Abstract
Twelve patients with T cell large granular lymphocyte leukemia and associated hematocytopenia were treated in a phase I dose-escalation trial with the murine monoclonal antibody Mikβ1. Mikβ1 identifies CD122, the β-subunit shared by the IL-2 and IL-15 receptors. At the doses administered in this study the antibody inhibited the actions of IL-15 on both natural killer and T cells and that of IL-2 when the intermediate-affinity IL-2 receptor was expressed. Mikβ1 treatment was not associated with significant toxicity or with the development of an immune response to the infused monoclonal antibody. At these doses of Mikβ1, >95% saturation of the IL-2/IL-15β receptor (CD122) on the surfaces of the leukemic cells was achieved. Furthermore, in seven patients this led to the down-modulation of the receptor from the surfaces of the leukemic cells. Nevertheless, no patients manifested a reduction in peripheral leukemic cell count or an amelioration of their hematocytopenia. This latter observation may reflect the fact that the monoclonal T cell large granular lymphocyte leukemia leukemic cells of the patients did not produce IL-2 or IL-15 or require their actions for cell survival. In light of the lack of toxicity and lack of immunogenicity of the antibody observed in the present study and the role for IL-15 in the pathogenesis of autoimmune diseases, clinical trials should be performed using the humanized version of Mikβ1 in groups of patients with human T cell lymphotropic virus I-associated myelopathy/tropical spastic paraparesis, rheumatoid arthritis, multiple sclerosis and refractory celiac disease.
Keywords: IL-2/IL-15β receptor (CD122), natural killer cells, cytokine
Interleukin-15 is an inflammatory cytokine that stimulates T and natural killer (NK) cell activity and induces expression of TNF-α, IL-1β, and inflammatory chemokines (1-4). IL-15 inhibits the self-tolerance mediated by IL-2-induced activation-induced cell death (5). In addition, IL-15 facilitates the survival of CD8+ memory T cells, including self-directed memory cells (5-7). The heterotrimeric IL-15 receptor includes a private IL-15-specific receptor subunit, IL-15Rα, together with the IL-2/IL-15Rβ subunit that is shared with IL-2 and the common γ-chain receptor subunit that is also used by IL-2, IL-4, IL-7, IL-9, and IL-21 (3, 8). In contrast to other interleukins, IL-15 acts as a membrane-associated cytokine. IL-15 and its private receptor subunit, IL-15Rα, are components of an immunological synapse that develops between antigen-presenting cells and effector NK and CD8 T cells (9, 10). The expression of IL-15Rα and associated IL-15 are coordinately induced on antigen-presenting cells through the stimulation of Toll-like and IFN receptors. IL-15 receptor and IL-15α form stable complexes on the cell surfaces of activated antigen-presenting cells. Upon cell-cell interaction these complexes on activated monocytes and dendritic cells present IL-15 in trans to resting NK and CD8+ T cells that express only IL-2/IL-15Rβ and the common γ-chain but not IL-15Rα (9). Abnormalities of IL-15 expression have been described in patients with select lymphoid malignancies and autoimmune diseases including rheumatoid arthritis, multiple sclerosis, psoriasis, and inflammatory bowel disease, as well as in diseases associated with human T cell lymphotropic virus I (HTLV-I) (3, 8, 11-16).
A number of approaches have been developed to treat autoimmune diseases by blocking IL-15 action (8, 12-16). Our efforts to inhibit the actions of IL-15 for the therapy of autoimmune diseases and HTLV-I-associated disorders have focused on the Mikβ1 monoclonal antibody that interacts with the IL-2/IL-15Rβ (CD122) receptor subunit (17, 18). We demonstrated that this monoclonal antibody prevents the transpresentation of IL-15 to NK and CD8+ T cells and thereby inhibits IL-15-mediated effects (9, 18, 19). In the present study we translate these observations concerning IL-15 blockade into clinical trials using the monoclonal antibody Mikβ1.
The present study was directed toward the evaluation of the safety and efficacy of the murine monoclonal antibody Mikβ1 in patients with T cell large granular lymphocyte leukemia (T-LGL) associated with hematocytopenia. The type of T-LGL that is the focus of the present study manifests the phenotype CD2+, CD3+, CD8+, CD57+, CD122+, CD4-, and CD25- (IL-2Rα) (20-25). After the addition of IL-2 or IL-15, the T-LGL cells develop lymphokine-activated killer activity. The majority of T-LGL leukemic cell populations, including all in the present study, were monoclonal as assessed by PCR or Southern blot analyses (26, 27). In most cases, T-LGL follows a chronic course characterized by peripheral blood lymphocytosis, with splenic and bone marrow infiltration with leukemic cells that is associated with neutropenia and recurrent infections. Other clinical manifestations include pure red cell aplasia and thrombocytopenia. Autoimmune phenomena including hemolytic anemia as well as rheumatoid arthritis occur in a proportion of patients and contribute to morbidity and mortality (20, 21).
The scientific basis for the present therapeutic study of Mikβ1 was that the monoclonal leukemic CD8+ lymphocytes of patients with T-LGL express large numbers of the IL-2/IL-15Rβ receptor subunit identified by Mikβ1 on their cell surfaces (21-23, 25, 28, 29). Resting normal NK and T-LGL cells did not express the IL-2Rα receptor subunit; however, it could be induced by the addition of IL-2 to these cells that express the β and common γ-receptor subunits, demonstrating that these receptors can signal in the initial absence of IL-2Rα (30). In subsequent studies it was shown that the IL-2 receptor β-chain plays a role in mediating signals for lymphokine-activated killer and NK cells in proliferation activities and that there is an induction of NK activity in LGL cells when they are activated by the anti-CD3 monoclonal antibody and by IL-2 that acts through the β-subunit (24). In addition, LGL cells are stimulated by IL-15, and this cytokine mediates its activity through subunits of the IL-2 receptor. In subsequent studies, the IL-2/IL-15Rβ subunit was shown to be a pivotal component of the heterotrimeric IL-15 receptor (1, 3, 19, 31). Furthermore, Mikβ1 blocks IL-15 action on T and NK cells as well as T-LGL (1, 3, 32).
