Abstract
We use chronologies of stable isotopes measured from elephant (Loxodonta africana) hair to determine migration patterns and seasonal diet changes in elephants in and near Samburu National Reserve in northern Kenya. Stable carbon isotopes record diet changes, principally enabling differentiation between browse and tropical grasses, which use the C3 and C4 photosynthetic pathways, respectively; stable nitrogen isotopes record regional patterns related to aridity, offering insight into localized ranging behavior. Isotopically identified range shifts were corroborated by global positioning system radio tracking data of the studied individuals. Comparison of the stable isotope record in the hair of one migrant individual with that of a resident population shows important differences in feeding and ranging behavior over time. Our analysis indicates that differences are the result of excursions into mesic environments coupled with intermittent crop raiding by the migrant individual. Variation in diet, quantified by using stable isotopes, can offer insight into diet-related wildlife behavior.
Keywords: 13-carbon, 15-nitrogen, chronology, human–elephant conflict
The stable isotope ratios of 13C/12C in hair records the diet of mammals (1–4). It is particularly useful in distinguishing diets of C3 browse versus C4 grass in tropical regions (5–7) because of the large difference in 13C/12C ratios between plants using the C3 and C4 photosynthetic pathways, respectively. In tropical regions, the C3 pathway is used primarily by trees and shrubs, whereas plants using the C4 pathway are principally grasses (8, 9).
Hair is a particularly useful indicator of diet change (3, 4) because the isotope turnover of mammal tissues is high enough to resolve short-term diet changes. Recent advances in methodology, progressed through the study of large mammals with controlled diet changes (10, 11), allows detailed reconstruction of the diet history of individual large mammals in wild populations (12, 13).
In this study, we determine the growth rates and stable 13C/12C and 15N/14N ratios in elephant hair collected between 2001 and 2004. We focus on the behavior of a resident population of Samburu National Reserve, Northern Kenya, for the time period of 2000 to 2002. We compare stable isotope results of this resident population with a migrant elephant (B1013) that visited Samburu Reserve up to several times each year. Differences in isotope ratios between the resident individuals and the migrant indicate different behaviors, including rapid migration across long distances by the migrant individual and differences in the fraction C4 biomass in the diet. The latter may be related to seasonal crop raiding, which can be quantified by using stable isotope ratios.
Materials and Methods
Global Positioning System (GPS) radio collars were fitted to elephants in Samburu National Reserve, Northern Kenya, between 2001 and 2004 (14). Collars were programmed to record positions at hourly intervals, offering detailed records of movement. Tail hairs from 35 elephants were collected over this period during immobilization operations while the collars were being fitted and for some, later, when collar batteries were being changed or when the collars were being removed. Of these 35 elephants, 7 have a secure isotopic dietary chronology over the period from late 2000 and extending through February 2002; GPS spatial information is available for all resident individuals from July 2001 to July 2002 and from February 2002 to July 2002 for the migrant B1013. We focus on comparing the behavior of the migrant B1013 to that of the resident Samburu group during this time interval.
The elephants tracked in this study used both the semiarid region in and around Samburu National Reserve and the mesic Imenti Forest on the flanks of Mt. Kenya. These two distinct ecotones are <60 km apart and are located between 37° and 38° east, just north of the equator. The elevation of the semiarid Samburu region is ≈900 m above sea level and dominated by acacia–comiphora savanna and scrub bushland. Rainfall averages ≈350 mm per year in this lowland region and occurs during biannual rainy seasons, which generally take place in April and November. The elevation of the Imenti Forest is ≈2,000 m above sea level and is dominated by evergreen and broad leaf deciduous tree species. Rainfall in this region also occurs biannually and averages ≈900 mm per year.
In this study, we used 10-day composite normalized differential vegetation index (NDVI) data, available through Satellite Probatoire d'Observation de la Terre (SPOT), to determine changes in season across the study area. NDVI is a remote sensing index value calculated as the ratio between red and near infrared reflection that is highly correlated with green (photosynthetically active) biomass (15, 16). Remotely sensed data provide a direct measure of photosynthetic activity over large spatial regions, offering advantages over the classically used point-sampled rainfall data in areas, like the study region, where weather stations are sparse. Isotope profiles for each elephant were compared with longitudinal 10-day NDVI records to determine the impact of seasonality on diet.
13C/12C and 15N/14N ratios of elephant hair and plant material were measured on an isotope ratio mass spectrometer after combustion in a flow-through modified Carlo–Erba system. Values are reported using the conventional permil (‰) notation, where
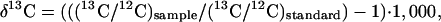 |
and an analogous terminology describes 15N/14N ratios. Standards are Vienna Pee Dee Belemnite (VPDB) and air for δ13C and δ15N, respectively. Uncertainties for average δ15N values for plants discussed in the text are reported as the standard error. Isotope enrichment between hair and diet is ≈3‰ for both δ13C and δ15N (7–9).
