. Investigations into the factors and variables that affect activation of ROCE and SOCE may therefore be fruitful avenues to improving our molecular knowledge of how ROCE or SOCE come about.
One such variable is involvement of tyrosine phosphorylation in the activation of ROCE and SOCE. The original observation that tyrosine phosphorylation may be an important regulator participating in activation of ROCE and SOCE in nonexcitable cells came from studies showing an inhibitory effect of tyrosine kinase inhibitors on these forms of Ca2+ entry in human foreskin fibroblasts (15). This finding was followed by studies that showed total loss of bradykinin-stimulated ROCE and partial absence of SOCE in embryonic fibroblasts derived from mice lacking the src tyrosine kinase (16). Furthermore, evidence has accumulated in the literature showing that tyrosine phosphorylation is a common consequence associated with stimulation of cells that signal through the heterotrimeric G protein with αqβγ subunit composition (Gq)-PLC-Ca2+pathway (17-19). We therefore became interested in the possibility that tyrosine phosphorylation may be an activating signal for one of the events that leads to ROCE and SOCE. Indeed, experiments parallel to ours showed that in HEK cells the activation of TRPC3 by a PLC-stimulating G protein-coupled receptor (GPCR) is inhibited by inhibitors of tyrosine kinases and that, when expressed in src kinase negative cells, TRPC3 is not activated by Gq-coupled receptor (20). These data recapitulated the earlier findings with an endogenous (bradikynin-activated) GPCR acting via Gq activation on the endogenous complement of the ROCE pathway (16).
The present work extends these observations in three ways. First, we sought to establish whether the site of action of the src kinase was at the level of TRPC3 or some other rate-limiting step in the pathway leading from a Gq-coupled GPCR to TRPC3, including other molecules that may form part of the ROCE channel to which TRPC3 belongs. Second, we tested whether the requisite for a tyrosine phosphorylation step was unique to TRPC3 or whether other TRPCs tested in the same cellular context shared with TRPC3 the dependence of their activation on the src tyrosine kinase. Third, we sought to learn whether tyrosine kinase activity is a universal feature of ROCE and/or SOCE. We report that TRPC3 is a direct target of the src tyrosine kinase, and, although all TRPCs can be shown to interact physically with the src tyrosine kinase in vitro, only TRPC3 depends on phosphorylation by src. Furthermore, although inhibition of tyrosine phosphorylation can be shown to abolish SOCE in one cell type, tyrosine phosphorylation appears to play much less of a role in another cell type. We propose that these results reflect molecular complexities in the regulation of ROCE and SOCE, which, in turn, reflect the complexity and diversity of the molecular components that make up the ROCE and SOCE pathways.
Experimental Procedures
Materials, reagents, cDNAs, transfection methods, and ratiometric imaging protocols were as described in refs. 8 and 11. [Ba2+]i was calculated assuming that the affinity of Fura-2 for Ba2+ and the spectroscopic properties of the Fura-2:Ba2+ complex are equal to those of the Fura-2:Ca2+ complex. The GST pull-down assays, coimmunoprecipitation assays, and Western blotting were as described in Boulay et al. (21) using the antibodies mentioned in the legend for Fig. 1.
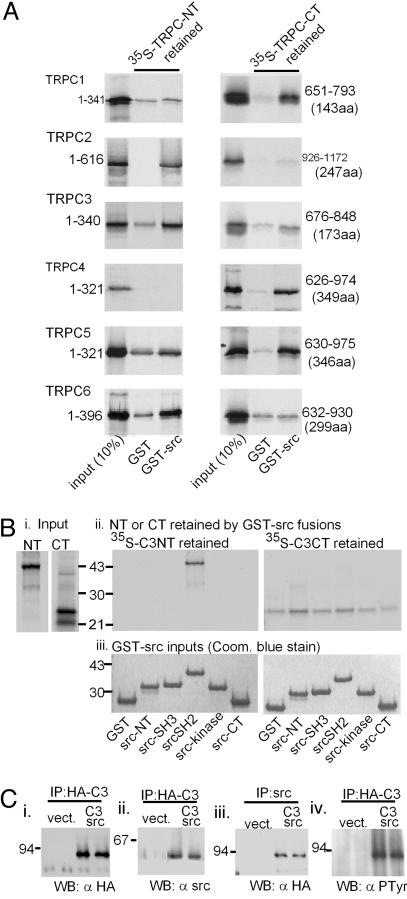
Fig. 1.