In the present study, we confirmed that the Mikβ1 antibody blocks IL-15 action in NK and T cell lines. Furthermore, in IL-2Rα-nonexpressing cells such as T-LGL cells, Mikβ1 inhibited the action of IL-2 as well. The administration of the antibody in the clinical setting was associated with the modulation of the target antigen IL-2/IL-15Rβ from the surfaces of the leukemic cells. The administration of Mikβ1 to the patients was not associated with significant toxicity, nor did it elicit an antibody response to the infused monoclonal antibody. However, the patients with T-LGL did not manifest a clinical response to a short course of murine Mikβ1.
Results
Preclinical Specificity of Mikβ1 for IL-2/IL-15Rβ (CD122) and Reactivity with Normal Human Tissues. The murine Mikβ1 antibody was the standard monoclonal antibody used in the International Leukocyte Differentiation Antigen Workshop that first defined CD122. In the present study Mikβ1 did not react with the unmodified 32D murine hematopoietic cell line lacking expression of human CD122, but it did bind to this cell line after transfection with an expression construct encoding human IL-2R/IL-15Rβ (Fig. 3, which is published as supporting information on the PNAS web site).
Distribution analyses to define the tissue targets of Mikβ1 antibody binding were performed on three sets of human tissue specimens obtained from autopsy by standard indirect immunofluorescence analyses. No binding of Mikβ1 was demonstrated to the following tissues: adrenal, bladder, bone marrow, brain, breast, sternum, cervix, esophagus, eye, heart, kidney, large intestine, liver, lung, lymph node, ovary, pancreas, parathyroid, parotid, pituitary, prostate, skin, small intestine, cervical cord, spleen, stomach, testis, thymus, thyroid, tonsils, or uterus. Three of three skeletal muscle specimens showed 1+ to 2+ reactivity. However, no RNA message encoding IL-2/IL-15Rβ (CD122) could be demonstrated by Northern blot analysis in RNA-extracted skeletal muscle from a cynomolgus monkey. Mikβ1 reacted with Epstein-Barr-virus-transformed B cell lines, phytohemagglutinin-activated lymphocytes, and the majority of NK cell lines. Thus, the epitope identified by Mikβ1 appears limited to expression in muscle cells, NK cells, T-LGL, and activated B and T lymphocytes.
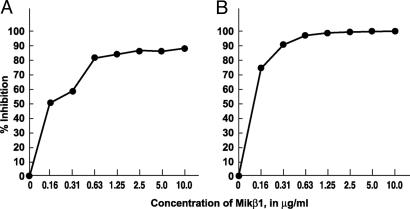
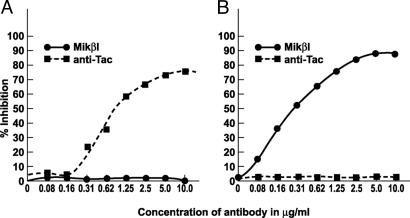
Mikβ1 as an Inhibitor of IL-15- and IL-2-Induced Proliferation of Cytokine-Dependent Cell Lines. To examine the specificity of the antibodies used in these studies, proliferation assays were performed with the cytokine-dependent 32Dβ and the Kit-225 T cell line (both IL-2- and IL-15-responsive). With the 32Dβ cell line that expresses only intermediate cytokine affinity, IL-2 and IL-15 receptors involving the common γ-chain, and IL-2/IL-15Rβ subunits, IL-2-mediated proliferation was inhibited by the addition of 10 μg/ml Mikβ1 (Fig. 1A). Furthermore, IL-15-mediated proliferation of 32Dβ was inhibited by Mikβ1 (Fig. 1B). The antibody did not inhibit the proliferation of 32Dβ mediated by IL-3 (data not shown). In the case of Kit-225, a cell line that expresses IL-2Rα as well as IL-2/IL-15Rβ and the common γ-chain Mikβ1, did not inhibit IL-2-mediated proliferation in this IL-2 high-affinity IL-2R-expressing cell line, whereas anti-Tac blocked such IL-2-mediated proliferation (Fig. 2A). IL-15-induced proliferation of Kit-225 was inhibited by Mikβ1 but not by anti-Tac (Fig. 2B). Thus, with the Kit-225 cell line each antibody blocked only the proliferation mediated by its cognate cytokine. These studies demonstrate the fidelity of each antibody and suggest that the proliferation inhibition observed is due to blockade of receptor for the targeted cytokine.
Fig. 1.
Inhibition of IL-2- and IL-15-induced proliferation of 32Dβ cells by Mikβ1. (A) Effect of Mikβ1 on IL-2 (1 ng/ml)-induced proliferation. (B) Dose-response effects of the addition of the Mikβ1 antibody on IL-15 (20 ng/ml)-induced proliferation in 32Dβ cells. In these cells, which express only IL-2/IL-15Rβ and common γ-chain, both IL-2- and IL-15-induced proliferation were inhibited by the addition of Mikβ1.
Fig. 2.
Inhibition of IL-15- but not IL-2-induced proliferation of Kit-225 cells by Mikβ1. (A) Dose-response effects of the addition of 10 ng/ml Mikβ1 antibody or anti-Tac antibody on IL-2 (500 pg/ml)-induced proliferation of Kit-225 cells. (B) Effect of 10 ng/ml anti-Tac or Mikβ1 on IL-15 (3 ng/ml)-induced proliferation of Kit-225 cells that express IL-2Rα as well as IL-2/IL-15Rβ and the common γ-chain. In these Kit-225 cells, which express the high-affinity receptor for IL-2, Mikβ1 inhibited the proliferation induced by IL-15 but not that induced by IL-2.