Elephant hair was serially sectioned in 5-mm intervals before stable isotope analysis. For one hair from B1013, sectioning was performed at ≈1-mm intervals for detailed isotope profiles over selected intervals. Tail hair growth rates in Kenya elephants were established in three ways: (i) using overlapping stable isotope patterns in hair collected from the same individual two or more times (12); (ii) using overlapping isotope patterns from individuals having similar geographical ranges but collected at different times (13); and (iii) using isotope data along with visual sightings. One individual, B1013, had only a single hair that was collected during collaring operations; its growth rate of hair was calibrated by comparing δ15N values with visual sightings in Samburu National Reserve.
Diet was estimated using the isotope turnover pool model of Ayliffe et al. (10, 12). We use end-member values of -27‰ and -13‰ for C3 and C4 plants, respectively, which represent average ecosystem values for Samburu National Reserve and nearby regions (7).
Results and Discussion
Growth Rates of Elephant Hair. Hair growth rates for male and female elephants are 0.55 ± 0.11 mm/day (9 hairs from 5 individuals) and 0.81 ± 0.13 (23 hairs from 11 individuals), respectively, and are significantly different (ANOVA, P < 0.0001). Within one individual, the growth rate of different hairs may vary by up to 30%, but growth rates for a given hair appears to be constant to within 5–10%, which agrees with previous observations for equids (10, 11). The overall range in tail hair growth rates is from 0.4 to 1.1 mm per day, with single hair lengths sometimes exceeding 500 mm, thus preserving diet or migration information for periods often exceeding 1 year for a single hair.
Spatial Distribution and Stable Isotope Ratios. GPS radio collars were fitted to the seven elephants (four female, three male) of this study in Samburu National Reserve in Northern Kenya in 2001 and 2002 (14). Six of the seven elephants were visually observed to be in Samburu National Reserve or in the immediate vicinity during the observation period from early 2001 to July 2002, and this was corroborated with the GPS information; this group constitutes the Samburu resident elephants. However, one elephant, an ≈40-year-old bull elephant (B1013), collared on February 3, 2002, had a very different movement history than the Samburu resident elephants for the period from February 2002 to July 2002. The GPS collar recorded the hourly positions of B1013's movements between February 3, 2002 and July 18, 2002, when the collar failed. Unfortunately, B1013 was shot and killed shortly thereafter, and a second hair could not be recovered. During this period, B1013 made three major trips, shifting from the arid lowlands of Samburu National Reserve to the mesic Imenti upland forest near Mt. Kenya or vice versa. The periods spent in the lowlands correspond to the mid- and late rainy season, and periods spent in the forest were during the dry season. Each range shift was accomplished in <15 h, covering a straight-line distance over 40 km, a behavior pattern described as “streaking” (14). It is assumed that B1013 moved in the same pattern in 2001, when the isotope data are available from a single hair, as from February 2002 to July 2002, when the GPS data are available.
Stable isotope analyses at 5-mm intervals (δ13C and δ15N) of the hair collected on February 3, 2002 showed that this individual occupied two isotopically distinct regions (Fig. 1B). The δ15N records environmental information, including δ15N values related to regional vegetation. The hair segments with elevated δ15N values (from 8‰ to 10.5‰) corresponded to values observed in resident elephants of Samburu National Reserve (average11‰ for the same time period). Plants in the mesic highlands of the Aberdares and Mt. Kenya region have δ15N values averaging 1.7 ± 0.5‰ (n = 21), whereas those in xeric lowlands of Samburu National Reserve average 7.9 ± 0.7‰ (n = 14); these values are significantly different (ANOVA; P < 0.0001). Enrichment in δ15N for hair compared with diet is ≈3‰, suggesting that equilibrium δ15N values for these two regions should be ≈5‰ and 11‰, respectively. Moving as he did between the two regions, B1013 never attained isotope equilibrium with the environment in either region. Thus, the movement data and the isotope data show that B1013 occupied the highlands (where the δ15N values are low) in the dry season, and in the rainy season he moved to the lowlands, where δ15N values are elevated.
Fig. 1.
