Abstract
Presenilins (PS) are required for γ-secretase cleavage of multiple type I membrane proteins including the amyloid precursor protein and Notch and also have been implicated in regulating intracellular protein trafficking and turnover. Using genetic and pharmacological approaches, we reveal here a unique function of PS in the pigmentation of retinal pigment epithelium and epidermal melanocytes. PS deficiency leads to aberrant accumulation of tyrosinase (Tyr)-containing 50-nm post-Golgi vesicles that are normally destined to melanosomes. This trafficking is γ-secretase-dependent, and abnormal localization of Tyr in the absence of PS is accompanied by the simultaneous accumulation of its C-terminal fragment. Furthermore, we show that the PS1M146V familial Alzheimer's disease mutation exhibits a partial loss-of-function in pigment synthesis. Our results identify Tyr and related proteins as physiological substrates of PS and link γ-secretase activity with intracellular protein transport.
Keywords: γ-secretase, pigmentation, melanocyte, knock-out, knock-in
Mammalian presenilins (PS) consists of two homologous proteins, PS1 and PS2. They are indispensable for processing the amyloid precursor protein (APP) at the γ-secretase site to generate Aβ40 and Aβ42, peptides that constitute the principal components of the β-amyloid plaques characteristic of Alzheimer's disease (1). Mutations in PS1 and PS2 lead to autosomal dominant inheritance of familial Alzheimer's disease (FAD). More than 100 mutations have been identified in PS1 and PS2, and they spread throughout the entire molecules. These mutations are known to alter the γ-secretase activity, often resulting in increased Aβ42 production at the expense of Aβ40 (2, 3). Through similar mechanisms defined as regulated intramembrane proteolysis (4), PS are required for processing and signaling of Notch (5), and this pathway likely contributes to various PS developmental activities (6).
The PS-dependent γ-secretase activity requires the formation of a high-molecular-weight complex containing nicastrin, Aph1, and Pen2 (6). The active complex is assembled in a sequential and interdependent manner through the endoplasmic reticulum and Golgi compartments and requires posttranslational modifications. The γ-secretase cleavage is preceded by extracellular processing and exhibits relaxed sequence specificity. Nicastrin recently was shown to function as the receptor for the γ-secretase substrates (7). Besides APP and Notch, PS has been implicated in the processing of a growing list of type I membrane proteins (reviewed in ref. 8). However, with the exception of Notch, the physiological significance of these proteolytic events remains speculative.
In addition to its γ-secretase activity, PS has been documented to regulate intracellular protein trafficking (9). PS1 deficiency has been reported to result in aberrant trafficking and maturation of APP (10, 11), Notch (12, 13), tyrosine kinase receptor TrkB (14), β- and δ-catenin (15, 16), and nicastrin (17–19). Reports by Esselens et al. (20) and Wilson et al. (21) implicated a role of PS1 in the turnover of telencephalin and α- and β-synucleins, respectively. They showed that these molecules accumulate in degradative organelles resembling autophagosomes in PS1-null neurons, but not by γ-secretase inhibitor treatment (20, 21). Thus, this activity is likely mediated through a γ-secretase independent mechanism.
Similar to APP and Notch, tyrosinase (Tyr) (monophenyl monooxidase, EC 1.14.18.1), along with two Tyr-related proteins, Tyr-related protein-1 (Tyrp1) and 3,4-dihydroxyphenylalanine (DOPA) chrome tautomerase/Tyr-related protein-2 (DCT/Tyrp2), are type I membrane proteins specialized in pigment synthesis (22). Tyr catalyzes the conversion of tyrosine to DOPA, which is an essential and rate-limiting step in melanin synthesis. Subsequent metabolism of DOPA and its derivatives by Tyr, Tyrp1, and DCT/Tyrp2 result in the synthesis of eumelanin (23). These reactions are conducted within the melanosomes of vertebrate pigment cells; the latter consist of retinal pigment epithelium (RPE) and epidermal melanocytes. Melanosomes are endosomal/lysosomal-related organelles. They progress through a series of maturation stages, ranging from stage I premelanosomes, which are coated endosomes lacking pigmentation, to stages II, III, and IV melanosomes, which are striated with increased melanin contents (24). Under normal conditions, Tyr is glycosylated through the secretory pathway, budded off from the trans-Golgi network as 50-nm vesicles, and transported to melanosomes to undergo pigment synthesis and further maturation (25, 26).
Proper regulation of Tyr processing and trafficking is essential for pigment synthesis. Mutations in tyrosinase (Tyr) and Tyrp1 result in retention of immature Tyr in the endoplasmic reticulum and are associated with oculocutaneous albinism type 1 and 3, respectively (27). In addition to the melanocyte-specific proteins, numerous other molecules, including both highly conserved adaptor proteins, soluble N-ethylmaleimide sensitive factor attachment protein receptors, and small GTPases of the Ras superfamily, as well as unique vertebrate-specific protein complexes termed biogenesis of lysosome-related organelles complexes, are known to regulate Tyr trafficking and pigment processes (reviewed in refs. 24 and 28). In addition to melanosomes, these proteins also play important roles in regulating other endosomal/lysosomal-related organelles that are essential for immune or neuronal functions.