Interactions of src with TRPC channel proteins. (A) Src interacts with all members of the TRPC family of TRP-related channels. N termini (NT) and C termini (CT) of TRPC1-TRPC6 were translated in vitro in a reticulocyte lysate in the presence of 35S-labeled methionine and cysteine and incubated with a GST fusion protein of src bound to glutathione Sepharose. After washing, the radioactive N and C termini retained by the GST-src fusion protein were separated by SDS/PAGE and quantified by autoradiography. Samples equivalent to 10% of the N and C termini offered to the GST-src fusion protein were electrophoresed along-side. (Bi-Biii) The N terminus of TRPC3 interacts preferentially with the src homology 2 (SH2) domain of src.(Bi and Bii) Input components. (Bii) Retention of the 35S-labeled N terminus (Left) or C terminus (Right) of TRPC3 by the different domains of src (fused to GST). coom., Coomassie. (Ci-Civ) TRPC3 and src interact upon coexpression in COS-7 cells, and the TRPC3 associated with src reacts with anti-phosphotyrosine antibodies. COS-7 cells were transfected with empty vectors (vect) or vectors carrying insert coding for HA-tagged TRPC3 and src (C3 src). Lysates from the transfected cells were then immunoprecipitated with either anti-HA 12CA5 or anti-src mAb327 monoclonal antibodies. The precipitates were washed and subjected to 10% SDS/PAGE in duplicate, followed by electrophoretic transfer onto nitrocellulose membranes and immunostaining with 12CA5 α-HA, mAb327 α-src, or 4G10 (α-phosphotyrosine, Upstate Biotechnology, Lake Placid, NY) antibodies. IP, immunoprecipitation; WB, Western blot.
Interacting cellular proteins were crosslinked by overlaying transfected COS-7 cells in 100-mm dishes with 5 ml of 2 mM dithiolbis(succinimidylpropionate) (Pierce) in PBS (GIBCO) solution for 30 min at room temperature, followed by addition of an equal volume of 20 mM Tris·HCl, pH 7.4, for 15 min. The cells were lysed with 500 μl of ice-cold src lysis buffer (1% Triton X-100/150 mM NaCl/5 mM EDTA/5 μg/ml aprotinin/5 μg/ml leupeptin/1 μg/ml trypsin inhibitor). The lysates were cleared by incubation for 20 min on ice with 25 μl of a 1:1 slurry of protein-A Sepharose (Amersham Pharmacia) followed by centrifugation at 10,000 × g for 5 min. Cleared lysates were then used as sources for immunoprecipitation with the antibodies indicated in Fig. 1 and subjected to Western blot analysis.
GST fusion proteins were phosphorylated in vitro by immunoprecipitated src as follows. Src was expressed transiently in COS-7 cells and isolated from 500 μl of lysates prepared as described above (without the crosslinking step) by immunoprecipitation with mAb 327 bound to protein A Sepharose. The immunocomplex was washed once and resuspended in 150 μl of kinase buffer (10 mM Tris, pH 7.5/5 mM MnCl2/100 μM NaV04/1 mM NaF/1 mM sodium pyrophosphate). GST fusion proteins (≈1 μg in 5 μl of kinase buffer) were mixed with 5 μl of src immunocomplex in kinase buffer and brought to a final volume of 35 μl. The kinase reaction was started with the addition of 10 Ci/pmol (1 Ci = 37 GBq) [γ-32P]ATP at 1 μM and incubated for 60 min at room temperature. The reactions were terminated by addition of 40 μl of 2× Laemmli's sample buffer with 20% 2-mercaptoethanol and subjected to SDS/PAGE and autoradiography. For positive control, the GST fusion proteins were replaced with enolase (Sigma).
For confocal microscopy, HEK cells expressing hemagglutinin (HA)-tagged TRPC3 and its variants were fixed with 4% paraformaldehyde and immunostained with HA-Alexa Fluor 433 antibody. Images were acquired with a Carl Zeiss LSM 51M laser scanning microscope at a z resolution of 0.6 μm.
Results
TRPCs Interact with src and TRPC3 Is Phosphorylated at 4 of 11 N-Terminal Tryrosines. We first investigated whether TRPCs (including TRPC3) interacted with src in in vitro binding assays. The N and C termini of the TRPC channels were labeled with 35S by in vitro translation with a reticulocyte lysate-based transcription-translation system (TnT, Promega) and incubated with a GST-src fusion protein bound to glutathione agarose beads. The retention of the labeled TRPC fragment was visualized by autoradiography after electrophoretic separation. The GST-src fusion protein but not GST alone interacted in a stable manner with each of the tested TRPCs either by interacting with the N terminus (Fig. 1A Left, TRPC1, TRPC3, and TRPC6), the C terminus (Fig. 1A Right, TRPC2 and TRPC4), or both termini (Fig. 1A, TRPC5). Given the previous report that TRPC3 depends on src for its activation, we focused next on TRPC3-src interaction. Fig. 1B shows that TRPC3-src interaction occurs through the src homology 2 domain of src. A weak interaction could also be seen between the C terminus and GST-src. This interaction was highly variable and, thus, was not explored further.