NDVI and stable isotope record of B1013 compared with other elephants. (A) Average 10-day composite NDVI values for the Samburu National Reserve region from 2001 through 2002, where elevated values indicate increased vegetative productivity occurring during wet seasons. (B) δ15N of hair from B1013 (black line; n = 83) and observations of B1013 in Samburu National Reserve (black rectangles; n = 17) during 2001 and 2002 compared with the range of average values of δ15N (±2σ; gray region; n = 520) in hairs of six resident elephants of Samburu National Reserve. Chronology was established as described in the text. Each average value (with standard deviation) of the Samburu National Reserve residents was averaged during the 10-day intervals coinciding with NDVI composite data. (C) δ13C from hair of B1013 (open circles; n = 83) compared with the average δ13C (±2σ; gray region; n = 520) values of hair from six resident elephants of Samburu National Reserve. (D) Estimated C4 component of diet of B1013 (open circles; n = 83) compared with the average of six resident elephants of Samburu National Reserve. Gray area shows the diet (±2σ; gray region, n = 520) of six resident elephants of Samburu National Reserve. Diet as percent C4 biomass was estimated as in ref. 12.
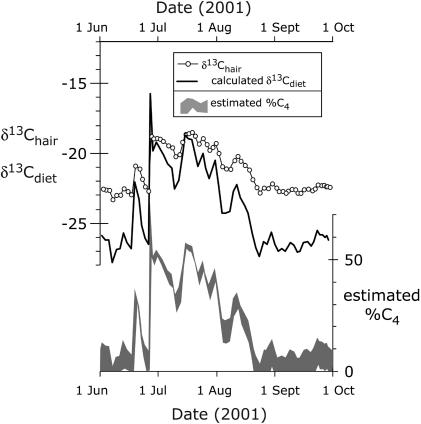
Seasonal Diet Changes. According to the chronology established here, B1013 had four periods of increased consumption of C4 grass. Three of these periods were associated with wet seasons and periods of elevated remotely sensed NDVI values, representing increased vegetative productivity. In general, African elephants have a diet dominated by browse (6) and consume grass only as a windfall when it is in new growth. These periods of grass consumption occurred when B1013 was observed in Samburu National Reserve and when NDVI values exceeded 0.3. This pattern of elevated C4 consumption during wet seasons was comparable with observed patterns in resident Samburu elephants (Fig. 1C); during these intervals, B1013 had similar C4 grass consumption as the other six elephants. The single exception is the highest C4 consumption peak, which occurred during the dry season between June 18 and August 16, 2001. By comparison, the other six elephants had a low fraction of C4 grass consumption during this dry season. During this interval, while B1013 was in the Imenti Forest, average NDVI was low, yet C4 grasses made up 36% of the diet integrated over this interval, with peak values approaching 50% C4 biomass. NDVI in the Imenti region was below the yearly average from June 11 to November 21, 2001, corresponding with the dry season period when vegetative productivity is decreased.
GPS tracking data from the subsequent period spent in Imenti reveals that, while in residence of the Imenti Forest, B1013 was outside the forest reserve boundaries only during nighttime hours (Fig. 2). Most elephant crop raiding occurs at night, apparently to avoid human interaction (17, 18). Subsistence farming occurs adjacent to the Imenti Forest (19), and this region, around the time of the data collection, was one of the major crop raiding conflict zones in Kenya. Thus, it appears that this isotope signal is a quantifiable record of crop raiding (Fig. 3). While in the Imenti Forest, C3 vegetation was the principal food available, and therefore the C4 component of diet was most likely obtained from adjacent crops, evidently at night. During the dry season between June and August of 2001, our study indicates that B1013 was supplementing his C3 forest diet with nocturnal crop raiding of C4 maize. Fig. 3 shows a detailed stable isotope profile along with the estimated fractionation C4 intake during this unusual C4 diet period during the long dry season of 2001.
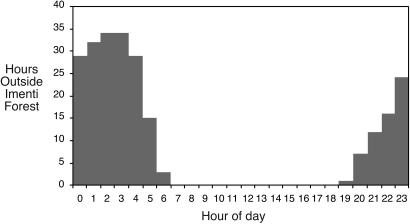
Fig. 2.
Histogram of hours spent by B1013 outside Imenti Forest. In total, 2,179 h of GPS positions were recorded when B1013 was in or around the Imenti Forest. During this period, 236 h of GPS positions (10.8%) were recorded outside the designated forest boundaries in areas dense with subsistence farming. Being essentially on the equator, the sun rises and sets at 6:30 every day with little variation. All but 2 of the 236 h spent outside the Imenti Forest were during night hours during this interval.
Fig. 3.
Detail of diet for B1013 from June 1 to October 1, 2001 using ≈1-mm hair segments. The diet estimate of C4 fraction uses C3 and C4 endmember mixing lines for both mesic (upper boundary) and xeric environments (lower boundary). These have average δ13C values of -28‰ and -12‰ for mesic environments and -26‰ and -13‰ for xeric environments, respectively. Average interval of time is 1.8 days (maximum, 2.4 days; minimum, 0.9 days).