Mikβ1 Led to the Saturation of CD122 or Its Modulation from the Cell Surface. The patients initially manifested strong expression of CD122 when assessed by analysis with Mikβ1 and with Mikβ3 that defines an epitope distinct from that identified by Mikβ1. The reactivity with fluorochrome-labeled Mikβ1 was virtually eliminated in the patients during the 48 h after infusion of 1.5 mg/kg monoclonal antibody. The reactivity of Mikβ3 with the leukemic cells was not inhibited by the presence of nonfluorochrome-labeled Mikβ1 when the antibody was added in vitro immediately before the infusions. However, in 7 of the 12 patients who were reanalyzed 48 h after the administration of Mikβ1, there was a marked reduction in the reactivity using immunofluorescence analyses with both Mikβ1 and Mikβ3. Because there was no reduction in the number of leukemic cells as assessed by the CD2+, CD8+, CD57+ phenotype analyses, the reduced reactivity did not reflect the elimination of the target cells. The reduction in reactivity with directly labeled murine Mikβ1 could theoretically have reflected saturation of the receptor with the infused monoclonal antibody. However, the loss of reactivity with Mikβ3 that was observed in seven patients cannot be explained by this mechanism. Rather, these losses of reactivity appear to reflect down-modulation of CD122 from the surface of the leukemic cells, possibly by monoclonal antibody-mediated internalization of the receptor. This finding suggests that the maintenance of CD122 is not required for the survival of the T-LGL cells at least for the period involved in the present study. There was reexpression of IL-2/IL-15Rβ with both Mikβ1 and Mikβ3 when assayed 4-6 weeks after the infusions.
Response and Toxicity to Murine Mikβ1 in Patients with T-LGL. All patients manifested stable disease. None developed a reduction in the peripheral leukemic count or an amelioration of their hematocytopenia.
No toxicity in terms of clinical hematological or clinical chemical analysis was observed after a single i.v. dose administration of 2.0 mg/kg of a humanized Mikβ1 preparation to each of three cynomolgus monkeys in a formal toxicological analysis. In addition, no antibody-related abnormalities were observed in these animals at autopsies performed 43 days after the Mikβ1 administration. No serious adverse events were observed in any patient in the present trial as assessed by clinical evaluation or routine hematological and clinical chemistry tests. With the exception of grade 2 fever observed in two patients immediately after the monoclonal antibody administration and grade 2 elevation of bilirubin in one of these individuals, no other adverse events were observed.
Pharmacokinetics of Mikβ1. In preclinical studies, murine Mikβ1 and murine anti-Tac (anti-IL-2Rα, anti-CD25 antibody) were radiolabeled with 125I and 131I, respectively, and the mixture was administered to cynomolgus monkeys. The terminal half-life of decline from the serum of radiolabeled Mikβ1 was 36 h, and that of murine anti-Tac was 40 h.
In our clinical trial at the 1.5 mg/kg dose in patients with T-LGL, Mikβ1 levels were quantitated in the serum in serial time points after the infusion of the antibody. The peak serum levels were 23-37 μg/ml, and the serum antibody concentration declined to a level of 8.9-11.6 μg/ml 48 h after the infusion and immediately before the next infusion.
Clinical Immunogenicity of Murine Mikβ1. The immunogenicity of murine Mikβ1 was assessed in cynomolgus monkeys and in patients by using a sensitive ELISA. Six animals undergoing a cardiac allograft received murine Mikβ1 at a dose of 1 mg/kg every other day for 5 doses. None of the monkeys in the study developed antibodies to murine Mikβ1. In the human clinical trial murine Mikβ1 was administered i.v. on four occasions (days 1, 4, 7, and 10) to 12 patients with T-LGL. None of these 12 patients developed an antibody response to the infused murine Mikβ1 when assessed between days 6 and 10 and at 4-6 weeks after therapy. Thus, murine Mikβ1 does not appear to be a strongly immunogenic monoclonal antibody, and the lack of efficacy did not reflect the production by the patients of antibodies directed toward this murine monoclonal antibody.
Lack of IL-2- and IL-15-Mediated Autocrine or Paracrine Proliferation of the T-LGL. The murine Mikβ1 monoclonal antibody does not fix complement, nor does it demonstrate antibody-dependent cellular cytotoxicity with human mononuclear cells. Thus, it is not an antibody that is cytotoxic to CD122-expressing cells. The scientific hypothesis that was evaluated in the present study was that T-LGL cells might depend for their survival on either IL-2 or IL-15, cytokines that are involved in the generation and maintenance of NK and NK-T cells. In an effort to define whether the leukemic cells were dependent on IL-2 or IL-15, a series of studies were performed to determine whether the T-LGL were responding in an autocrine or paracrine fashion to these cytokines.
The ex vivo proliferations of the patient's peripheral blood mononuclear cells (PBMC), including the T-LGL, were evaluated by culturing 105 PBMC in the absence of added antigen, mitogen, or cytokine. Proliferation was evaluated by [3H]thymidine uptake into PBMC that had been cultured ex vivo for 6 days. No meaningful spontaneous proliferation was observed by the cells examined from 10 patients. In particular, there was <2,000 cpm of [3H]thymidine uptake per 105 cells, a very low uptake value that was not greater than that seen with the ex vivo cultured PBMC from 10 normal individuals. This finding contrasts with the mean of 40,200 ± 6,000 cpm [3H]thymidine observed per 105 cells in comparable studies of PBMC from patients with the neurological disease HTLV-I-associated myelopathy/tropical spastic paraparesis, a disease known to manifest autocrine IL-2/IL-2 receptor-mediated as well as IL-15/IL-15 receptor-mediated proliferation of PBMC ex vivo. (14, 33).