To test the relevance of the TRPC3 interaction with src seen in the GST pull-down experiments in the context of a living cell, we coexpressed TRPC3 and src in COS-7 cells and asked whether expressed proteins could be coimmunoprecipitated from cell lysates. We found that TRPC:src complexes can be immunoprecipitated by using antibodies directed against TRPC3 (tagged with the HA epitope) or the src protein (Fig. 1C). Thus, immunoprecipitating HA-tagged protein brought down not only protein corresponding to the relative molecular weight of TRPC3-HA (Fig. 1Ci) but also protein immunoreactive for src (Fig. 1Cii). In addition, immunoprecipitating src brought down HA-tagged TRPC3 (Fig. 1Ciii), and the TRPC3-HA immunoprecipitated from cells expressing src reacted positive for phosphotyrosine (Fig. 1Civ). These experiments argue strongly for a direct interaction between TRPC3 and src in a cellular context and for TRPC3 being a substrate for the src tyrosine kinase.
The susceptibility of the interacting TRPC3 N terminus to be phosphorylated by src was directly tested by incubating the N terminus fused to GST with src in the presence of [γ-32P]ATP and Mn2+ using as a source of active src lysates from COS-7 cells in which src had been transiently expressed. As illustrated in Fig. 2B, lane 4, the ≈63-kDa GST-C3 N terminus [TRPC3(1-370)] fusion protein was intensely phosphorylated by the kinase activity immunopurified from COS-7 cell lysates. Lanes 1 and 2 of Fig. 2B show the results from negative (GST only) and positive (enolase) control reactions. We concluded that the TRPC3 N terminus is a target of the tyrosine kinase activity of src.
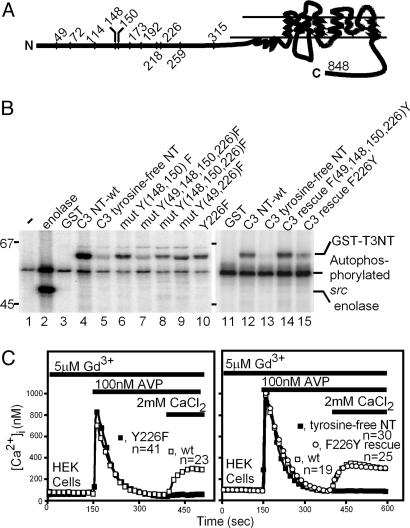
Fig. 2.
In vitro phosphorylation of TRPC3 with unmodified N terminus (NT) or with N termini in which the indicated tyrosines had been mutated to phenylalanine. (A) Diagram of the location of the 11 N-terminal tyrosines in the 848-aa TRPC3 protein. (B) Autoradiogram of the N terminus-GST fusion proteins subjected to an in vitro tyrosine kinase reaction using [γ-32P]ATP and src isolated by immunoprecipitation from lysates of COS-7 cells transfected with the pcDNA3 expression vector carrying the chicken src cDNA. The products from the tyrosine kinase reaction were separated by SDS/PAGE and analyzed by autoradiography. Lanes 1 and 2 show negative (GST only) and positive (GST fusion protein replaced with enolase) controls, respectively. (C) Y226 is required for activation of TRPC3 by a Gq-coupled receptor. Fura-2-loaded HEK cells expressing TRPC3 or the indicated mutants and cotransfected V1aR were stimulated with AVP in the absence of extracellular Ca2+ or Ba2+ to activate the Gq-PLCβ signaling system and deplete internal Ca2+ stores. At the time indicated by the bar, the Ca2+-free medium was exchanged with the same medium plus 2 mM CaCl2 to record ROCE. Changes in [Ca2+]i were monitored in individual cells by ratiometric fluorescence spectroscopy and video microscopy as described in ref. 11. GdCl3 at 5 μM was present throughout to suppress Ba2+ entry through endogenous ROCE and SOCE channels. (Left) A single mutation, Y226F, abrogates activation of TRPC3. (Right) Mutating all 11 N-terminal tyrosines to phenylalanine (tyrosine-free NT) abolishes receptor-operated cation entry; reintroduction of only Y226 into the tyrosine-free NT of TRPC3 (F226Y rescue), restores receptor-operated cation entry.
The N terminus of TRPC3 (848-aa splice variant, GenBank accession no. U47050) has 11 tyrosines (Fig. 2 A). Through systematic site-directed mutation of these 11 tyrosines singly and in groups (Fig. 2B), we identified Y49, Y148, Y150, and Y226 as src substrates. Thus, a tyrosine-free N terminus in which all 11 tyrosines had been mutated to phenylalanine was no longer a src substrate (Fig. 2B, lanes 5 and 13), and reintroduction of tyrosines only at positions 49, 148, 150, and 226 [Fig. 2B, lane 14: rescue F(49,148,150,226)Y] yielded a substrate equally as phosphorylated as the wild-type N terminus shown in lane 11. Removal of only Y226 (lane 10) resulted in partial loss of phosphorylation; Y226 contributed partially to the phosphorylation of the N-terminal tyrosines (lane 15).