Quantification of longitudinal diet records from hair in wild animals can offer important information to conservationists and wildlife managers. This article provides an example of one of the many possible uses that stable isotopes can provide in resolving the elephant–human conflict issue. Despite the recording of conflict incidences, it is often difficult to understand elephant decisions made at an individual level and to quantify the dietary importance of crop raiding by elephants. Combining studies of elephant movement patterns and overall diet with incidences of raiding injects additional scientific data into the highly topical discussion of human–elephant conflict alleviation.
Acknowledgments
We thank J. R. Ehleringer and the Stable Isotope Ratio Facility for Environmental Research (SIRFER) at the University of Utah, the government of Kenya for permission to work in Kenya, the Kenya Wildlife Service for facilitation, Save The Elephants for technical and logistical field support, and the Packard Foundation.
Author contributions: T.E.C., G.W., H.B.R., F.V., and I.D.-H. designed research; T.E.C., G.W., H.B.R., F.V., C.E.C., T.J.R., and I.D.-H. performed research; T.E.C., G.W., and I.D.-H. analyzed data; and T.E.C. and G.W. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: GPS, Global Positioning System; NDVI, normalized differential vegetation index.
References
- 1.DeNiro, M. J. & Epstein, S. (1978) Geochim. Cosmochim. Acta 42, 495-506. [Google Scholar]
- 2.DeNiro, M. J. & Epstein, S. (1981) Geochim. Cosmochim. Acta 45, 341-351. [Google Scholar]
- 3.White, C. D. (1993) J. Archaeol. Sci. 20, 657-666. [Google Scholar]
- 4.Macko, S. A., Engel, M. H., Lubec, V. G., O'Connell, T. C. & Hedges, R. E. M. (1999) Philos. Trans. R. Soc. London B 354, 65-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee-Thorp, J. & van der Merwe, N. J. (1987) S. Afr. J. Sci. 83, 712-715. [Google Scholar]
- 6.van der Merwe, N. J., Lee-Thorp, J. A., Thackeray, J. F., Hall-Martin, A., Kruger, F. J., Coetzee, H., Bell, R. H. V. & Lindeque, M. (1990) Nature 346, 744-746. [Google Scholar]
- 7.Cerling, T. E., Harris, J. M. & Passey, B. H. (2003) J. Mammal. 84, 456-471. [Google Scholar]
- 8.Bender, M. M. (1971) Phytochemistry 10, 1239-1245. [Google Scholar]
- 9.Sage, R. F., Li., M. & Monson, R. K. (1999) in C4 Plant Biology, eds. Sage, R. F. & Monson, R. K. (Academic, San Diego), pp. 551-584.
- 10.Ayliffe, L. K., Cerling, T. E., Robinson, T., West, A. G., Sponheimer, M., Passey, B. H., Hammer, J., Roeder, B., Dearing, M. D. & Ehleringer, J. R. (2004) Oecologia 139, 11-22. [DOI] [PubMed] [Google Scholar]
- 11.West, A. G., Ayliffe, L. K., Cerling, T. E., Robinson, T. F., Karren, B., Dearing, M. D. & Ehleringer, J. R. (2004) Funct. Ecol. 18, 616-624. [Google Scholar]
- 12.Cerling, T. E., Passey, B. H., Ayliffe, L. K., Cook, C. S., Ehleringer, J. R., Harris, J. M., Dhidha, M. B. & Kasiki, S. M. (2004) Palaeogeogr. Palaeoclimatol. Palaeoecol. 206, 367-376. [Google Scholar]
- 13.Cerling, T. E. & Viehl, K. (2004) Afr. J. Ecol. 42, 88-92. [Google Scholar]
- 14.Douglas-Hamilton, I., Krink, T. & Vollrath, F. (2005) Naturwissenschaften 92, 163-168. [DOI] [PubMed] [Google Scholar]
- 15.Goward, S. N. & Prince, S. D. (1995) J. Biogeogr. 22, 549-564. [Google Scholar]
- 16.Diallo, O., Diouf, A., Hanan, N. P., Niaye, A. & Prevost, Y. (1991) Int. J. Remote Sensing 12, 1259-1279. [Google Scholar]
- 17.Thouless, C. R. (1994) Oryx 28, 119-127. [Google Scholar]
- 18.Hoare, R. E. (1995) Pachyderm 19, 54-63. [Google Scholar]
- 19.Gathaara, G. N. (1999) Aerial Survey of the Destruction of Mt. Kenya, Imenti and Ngare Ndare Forest Reserves (Kenya Wildlife Service, Nairobi, Kenya) p. 33