The expression of IL-2 and IL-15 mRNA was evaluated in the PBMC from 12 patients with T-LGL and 8 normal controls by using real-time PCR. Either no or only minimal IL-2 and IL-15 mRNA expression was observed in the patients examined. The mean mRNA expression for IL-15 for the patients was not different from that of normal control PBMC and was <10% of that of the positive control cell line HUT-102. Furthermore, the IL-2 mRNA expression was comparable to normal PBMC and was <5% of normal PBMC stimulated with ionomycin/PMA. Furthermore, little or no secreted IL-2 and IL-15 was detected by ELISA performed on the 6-day culture supernatants of the PBMC of the six patients with T-LGL studied. In addition, these supernatants did not stimulate the proliferation of the IL-2- and IL-15-responsive cytokine-dependent cell line NK-92. Taken as a whole these studies suggest that the monoclonal T-LGL leukemic cells of the patients examined were not in an autocrine IL-2- or IL-15-mediated self-stimulatory phase, nor were they actively responding to IL-2 or IL-15 produced by the patients' nonleukemic cells. Thus, the patients with T-LGL did not fulfill the basic premise of the study that demanded that there was IL-2- or IL-15-induced proliferation of leukemic LGL that in turn could be inhibited by the administration of an anti-IL-2/IL-15Rβ-directed monoclonal antibody.
Discussion
We evaluated the toxicity, pharmacokinetics, and activity of the murine monoclonal antibody Mikβ1 directed toward IL-2/IL-15Rβ (CD122) in patients with CD122-expressing T-LGL with hematocytopenia. No dose-limiting toxicity beyond a common toxicity criteria grade 2 increase in serum bilirubin in one case and grade 2 fever in two patients was observed after four administrations of Mikβ1 up to a dose of 1.5 mg/kg. Furthermore, the murine monoclonal antibody did not elicit an immune response. On the basis of the pharmacokinetic and pharmacodynamic studies, the 1.5 mg/kg dose was sufficient to maintain unbound antibody in the circulation until the subsequent administration of the antibody on this 1-, 4-, 7-, and 10-day schedule. Furthermore, it was sufficient to saturate and/or modulate the CD122 receptor from the leukemic cell surfaces. The murine Mikβ1 was specific for the IL-2/IL-15Rβ receptor in that it blocked the proliferative effect of IL-15 when assessed using cytokine-dependent T and NK cell lines. The antibody did not provide effective blockade of IL-2 when the high-affinity heterotrimeric IL-2 receptor was expressed. However, the antibody blocked the action of IL-2 in cells such as T-LGL cells that express the intermediate-affinity IL-2 receptor that does not include IL-2Rα but involves only the IL-2/IL-15Rβ receptor subunit and the common γ-subunit. The T cells of the three T-LGL patients studied manifested ex vivo proliferation in response to 5,000 pg of IL-2 and to 20,000 pg of IL-15. These cytokine-induced proliferations were abrogated by the addition of 10 μg/ml Mikβ1 to the cultures.
Despite its capacity to block IL-2- and IL-15-induced stimulation of IL-2/IL-15β-expressing and common γ-chain-expressing T-LGL cells, no therapeutic efficacy was observed after administration of four doses of murine Mikβ1 over a 10-day period. A number of factors may underlie this lack of efficacy. One potential factor is that the time period of blockade of the IL-2/IL-15 receptor achieved (10-12 days) may not be sufficient for effective action. In support of this possibility is the observation that, even in patients who ultimately responded favorably to cyclosporin A administration, a period of ≈3 months of therapy may be required for the initial response. A second factor is that use of murine Mikβ1 does not fix complement and does not manifest antibody-dependent cellular cytotoxicity with human mononuclear cells (18). To address these issues of pharmacokinetics and function, a humanized form of Mikβ1 was generated that manifests antibody-dependent cellular cytotoxicity with human mononuclear cells (18). An additional advantage in the use of humanized as opposed to murine Mikβ1 is in the pharmacokinetics of the antibodies. Murine Mikβ1 in cynomolgus monkeys had a terminal t1/2 of survival in the serum of 36 h as compared with 96 h with the humanized version. These differences parallel those with anti-Tac (anti-IL-2Rα, CD25) where the murine version had a terminal t1/2 of 40 h and the humanized version had a terminal t1/2 of 103 h in cynomolgus monkeys. In studies in humans, humanized anti-Tac manifested a terminal t1/2 in its serum die-away curve of 21 days (34). We hope that a comparable prolongation of survival of the humanized version of the Mikβ1 monoclonal antibody will be observed in humans. This prolonged action may maintain the long-term saturation of the CD122 receptor that may be required for effective therapy in T-LGL. These characteristics manifested by humanized Mikβ1, but not the murine form, were associated with greater efficacy in inhibiting cardiac allograft rejection in a cynomolgus monkey model. In particular, the humanized but not the murine version of Mikβ1 was effective in prolonging cardiac allograft survival in cynomolgus monkeys (19).
A dominant factor that may underlie the lack of efficacy of this therapeutic modality in patients with LGL is that the basic premise of the study was not fulfilled by the monoclonal T-LGL cells studied. The premise was that IL-2 and IL-15 are required for the survival and function of the T-LGL cells and that Mikβ1 would block the interactions of IL-2 and IL-15 with the IL-2/IL-15Rβ receptor. However, in the present study the cells examined during the monoclonal leukemic phase of the disease did not appear to produce or require IL-2 or IL-15 for their survival. In particular, the T-LGL cells did not manifest ex vivo spontaneous proliferation, a hallmark of leukemic cells that are in a cytokine-mediated autocrine phase of the disease. Furthermore, the leukemic cells did not produce biologically meaningful quantities of mRNA encoding IL-2 or IL-15, nor did they secrete these cytokines into the media during ex vivo cultures. Thus, to be effective in patients in the noncytokine-dependent phase of T-LGL, the anti-CD122 antibody would have to have manifest cytotoxic activity. This requirement is not met by murine Mikβ1; however, it is a characteristic of humanized Mikβ1 that manifests antibody-dependent cellular cytotoxicity (18).