In this work, we reveal an indispensable role of PS in Tyr trafficking and pigmentation by a γ-secretase-dependent mechanism, and we document a partial loss-of-function by the PS1M146V FAD mutation in vivo.
Materials and Methods
Abs and Inhibitors. Rabbit polyclonal antisera against Tyrp1 (αPEP1), DCT/Tyrp2 (αPEP8), and C- and N-terminal Tyr αPEP-7 and αPEP-5, respectively, were generously provided by V. J. Hearing (National Institutes of Health, Bethesda). The PS1 Ab AB14 is described in ref. 29. Mouse monoclonal anti-Pmel17 Ab was obtained from Dako. The γ-secretase inhibitor N-[N-(3,5-dif luorophenacetyl-l-alanyl)]-S-phenylglycine t-butyl ester (DAPT) was purchased from Calbiochem. DOPA was available from Sigma.
Mice and Cell Culture. The PS1 and PS2 knock-out, PS1M146V knock-in, and PS rescue animals have been described (30–33). They were backcrossed onto C57BL/6J background to yield animals of black coat colors. Primary melanocytes were established from neonatal skins of C57BL/6J mice. Skins were incubated in 0.25% trypsin for 2 h at 37°C. The epidermis was separated from dermis layer and vortexed for 30 sec to separate the melanocytes from the epidermal cell layer. The melanocytes were plated in melanocyte growth medium, which consisted of M154 basal medium (Cascade Biologicals, Portland, OR) supplemented with 4% heat-inactivated FBS, 1× antibiotic/(antimycotic) solution (Invitrogen), and human melanocyte growth supplement (Cascade Biologicals). All cultures were maintained in a tissue culture incubator at 37°C with 5% CO2. The γ-secretase inhibitor treatment began 1 d after plating of the melanocytes, and the medium was changed every 3–4 d. Concentration at 500 nM was used unless otherwise indicated. Immunostaining and Western blotting was carried out 2 d after treatment, whereas EM and melanin content assay was performed after 2 wk of treatment. DOPA histochemistry was done by incubating the fixed cells with 0.1% solution of DOPA for 2 h twice at 37°C.
Melanin Assay. Cells were rinsed with PBS and lysed with an extraction buffer (50 mM Tris, pH 7.5/2 mM EDTA/150 mM NaCl/1% Triton X-100) containing protease inhibitor mixture at 4°C. Cell pellet was collected and assayed for melanin content by rinsing twice with ethanol–ether (1:1), dissolving in 2 M NaOH solution, and measuring for A at 490 nm.
Histology and Immunofluorescence. Histology and immunohistochemical staining of mouse tissues were performed as described in ref. 33. Immunohistochemical staining was performed on paraffin-fixed sections. Sections were blocked with 5% goat serum, incubated with primary anti-PS1 Ab AB14 (1:250 dilution) at 4°C overnight, washed in PBS, incubated with 1/1,000 Alexa Fluor-594-conjugated secondary Ab (Molecular Probes) for 1 h at room temperature, washed in PBS, and mounted in glycerol/PBS. Digital images were obtained with a Zeiss confocal microscope (Axioskop 2).
Mouse melanocyte monolayers were seeded onto gelatin-coated six-well Lab-Tek chamber slides (Nunc). The cells were fixed for 10 min in 2% formaldehyde in PBS. Cells were incubated for 1 h at room temperature in a mixture of mouse monoclonal and rabbit polyclonal Abs (diluted in 5% goat serum in PBS). After the incubation, unbound Abs were removed by washing with PBS three times for 5 min each. Cells then were incubated for 30 min with Oregon Green 488 goat anti-rabbit IgG, and images were captured by the confocal microscope (Zeiss).
Western Blotting. Protein extracts were separated by SDS/PAGE and electroblotted onto 0.2-μm nitrocellulose membranes (Schleicher & Schuell). Nonspecific sites were blocked by incubation in 5% (wt/vol) nonfat dry milk and 0.1% Tween 20. The membranes were incubated with primary antiserum followed by horseradish peroxidase-conjugated secondary Abs and visualized with the enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech).
EM. Melanocytes were seeded onto gelatin-coated eight-well Lab-Tek chamber slides (Nunc). The cells were fixed in wells with one-half-strength Karnovsky's fixative in 0.2 M sodium cacodylate buffer (pH 7.2) for 30 min at room temperature. Cells then were postfixed with 1% osmium tetroxide containing 1.5% potassium ferrocyanide for 30 min, washed, stained en bloc with 0.5% uranyl acetate for 30 min, dehydrated, and embedded in Eponate 12. Cells were sectioned on an MT 6000-XL ultramicrotome (RMC, Tucson, AZ), stained with aqueous solutions of uranyl acetate (2%) and lead citrate (0.3%) for 15 min each, and then viewed and photographed with a Hitachi transmission electron microscope. All tissue-processing supplies were purchased from Ted Pella (Tustin, CA).