Functional Correlate to Lack of Phosphorylation of TRPC3 Y226. Expression of TRPC3 with mutated N-terminal tyrosines followed by the standard test for activation by a cotransfected GPCR, either the muscarinic M5 acetylcholine receptor (M5R) or the type 1a vasopressin receptor (V1aR), showed that TRPC3 activation was lost whenever Y226 was mutated to phenylalanine either singly (Fig. 2C Left) or as part of the removal of all N-terminal tyrosines (Fig. 2C Right). Moreover, the activation of an N-terminally tyrosine-free TRPC3 was restored by reintroducing only Y226 (Fig. 2C Right, ○).
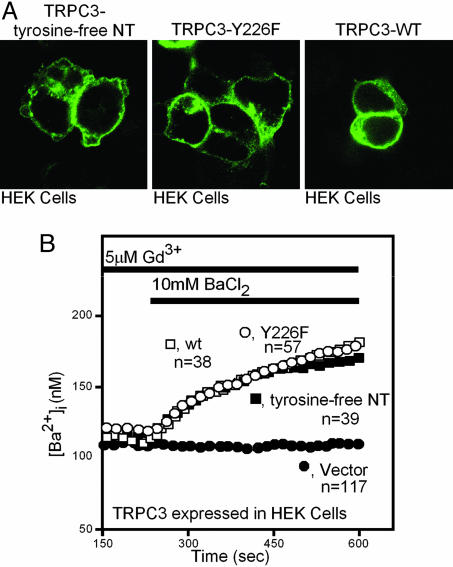
These experiments showed that TRPC3 critically depends on the presence of Y226, a substrate of the src tyrosine kinase. However, the failure of agonists to activate Ca2+ influx in cells expressing the mutant TRPC3s could also be attributed to structural interference. Two types of experiments showed that TRPC3 with mutated Y226 reaches the plasma membrane and is capable of forming divalent cation-permeable ion channels. In the first experiment, we tagged the C termini of the nonresponsive N-terminally tyrosine-free TRPC3 molecules, Y226F mutant TRPC3 molecules, and wild-type TRPC3 molecules with the HA epitope and visualized their subcellular distribution by confocal microscopy. We saw no significant difference in their localization within the cells, including their periphery (Fig. 3A). The second experiment took advantage of the unique property of TRPC3 to exhibit a low level of constitutive activity, which is readily apparent if influx is tested with 10 mM BaCl2 instead of 2 mM CaCl2. Indeed, basal Ba2+ entry into HEK cells expressing TRPC3 did not differ among wild-type TRPC3, the TRPC3 with an N terminus free of tyrosines, and TRPC3 with a single Y226F mutation (Fig. 3B).
Fig. 3.
Mutating N-terminal (NT) tyrosines in TRPC3 has no effect on its subcellular localization or its basal divalent cation channeling activity. (A) HA-tagged wild-type or mutated TRPC3 were transiently expressed in HEK cells, and their subcellular localization was analyzed 24 h after transfection by confocal microscopy. (B) Divalent cation entry into HEK cells mediated by wild-type TRPC3 or TRPC3 with either the Y266F mutation or a tyrosine-free N terminus was recorded as described for in Fig. 2C, except that the cells were not stimulated with agonist and 10 mM BaCl2 was substituted for 2 mM CaCl2.
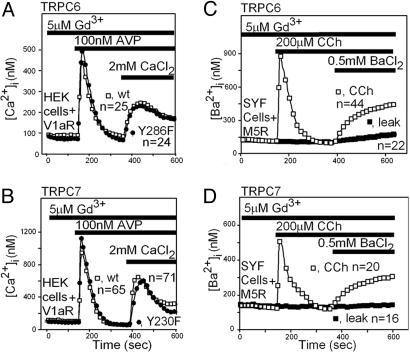
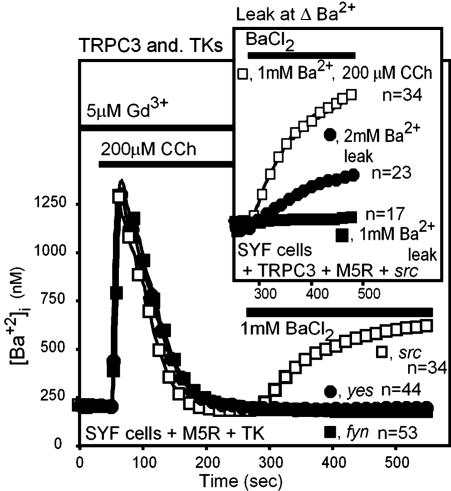
The cognate of TRPC3 Y226 is present in the closely related TRPC6 and TRPC7 but absent in other TRPCs. We therefore tested whether mutation of the TRPC6 and TRPC7 “Y226” tyrosines (TRPC6 Y284 and TRPC7 Y230) would affect the behavior of these channels. Contrary to our expectation, neither mutation affected the behavior of TRPC6 and TRPC7 (Fig. 4 A and B). Moreover, expression of TRPC6 and TRPC7 in embryonic fibroblasts lacking the src, yes, and fyn tyrosine kinases (SYF cells) led to normal TRPC6- and TRPC7-mediated ROCE, indicating that these channels do not depend on these kinases for their activity. In contrast and in agreement with published observations (20), TRPC3 could not be activated by a GPCR coupled by Gq in SYF cells (Fig. 5) unless they were supplemented with src. Furthermore, neither yes nor fyn could substitute for src (Fig. 5). In these experiments, TRPC3 activation was evaluated at 1 mM BaCl2 to minimize interference from its basal activity (Fig. 5 Inset).