Potentially more favorable targets for the action of Mikβ1, especially its humanized version, would be in the therapy of autoimmune diseases wherein disorders of IL-15 action have been reported and where it has been suggested that the interaction of IL-15 with its receptor subunits plays a pivotal role in the generation and maintenance of the autoimmune disorder (8, 11-16). For example, excessive IL-15 action has been demonstrated in patients with rheumatoid arthritis and has been proposed to be a required element in the pathogenesis of rheumatoid arthritis, inflammatory bowel disease, celiac disease, psoriasis, and multiple sclerosis (4, 8, 11-16, 35, 36). Furthermore, diseases casually associated with infection with the retrovirus HTLV-I overexpress IL-15 and its receptor (14). Ex vivo studies of PBMC from these patients suggest that the blockade of IL-2 and IL-15 actions by human Mikβ1 may be effective. For example, the spontaneous ex vivo proliferation of PBMC from patients with HTLV-I-associated myelopathy/tropical spastic paraparesis was inhibited, in part, by human Mikβ1 (14). Furthermore, by using tetramer technology, the addition of Mikβ1 to cells cultured ex vivo for 6 days from HTLV-I-associated myelopathy/tropical spastic paraparesis patients led to a decline in the persistence of tax antigen-specific CD8+ cells, which have been suggested to play a role in the damage to the central nervous system (14).
Abnormalities of IL-15 have also been reported in association with the development and progression of rheumatoid arthritis. McInnes et al. (11) reported elevated levels of IL-15 in this disease. Furthermore, they demonstrated that IL-15 induced TNF-α in select circumstances and suggested that IL-15 may precede TNF-α in the cytokine cascade (11). TNF-α-directed therapy has proven to be of value in the treatment of patients with refractory rheumatoid arthritis (37). However, such therapy directed toward TNF-α has the limitation that TNF-α does not act on memory CD8 T cells that may be directed toward normal host tissues. An advantage of IL-15-receptor-directed rather than TNF-α-directed therapy would be that administration of a monoclonal antibody targeting IL-15 or its receptor not only might yield antiinflammatory effects but might also terminate the IL-15-mediated persistence of CD8 memory cells, including those directed toward host tissues. In light of the lack of toxicity and lack of immunogenicity of the antibody observed in the present study and the role for IL-15 in the pathogenesis of autoimmune diseases, clinical trials are needed using the humanized version of Mikβ1 in groups of patients with HTLV-I-associated myelopathy/tropical spastic paraparesis, rheumatoid arthritis, multiple sclerosis, and refractory celiac disease.
Materials and Methods
Patient Population. The eligibility requirements were as follows: (i) histologically confirmed T-LGL; (ii) clinically significant hematocytopenia defined as an absolute granulocyte count of <1,000 per mm3, a hemoglobin count of <8.0 g/dl, or a platelet count of <50,000 per mm3;(iii) circulating mononuclear cells that contain a monoclonal T cell population as demonstrated by Southern blot or PCR analysis of T cell antigen receptor β-chain gene rearrangement; and (iv) a leukemic cell phenotype of CD3+, CD8+, CD122+ (IL-2/IL-15Rβ), and CD4-. Patients with or without previous chemotherapy were eligible for inclusion in the study.
Twelve patients (median age, 57 years; range, 22-77 years; see Table 1) were treated. The patients' white blood cell count ranged from 1,760 to 15,700 per mm3 (geometric mean, 4,994), and the lymphocyte counts ranged from 1,152 to 13,172 per mm3. The neutrophil count ranged from 0 to 1,710 per mm3. The number of leukemic cells characterized as CD8+, CD122+ ranged from 632 to 12,408 per mm3 (geometric mean, 3,071) (Table 2). The phenotype was CD2+, CD3+, CD7+ (11 of 12 patients), CD4-, CD8+, CD122+, CD57+ (11 of 12 patients), HLA-DR+ (8 of 10 patients). The CD4:CD8 ratio ranged from 0.06 to 0.38 (geometric mean, 0.19). The cells of eight of the patients were CD16+, and the cells of one of the patients were CD56+. The circulating mononuclear cells of all of the patients showed high-level expression of the CD122 (IL-2/IL-15Rβ), the target of the Mikβ1 monoclonal antibody.
Table 1. Characteristics of patients with T-LGL before therapy with Mikβ1.
| Patient no. | Age, years | Sex | Race | Receiving G-CSF before study entry | No. of white blood cells per mm3 | No. of neutrophils per mm3 | Anemia | Thrombocytopenia | Rheumatoid arthritis | History of skin rash | History of infection | TCR rearrangement |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | F | H | N | 13,000 | 110 | N | N | Y | Y | Y | Y |
| 2 | 60 | F | W | N | 2,600 | 406 | Y | N | N | N | Y | Y |
| 3 | 65 | F | B | N | 4,200 | 0 | N | N | N | N | Y | Y |
| 4 | 60 | M | W | N | 3,800 | 234 | N | N | N | N | Y | Y |
| 5 | 58 | F | W | N | 6,900 | 42 | N | N | N | Y | Y | Y |
| 6 | 61 | M | W | Y | 3,900 | 62 | Y | N | Y | Y | Y | Y |
| 7 | 52 | M | W | Y | 1,760 | 23 | N | N | N | N | Y | Y |
| 8 | 62 | M | W | N | 15,700 | 435 | Y | N | N | N | N | Y |
| 9 | 53 | F | W | N | 8,900 | 885 | Y | N | Y | N | Y | Y |
| 10 | 77 | M | W | N | 11,800 | 1,710 | Y | N | N | Y | Y | Y |
| 11 | 22 | F | W | N | 3,360 | 978 | N | N | N | N | Y | Y |
| 12 | 48 | M | W | N | 1,700 | 303 | Y | Y | Y | N | N | Y |
F, female; M, male; H, Hispanic; B, black; W, white; N, no; Y, yes; TCR, T cell antigen receptor.
Table 2. Immunophenotype of patients with T-LGL before therapy with Mikβ1.
| Patient no. | CD2 | CD3 | CD4 | CD7 | CD8 | CD4/8 ratio | CD16 | CD56 | CD57 | CD122 | HLA-DR | No. of white blood cells per mm3 | No. of lymphocytes per mm3 | No. of CD8+ CD122+ cells per mm3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | – | + | + | 0.17 | – | – | + | + | + | 7,200 | 6,400 | 4,096 |
| 2 | + | + | – | – | + | 0.08 | + | – | + | + | + | 2,200 | 1,628 | 1,389 |
| 3 | + | + | – | + | + | 0.30 | + | – | + | + | + | 4,100 | 3,690 | 2,553 |
| 4 | + | + | +/– | + | + | 0.38 | – | – | + | + | – | 2,600 | 1,950 | 1,342 |
| 5 | + | + | – | + | + | 0.36 | – | – | + | + | + | 4,200 | 3,440 | 2,368 |
| 6 | + | + | – | + | + | 0.10 | + | – | + | + | + | 8,210 | 5,940 | 5,643 |
| 7 | + | + | +/– | + | + | 0.35 | – | – | + | + | + | 1,760 | 1,240 | 632 |
| 8 | + | + | – | + | + | 0.09 | + | + | + | + | + | 14,800 | 13,172 | 12,408 |
| 9 | + | + | – | + | + | 0.07 | + | – | + | + | + | 3,030 | 1,673 | 1,531 |
| 10 | + | + | – | + | + | 0.06 | + | – | + | + | + | 11,000 | 8,921 | 8,302 |
| 11 | + | + | – | + | + | 0.70 | + | – | – | + | NA | 3,360 | 1,636 | 761 |
| 12 | + | + | – | + | + | 0.41 | + | – | + | + | NA | 1,580 | 1,152 | 766 |
NA, not available.
Treatment Plan. Patients with T-LGL were entered at three sequential dose levels of administered Mikβ1. Four patients in group 1 received 0.5 mg/kg. Three patients in group 2 received 1.0 mg/kg, whereas five patients in group 3 received 1.5 mg/kg i.v. on days 1, 4, 7, and 10. Adverse events were assessed by using the National Cancer Institute common toxicity criteria. The protocol was approved by the National Cancer Institute's Institutional Review Board, and the patients gave informed consent.
Monoclonal Antibody Production. The production and characterization of murine Mikβ1 were described in ref. 18.
Functional Assay of Mikβ1 as an Inhibitor of IL-15- and IL-2-Induced Proliferation of a Cytokine-Dependent Cell Line. To examine the specificity of the antibodies used in these studies, proliferation assays were performed with the cytokine-dependent cell lines 32Dβ and Kit-225, which were both IL-2- and IL-15-responsive (1, 32). Cells were seeded for tissue culture with no cytokine or with exogenous IL-2 (a gift from Hoffmann-La Roche) or IL-15 (PeproTech, Rocky Hill, NJ). The concentration of IL-2 used with 32Dβ that does not express IL-2Rα was 1 ng/ml, whereas that for Kit-225 that expresses the high-affinity IL-2 receptor was 500 pg/ml. The concentration of IL-15 used with the 32Dβ cell line was 20 ng/ml, and that for Kit-225 was 3.0 ng/ml. Serial 2-fold dilutions of humanized anti-Tac or Mikβ1 from 10 to 0.08 μg/ml were added to the cell lines supplemented with IL-2 or IL-15. Proliferation was measured by [3H]thymidine uptake in the presence and absence of the antireceptor antibodies after 16- to 18-h cultures of the cells with cytokine with [3H]thymidine addition during the final 4 h.
Receptor Saturation During Monoclonal Antibody Therapy with Mikβ1. The relative IL-2/IL-15β receptor occupancy by Mikβ1 was studied in patients before and after therapy by flow cytometric analysis with two different fluorochrome-labeled antibodies, Mikβ1 and Mikβ3. The two antibodies identify noncompeting epitopes on the IL-2/IL-15Rβ chain. Before infusion both antibodies were capable of detecting the β-chain of the IL-2/IL-15 receptor on the leukemic cell surfaces. After infusion, if saturation was achieved, the occupancy of the receptor by the infused Mikβ1 would block the ability of fluorochrome-labeled Mikβ1 to bind to the circulating cells. Saturation of the receptor or modulation of the receptor from the cell surfaces by the infused Mikβ1 was assessed by comparing the relative mean fluorescence intensity of binding of the fluorochrome-labeled Mikβ1 observed before and after therapy. Mikβ3 binding would not be affected unless there were destruction of the leukemic cells or modulation of the receptor from the cell surfaces mediated by their interaction with Mikβ1. The binding of Mikβ3 and assessment of tumor cell number were used to detect such receptor internal modulation.
Assays for IL-2 and IL-15 Production by T-LGL Cells. The patient's PBMC were studied ex vivo by real-time PCR by using the TaqMan procedure to quantitate mRNA encoding IL-2 and IL-15. The quantity of IL-2 and IL-15 produced and secreted by the patients' cells after 6 days of ex vivo culture in RPMI medium 1640 with 10% FBS were quantitated by using IL-2- and IL-15-specific ELISA plates according to the manufacturer's instructions (R & D Systems).
Real-Time Quantitative PCR. The patient's PBMC were studied ex vivo by real-time PCR by using the ABI Prism 7700 sequence detection system (Applied Biosystems) to quantitate RNA encoding IL-2 and IL-15. Predesigned primers and probes for human IL-2, human IL-15, and human hypoxanthine phosphoribosyltransferase used in this assay were purchased from Applied Biosystems.
Molecular Genetic Analysis of T Cell Antigen Receptor Gene Rearrangements to Define Clonality. Southern blot analysis of T cell antigen receptor gene rearrangements demonstrating clonality were performed on 10 patients as described in ref. 38. The clonality in the two other patients was demonstrated by PCR analysis.