Fig. 4.
TRPC6 and TRPC7 do not share with TRPC3 a similar dependence on tyrosine phosphorylation. (A and B) Mutating the tyrosines of TRPC6 (A) and TRPC7 (B) that are cognates of TRPC3 Y226 has no effect on their ability to respond to the activation of a Gq-coupled receptor. ROCE mediated by TRPC6, TRPC7, and the indicated mutant forms of these TRPCs was recorded as described for Fig. 2C. (C and D) TRPC6 (C) and TRPC7 (D) do not require src, yes, or fyn for activation by the Gq-PLCβ signaling pathway. TRPC cDNAs were cotransfected with M5R into SYF embryonic fibroblasts and tested for activation by CCh. For these experiments, ROCE was assessed by measuring Ba2+ instead of Ca2+ influx.
Fig. 5.
Expression of src but not of yes or fyn in SYF cells rescues activation of TRPC3 by the Gq-PLCβ signaling pathway. (Inset) Lack of interference by the basal activity of TRPC3 in ROCE assessment by using 1 mM BaCl2 to monitor ROCE. TK, tyrosine kinase.
Heterogeneity of Channels Mediating ROCE and SOCE as Seen in Different Cells: Comparison of Endogenous ROCE and SOCE to ROCE Mediated by TRPC3, TRPC6, TRPC7, and TRPC5. TRPC3, TRPC6, and TRPC7 are closely related not only from a structural point of view (being >70% identical in the amino acid sequence that spans 150 aa on either side of Y226) but also from biochemical and functional points of view, because all three interact through their N termini with src in GST pull-down tests (Fig. 1), and all three are directly TRPCs activated by diacylglycerol (22). The failure to observe an effect upon mutating the cognates of TRPC3 Y226 in TRPC6 and TRPC7 (Fig. 4 A and B) and the lack of impairment of their activation by a Gq-coupled receptor in SYF cells (Fig. 4 C and D) was therefore surprising.
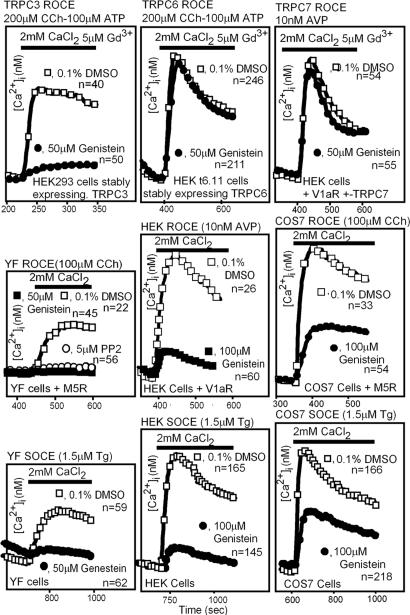
We next tested whether genistein would inhibit TRPC6- and TRPC7-mediated ROCE as seen with TRPC3. We again found that the activation of the TRPC3-related TRPC6 and TRPC7 differed from TRPC3 and were unaffected by genistein (Fig. 6 Top). The insensitivity of TRPC6 and TRPC7 activation to genistein is in sharp contrast to that seen with TRPC3 in the same cells (Fig. 6 Top Left). In other experiments, TRPC5-mediated ROCE in HEK cells was also found to be insensitive to genistein, thus resembling TRPC6 and TRPC7 in this respect (data not shown).
Fig. 6.
Endogenous ROCE, endogenous SOCE, and TRPC-mediated ROCE show different sensitivities to tyrosine kinase inhibition. (Top) ROCE mediated by TRPC3 but not by TRPC6 or TRPC7 is sensitive to inhibition by the nonselective tyrosine kinase inhibitor genistein in HEK cells. TRPC cDNAs were cotransfected with V1aR into HEK cells, and TRPC-mediated ROCE was recorded. (Middle) Variable effectiveness of genistein to inhibit the Ca2+entry phase mediated by endogenous ROCE channels of YF, HEK, and SOS-7 cells. YF mouse fibroblasts were the most sensitive to genistein, and COS-7 cells were the least sensitive. (Bottom) Variable effectiveness of genistein as an inhibitor of endogenous SOCE activated by thapsigargin-mediated store depletion in YF, HEK, and COS-7 cells. For all experiments, only the Ca2+ entry phase is shown. Tg, thapsigargin.
These differences prompted us to test whether natural ROCE and SOCE channels would show cell-to-cell differences in terms of their susceptibility to inhibition by genistein. Such differences were indeed found (Fig. 6 Middle and Bottom). Note that the 5 μM Gd3+ used to inhibit endogenous ROCE and SOCE when studying Ca2+entry mediated by transfected TRPCs (11, 22) was omitted from the external media when endogenous ROCE and SOCE were tested. Thus, YF cells (cells lacking yes and fyn), HEK cells, and COS-7 cells were plated without prior manipulations, loaded with Fura-2, and tested for SOCE activation by thapsigargin (Fig. 6 Bottom) or plated after transfection of a suitable Gq-coupled receptor and tested for ROCE activation by the transfected receptor [V1aR activated by arginine vasopressin (AVP) or M5R activated by carbachol (CCh)] (Fig. 6 Middle) in the absence and the presence of genistein. The degree to which genistein inhibited SOCE and ROCE was similar in each cell but differed when comparing one cell to another, from total inhibition in YF embryonic fibroblasts to ≈80-90% inhibition in HEK cells and ≈40-50% inhibition in COS-7 cells. SYF cells behaved like YF cells (data not shown).
Discussion
Two methodologies are currently used to study ROCE and SOCE into cells. One method is based on changes in [Ca2+]i (intracellular Ca2+ concentration) (or [Ba2+]i) monitored by using a Ca2+ indicator such as Fura-2. The other method involves recording by electrophysiological means the activities of the channels that are activated when ROCE and SOCE are induced. However, it is not clear whether both techniques look at the same event. For example, endogenous ROCE and SOCE channels feature very short openings and, especially when SOCE channels are studied, are often of very low amplitude and are slow in becoming apparent. ROCE and SOCE measured by means of Fura-2 fluorescence changes do not predict such differences. ROCE channels are often rapidly inactivated by Ca2+ (in the millisecond range), whereas feedback inhibition by entering Ca2+ is slow in ROCE and SOCE measured with Fura-2. In fact, Fura-2-based measurements of ROCE and SOCE do not lend themselves to observations in the millisecond range and reflect [Ca2+]i changes over tens of seconds. The gradual decreases in [Ca2+]i seen in Fura-2 experiments are very likely the sum of not only influx rates but also plasma membrane Ca2+ pump-mediated extrusion rates.
The present studies did not address these issues and focused on ROCE and SOCE as originally defined in studies that measured macroscopic ion fluxes with the Fura-2 Ca2+ indicator dye. In this context we probed the ROCE and SOCE phenomena with the nonselective tyrosine kinase inhibitor genistein and compared effects on endogenous ROCE and SOCE to its effect on TRPC-mediated Ca2+ entry. Our hope was that we might be able to establish correlations that would be informative not only about the mechanism of action by which genistein inhibits TRPC function but also about the nature of the channels that mediate native ROCE and SOCE.
We were able to elucidate the mechanism by which genistein inhibits ROCE mediated by TRPC3 by pinpointing the tyrosine phosphorylated by src that is required for its stimulation by the GPCR-Gq-PLCβ signaling pathway. Removal of this tyrosine, TRPC3 Y226, causes loss of the ability of TRPC3 to respond to the Gq-PLCβ signal (presumably diacylglycerol), because genistein inhibits activation not only by a receptor agonist but also by oleyl-acetyl-glycerol, a mimic of PLCβ activation (22). Src is a candidate kinase for enabling activation of TRPC3 (Fig. 1). PP2, another tyrosine kinase inhibitor that is more selective toward src than genistein, also inhibits activation of TRPC3 in HEK cells (20), and restitution of Y226 in a TRPC3 channel lacking all N-terminal tyrosines shown to be src substrates in in vitro kinase assays rescues activation by the Gq-PLCβ pathway (Fig. 2). Transfection of yes or fyn into SYF cells did not rescue stimulation of TRPC3 by the Gq-PLCβ pathway (Fig. 5). Thus, the closely related yes and fyn tyrosine kinases cannot substitute for src. Although functionally important, the interaction of src with TRPC3 was not very stable and, to be reliably observed, required chemical stabilization with a crosslinker (see Experimental Procedures) before cell solubilization and immunoprecipitation. Furthermore, phosphorylation of transfected TRPC3 was not observable unless exogenous src was cotransfected. Yet we conclude that activation of TRPC3 depends on src-mediated phosphorylation on Y226. Phosphorylation on Y226 is not required for channel assembly because Y226I TRPC3 shows a subcellular distribution indistinguishable from that of wild-type TRPC3 (Fig. 3A) and exhibits the same degree of basal activity as wild-type TRPC3 (Fig. 3B).