Spontaneous PBMC Proliferation in Patients with T-LGL. The spontaneous proliferation of PBMC from 10 patients with T-LGL and 10 normal controls was studied (33). PBMC obtained by Ficoll centrifugation were washed and cultured for 6 days in RPMI medium 1640 (BioSource, Rockville, MD), 10% FBS, 0.3 mg/ml glutamine, and incubated at 37°C in 5% CO2. PBMC were incubated with medium alone or with the addition of 10 μg/ml UPC10 antigen as a nonspecific murine IgG 2a Ig control antibody (Sigma), anti-Tac (anti-IL-2Rα, anti-CD25, Metabolism Branch, National Cancer Institute), or murine Mikβ1 (anti-IL-2/IL-15Rβ, anti-CD122). Cells were pulsed after 6 days of culture with 1 mCi of [methyl 3H]thymidine (1 Ci = 37 GBq), and cellular uptake of radioactivity was determined after an additional 4 h of culture.
Immune Responses to Murine Mikβ1. The development of patient antibodies to murine Mikβ1 was examined by using an antigen-bridging ELISA. Briefly, ELISA 96-well plates were coated with 50 ng of murine Mikβ1 (100:1) in PBS overnight at 4°C and then blocked with BSA. Test samples at various dilutions were added to the plates. Biotinylated Mikβ1 was then added to each well and quantitated by the addition of an alkaline phosphatase streptavidin reagent. This reagent was developed by the addition of the enzyme substrate nitrophenol phosphate. Patients were deemed to have seroconverted when the antiglobulin level after treatment was at least 250 ng/ml.
Murine Mikβ1 Serum Levels. Serum concentrations of murine Mikβ1 antibody used in human pharmacokinetic studies were determined by using an additional antigen-bridging ELISA. In this assay, 96-well plates were coated with affinity-purified goat anti-mouse IgG2a and blocked with BSA as above. Standards or test samples were added to the plates followed by a biotinylated noncompeting rabbit anti-mouse IgG2a to detect the bound monoclonal antibody. The assay was developed as above. Levels of Mikβ1 in patient sera were quantitated by comparison to a standard curve of known Mikβ1 concentrations diluted in normal human serum with a sensitivity of 200 ng/ml.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
Author contributions: J.C.M. and T.A.W. designed research; J.C.M., J.E.J., J.D.W., T.A.F., M.B., M.T., C.K.G., B.B., M.P., L.T., C.C.L., and W.G. performed research; T.A.W. analyzed data; and T.A.W. wrote the paper.
Conflict of interest statement: T.A.W. has been granted a patent for the use of antibodies for the IL-2/IL-15β receptor.
Abbreviations: LGL, large granular lymphocyte leukemia; T-LGL, T cell LGL; NK, natural killer; PBMC, peripheral blood mononuclear cell; HTLV-I, human T cell lymphotropic virus I.
References
- 1.Bamford, R. N., Grant, A. J., Burton, J. D., Peters, C., Kurys, G., Goldman, C. K., Brennan, J., Roessler, E. & Waldmann, T. A. (1994) Proc. Natl. Acad. Sci. USA 91, 4940-4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grabstein, K. H., Eisenman, J., Shanebeck, K., Rauch, C., Srinivasan, S., Fung, V., Beers, C., Richardson, J., Schoenborn, M. A., Ahdieh, M., et al. (1994) Science 264, 965-968. [DOI] [PubMed] [Google Scholar]
- 3.Waldmann, T. A. & Tagaya, Y. (1999) Annu. Rev. Immunol. 17, 19-49. [DOI] [PubMed] [Google Scholar]
- 4.Waldmann, T. A., Dubois, S. & Tagaya, Y. (2001) Immunity 14, 105-110. [PubMed] [Google Scholar]
- 5.Marks-Konczalik, J., Dubois, S., Losi, J. M., Sabzevari, H., Yamada, J., Feigenbaum, L., Waldmann, T. A. & Tagaya, Y. (2000) Proc. Natl. Acad. Sci. USA 97, 11445-11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang, X., Sun, S., Hwang, L., Tough, D. F. & Sprent, J. (1998) Immunity 8, 591-599. [DOI] [PubMed] [Google Scholar]
- 7.Ku, C. C., Murakami, M., Sakamoto, A., Kappler, J. & Marrack, P. (2000) Science 288, 675-678. [DOI] [PubMed] [Google Scholar]
- 8.Fehniger, T. A. & Caligiuri, M. A. (2001) Blood 97, 14-32. [DOI] [PubMed] [Google Scholar]
- 9.Dubois, S., Mariner, J., Waldmann, T. A. & Tagaya, Y. (2002) Immunity 17, 537-547. [DOI] [PubMed] [Google Scholar]
- 10.Lodoice, J. P., Burkett, P. R., Boone, D. L., Chien, M. & Ma, A. (2001) J. Exp. Med. 194, 1187-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McInnes, I. B., Leung, B. P., Sturrock, R. D., Field, M. & Liew, F. Y. (1997) Nat. Med. 3, 189-195. [DOI] [PubMed] [Google Scholar]
- 12.Villadsen, L. S., Schuurman, J., Beurskens, F., Dam, T. N., Dagnaes-Hansen, F., Skov, L., Rygaard, J., Voorhorst-Ogink, M. M., Gerritsen, A. F., van Dijk, M. A., et al. (2003) J. Clin. Invest. 112, 1571-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mention, J. J., Ahmed, M. B., Bégue, B., Barbe, U., Verkarre, V., Asnafi, V., Colombel, J. F., Cugnenc, P. H., Ruemmele, F. M., McIntyre, E., et al. (2003) Gastroenterology 125, 730-745. [DOI] [PubMed] [Google Scholar]
- 14.Azimi, N., Nagai, M., Jacobson, S. & Waldmann, T. A. (2001) Proc. Natl. Acad. Sci. USA 98, 14559-14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruchatz, H., Leung, B. P., Wei, X. Q., McInnes, I. B. & Liew, F. Y. (1998) J. Immunol. 160, 5654-5660. [PubMed] [Google Scholar]
- 16.Kim, Y. S., Maslinski, W., Zheng, X. X., Stevens, A. C., Li, X. C., Tesch, G. H., Kelley, V. R. & Strom, T. B. (1998) J. Immunol. 160, 5742-5748. [PMC free article] [PubMed] [Google Scholar]
- 17.Tsudo, M., Kitamura, F. & Miyasaka, M. (1989) Proc. Natl. Acad. Sci. USA 86, 1982-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hakimi, J., Ha, V. C., Lin, P., Campbell, E., Gately, M. K., Tsudo, M., Payne, P. W., Waldmann, T. A., Grant, A. J., Tsien, W.-H. & Schneider, W. P. (1993) J. Immunol. 151, 1075-1085. [PubMed] [Google Scholar]
- 19.Tinubu, S. A., Hakimi, J., Kondas, J. A., Bailon, P., Familletti, P. C., Spence, C., Crittenden, M. D., Parenteau, G. L., Dirbas, F. M., Tsudo, M., et al. (1994) J. Immunol. 153, 4330-4338. [PubMed] [Google Scholar]
- 20.Loughran, T. P., Jr. (1993) Blood 82, 1-14. [PubMed] [Google Scholar]
- 21.Loughran, T. P., Jr., & Starkebaum, G. (1987) Medicine 66, 397-405. [PubMed] [Google Scholar]
- 22.Broucet, J.-C., Sasportes, M., Flandrin, G., Preud'Homme, J. L. & Seligman, M. (1975) Lancet 306, 890-893. [DOI] [PubMed] [Google Scholar]
- 23.Loughran, T. P., Jr., Aprile, J. A. & Ruscetti, F. W. (1990) Blood, 75, 935-940. [PubMed] [Google Scholar]
- 24.Tagawa, S., Hatakcyama, M., Shibano, M., Taniguchi, T. & Kitani, T. (1988) Blood 71, 1161-1164. [PubMed] [Google Scholar]
- 25.Tsudo, M., Goldman, C. K., Bongiovanni, K. F., Chan, W. C., Winton, E. F., Yagita, M., Grimm, E. A. & Waldmann, T. A. (1987) Proc. Natl. Acad. Sci. USA 84, 5394-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waldmann, T. A., Davis, M. M., Bongiovanni, K. F. & Korsmeyer, S. J. (1985) N. Engl. J. Med. 313, 776-783. [DOI] [PubMed] [Google Scholar]
- 27.Vie, H., Chevalier, S., Garand, R., Moisan, J.-P., Praloran, V., Devilder, M.-C., Moreau, J. F. & Soulillou, J.-P. (1989) Blood 74, 285-290. [PubMed] [Google Scholar]
- 28.Semenzato, G., Pandolfi, F., Chisesi, T., De Rossi, G., Pizzolo, G., Zambello, R., Trentin, L., Agostini, C., Dini, E., Vespignani, M., et al. (1987) Cancer 60, 2971-2978. [DOI] [PubMed] [Google Scholar]
- 29.Zambello, R., Trentin, L., Pizzolo, G., Bulian, P., Masciarelli, M., Feruglio, C., Agostini, C., Raimondi, R., Chisesi, T. & Semenzato, G. (1990) Blood 76, 2080-2085. [PubMed] [Google Scholar]
- 30.Alileche, A., Goldman, C. K. & Waldmann, T. A. (2001) Biochem. Biophys. Res. Commun. 285, 1302-1308. [DOI] [PubMed] [Google Scholar]
- 31.Giri, J. G., Kumaki, S., Ahdieh, M., Friend, D. J., Loomis, A., Shanebeck, K., DuBose, R., Cosman, D., Park, L. S. & Anderson, D. M. (1995) EMBO J. 14, 3654-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guex-Crosier, Y., Raber, J., Chan, C. C., Kriete, M., Levin, W., Kersin, J. A., Pilson, R. S., Waldmann, T. A., Hakimi, J. & Roberge, F. G. (1997) J. Immunol. 158, 452-458. [PubMed] [Google Scholar]
- 33.Tendler, C. L., Greenberg, S. J., Blattner, W. A., Manns, A., Murphy, E., Fleisher, T., Hanchard, B., Morgan, O., Burton, S. D., Nelson, D. L., et al. (1990) Proc. Natl. Acad. Sci. USA 87, 5218-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vincenti, F., Kirkman, R., Light, S., Bumgardner, G., Pescovitz, M., Halloran, P., Neylan, J., Wilkinson, A., Ekberg, H., Gaston, R., et al. (1998) N. Engl. J. Med. 338, 161-165. [DOI] [PubMed] [Google Scholar]
- 35.Kivisakk, P., Matusevicius, D., He, B., Soderstrom, M., Frederikson, S. & Link, H. (1998) Clin. Exp. Immunol. 111, 193-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menton, J. J., Ben, A., Begue, B., Barbe, U., Verkarre, V., Asnafi, V., Colombel, J. F., Cugnenc, P. H., Ruemmele, F. M., McIntyre, E., et al. (2003) Gastroenterology 125, 730-745. [DOI] [PubMed] [Google Scholar]
- 37.Feldmann, M., Brennan, F. M. & Maini, R. N. (1996) Annu. Rev. Immunol. 14, 397-440. [DOI] [PubMed] [Google Scholar]
- 38.Waldmann, T. A., White, J. D., Goldman, C. K., Top, L., Grant, A., Bamford, R., Roessler, E., Horak, I., Zaknoen, S., Kasten-Sportes, C., et al. (1993) Blood 82, 1701-1712. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.