The finding that TRPC6 is active in SYF and YF cells was unexpected, because Hisatsune et al. (24) reported that TRPC6 is a substrate of fyn and that it behaves essentially the same as TRPC3 in in vitro and cell expression assays. Thus, TRPC6 is phosphorylated by coexpression with fyn in COS-7 cells and associates with fyn in GST pull-down assays by interaction of its N terminus with the src homology 2 domain of fyn (24). Furthermore, TRPC6 activation by a receptor-tyrosine kinase (RTK)-PLCγ pathway (triggered by EGF) is inhibited by PP2, a tyrosine kinase inhibitor selective for the src family of tyrosine kinases to which fyn belongs. Moreover, addition of fyn to inside-out membrane patches from cells expressing TRPC6 increased basal and oleyl-acetyl-glycerol-stimulated TRPC6 activity (24). Yet, we find that TRPC6 ROCE is activated in cells lacking not only fyn but also yes and src (Fig. 4C). We do not know whether this discrepancy is due to our use of the GPCR-Gq-PLCβ activation pathway instead of a RTK-PLCγ pathway or to the method used to assess TRPC6 ROCE, Fura-2 in our case and electrophysiological inside-out membrane patches in the case of Hisatsune et al. (24). If, however, the lack of sensitivity of TRPC6 to genistein in our experiments and TRPC6 being active in SYF cells are not due to the use of different activation pathways or the method used to assess TRPC6 activation, then the data may also indicate that, in addition to src, fyn, and yes, functionally there is at least one more “PP2-sensitive src-family tyrosine kinase” able to regulate TRPC6. The fact that TRPC1-TRPC7 all interacted with src in GST pull-down assays (Fig. 1 A) also raises the possibility that all TRPC channels depend on tyrosine phosphorylation for their functioning as an effector system for the activation of the GPCR-Gq-PLCβ/RTK-PLCγ pathways.
The interaction of src with N and C termini of TRPCs fell into three types (Fig. 1 A). Given the unexpected differences in the kinase(s) that may confer regulation to TRPC3 vs. those acting on TRPC6 and TRPC7, it is clear that the essential tyrosines involved and the specific phosphorylating kinase or kinases need to be determined in separate TRPC-specific studies. Indeed, although our studies have conclusively shown that src phosphorylates and enables TRPC3 to respond to the activating signal generated by the activation of the Gq-PLCβ pathway, they have not ruled out the possibility that another tyrosine kinase not expressed in SYF and YF cells may not also have the same enabling properties as src. In this sense, our studies support the postulate of Hisatsune et al. (24) that tyrosine phosphorylation may be an obligatory step for the activation of TRPC channels. Moreover, results presented in Fig. 6 showing various degrees of inhibition of ROCE and SOCE by genistein strongly argue not only that the type of kinase may vary but also that the makeup of endogenous ROCE and SOCE channels is likely to vary from cell to cell. Results from the studies presented above may aid not only in understanding the mechanisms and molecular makeup of channels responsible for ROCE and/or SOCE but also in establishing a bridge between phenomena measured on a time scale of tens of seconds using fluorescent indicator dyes and phenomena measured in time scales of milliseconds by electrophysiological means.
Acknowledgments
We thank Dr. Yasuo Mori (University of Kyoto, Kyoto) for the TRPC7 cDNA, Drs. Tohru Tezuka and Tadashi Yamamoto (University of Tokyo, Tokyo) for the fyn and yes cDNAs, Dr. Joan Brugge (Harvard Medical School, Boston) for the mAb327 anti-src monoclonal antibody, Drs. Gary St. John Bird and James Putney (National Institute of Environmental Health Sciences) for the TRPC3-expressing HEK clone, and Dr. Mohamed Trebak for helpful assistance throughout these studies. This work was supported by the Intramural Research Program of the NIH, NIEHS.
Author contributions: L.B. designed research; and B.T.K. and Y.L. performed research.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AVP, arginine vasopressin; CCh, carbachol; GPCR, G protein-coupled receptor; Gq, hetrotrimeric G protein with αqβγ subunit composition; HA, hemagglutinin; M5R, M5 muscarinic acetylcholine receptor; PLC, phospholipase C; ROCE, receptor-operated Ca2+ entry; SOCE, store-operated Ca2+ entry; SYF, src-, yes-, and fyn-deficient; TRPC, C-type transient receptor potential; V1aR, type 1a vasopressin receptor; YF, SYF expressing transfected src.
References
- 1.Putney, J. W., Jr. (1987) Am. J. Physiol. 252, G149-G157. [DOI] [PubMed] [Google Scholar]
- 2.Berridge, M. J. & Irvine, R. F. (1989) Nature 341, 197-205. [DOI] [PubMed] [Google Scholar]
- 3.Berridge, M. J. (1993) Nature 361, 315-325. [DOI] [PubMed] [Google Scholar]
- 4.Liao, C. F., Schilling, W. P., Birnbaumer, M. & Birnbaumer, L. (1990) J. Biol. Chem. 265, 11273-11284. [PubMed] [Google Scholar]
- 5.Hardie, R. C. & Minke, B. (1993) Trends Neurosci. 16, 371-376. [DOI] [PubMed] [Google Scholar]
- 6.Selinger, Z, Doza, Y. N. & Minke, B. (1993) Biochim. Biophys. Acta 1179, 283-299. [DOI] [PubMed] [Google Scholar]
- 7.Birnbaumer, L., Zhu, X., Jiang, M., Boulay, G., Peyton, M., Vannier, B., Brown, D., Platano, D., Sadeghi, H., Stefani, E. & Birnbaumer, M. (1996) Proc. Natl. Acad. Sci. USA 93, 15195-15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu, X., Jiang, M., Peyton, M. J., Boulay, G., Hurst, R., Stefani., E. & Birnbaumer, L. (1996) Cell 85, 661-671. [DOI] [PubMed] [Google Scholar]
- 9.Okada, T, Shimizu, S., Wakamori, M., Maeda, A., Kurosaki, T., Takada, N., Imoto, K & Mori, Y. (1998) J. Biol. Chem. 273, 10279-10287. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann, T., Schaefer, M., Schultz, G. & Gudermann, T. (2002) Proc. Natl. Acad. Sci. USA 99, 7461-7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yildirim, E., Kawasaki, B. T. & Birnbaumer, L. (2005) Proc. Natl. Acad. Sci. USA 102, 3307-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoth, M. & Penner, R. (1992) Nature 355, 353-356. [DOI] [PubMed] [Google Scholar]
- 13.Fasolato, C., Innocenti, B. & Pozzan, T. (1994) Trends Pharmacol. Sci. 15, 77-83. [DOI] [PubMed] [Google Scholar]
- 14.Fasolato, C., Hoth, M., Matthews, G. & Penner, R. (1993) Proc. Natl. Acad. Sci. USA 90, 3068-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang, Z., Tang, T., Tikunova, S., Johnson, J. D., Chen, Z., Qin, N., Dietrich, A., Stefani, E., Birnbaumer, L. & Zhu, M. X. (2001) Proc. Natl. Acad. Sci. USA 98, 3168-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, K.-M., Toscas, K. & Villereal, M. L. (1993) J. Biol. Chem. 268, 9945-9948. [PubMed] [Google Scholar]
- 16.Babnigg, G., Bowersox, S. R. & Villereal, M. L. (1997) J. Biol. Chem. 272, 29434-29437. [DOI] [PubMed] [Google Scholar]
- 17.Gutkind, J. S. & Robbins, K. C. (1992) Biochem. Biophys. Res. Commun. 188, 155-161. [DOI] [PubMed] [Google Scholar]
- 18.Lee, K.-M. & Villereal, M. L. (1996) Am. J. Physiol. 270, C1430-C1437. [DOI] [PubMed] [Google Scholar]
- 19.Igishi, T. & Gutkind, J. S. (1998) Biochem. Biophys. Res. Commun. 244, 5-10. [DOI] [PubMed] [Google Scholar]
- 20.Vazquez, G., Wedel, B. J., Kawasaki, B. T., St. John Bird, G. & Putney, J. (2004) J. Biol. Chem. 279, 40521-40528. [DOI] [PubMed] [Google Scholar]
- 21.Boulay, G., Brown, D. M., Qin, N., Jiang, M., Dietrich, A., Zhu, M. X., Chen, Z., Birnbaumer, M., Mikoshiba, K. & Birnbaumer, L. (1999) Proc. Natl. Acad. Sci. USA 96, 14955-14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofmann, T., Obukhov, A. G., Schaefer, M., Harteneck, C., Gudermann, T. & Schultz, G. (1999) Nature 397, 259-263. [DOI] [PubMed] [Google Scholar]
- 23.Zhu, X., Jiang, M. & Birnbaumer, L. (1998) J. Biol. Chem. 273, 133-142. [DOI] [PubMed] [Google Scholar]
- 24.Hisatsune, C., Kuroda, Y., Nakamura, K., Inoue, T., Nakamura, T., Michikawa, T., Mitsutani, A. & Mikoshiba, K. (2004) J. Biol. Chem. 279, 18887-18894. [DOI] [PubMed] [Google Scholar]