Results
PS Are Required for Pigmentation of Retinal Pigment Epithelium and Cutaneous Melanocytes. We developed a unique CNS-restricted PS “rescue” system in which the early lethal phenotype of the PS1 and PS2 double-null embryos could be partially rescued by neuronal expression of the human PS1 transgene (33). These rescued embryos could be readily identified because their eyes lacked pigmentation (Fig. 1A). Histological analysis of the eye at embryonic day 13.5 showed that all cellular structures, in particular the RPE cells, could be identified, and the morphology appeared to be normal (Fig. 1B). This result suggests that the defect lies specifically in melanin synthesis within the RPE. Immunohistochemical staining by using an anti-PS1 Ab documented that although PS1 protein could be detected in the littermate control, it was greatly reduced in the RPE of the rescue mutant (Fig. 1C). Thus, the pigment defect is caused by the loss of PS, and the mutant is termed PS-null in the context of RPE.
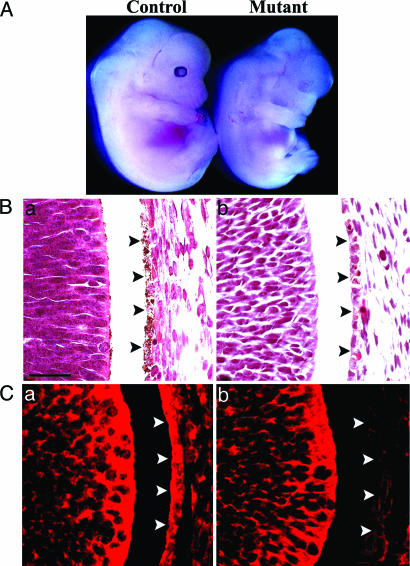
Fig. 1.
Loss of PS leads to defective pigmentation in RPE. (A) Dramatically reduced eye pigmentation in a CNS-restricted PS rescue embryo (Mutant, Right) compared with a littermate control (Left). (B) H&E staining of control (a) and PS mutant (b) eyes, showing virtually absent pigment granules in the PS mutant RPE (marked by arrowheads). (C) Immunohistochemical staining by using the anti-PS1 N-terminal Ab (AB14) documented the presence of PS1 protein in RPE (marked by arrowheads) in the control (a) but not the PS mutant (b). Embryonic stage 13.5 was used for all of the analysis. (Scale bar: 20 μm.)
In addition to RPE, neural-crest-derived pigment cells also comprise the cutaneous melanocytes of the skin and hair. Because both cell types share common melanin synthesis pathway, we decided to investigate whether PS is also required for the pigmentation of mouse cutaneous melanocytes by using specific PS inhibitors (34, 35). We cultured primary melanocytes from newborn WT C57BL/6J mouse skin and allowed the culture to mature and become pigmented (Fig. 2Aa). Addition of the γ-secretase inhibitor DAPT potently blocked the pigmentation (Fig. 2 Ab). The same result was obtained by using a different γ-secretase inhibitor, Compound 1 (36) (data not shown). This effect was dose-dependent, and 0.25 μm of DAPT resulted in almost complete blockage of pigment synthesis (Fig. 2B). These results establish an essential role of PS in the pigmentation of both RPE and melanocytes.
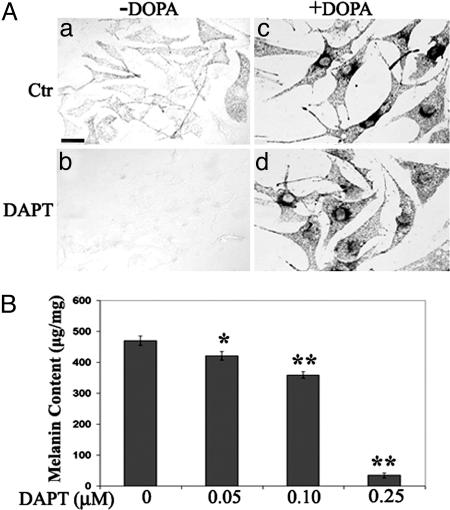
Fig. 2.
Effect of γ-secretase inhibitor treatment on melanin synthesis. (Aa) Control primary melanocytes became pigmented after 2 wk of culturing. (b) Complete blockage of pigment synthesis by 500 nM of DAPT. (c and d) Postfixation treatment of DOPA showed reaction product in both the control (c) and DAPT-treated culture (d), demonstrating that Tyr remained active in the presence of the inhibitor. (B) Quantification of melanin content (μg/mg of protein) documented dose-dependent inhibition of melanin synthesis by DAPT. *, P < 0.001; **, P < 0.0001 (Student's t test). (Scale bars: 20 μm.)
Of significance, histochemical staining by using the Tyr substrate DOPA efficiently induced pigment reaction product in inhibitor-treated cultures (Fig. 2Ad), demonstrating that catalytically functional Tyr was present and that the overt cellular morphology and viability was not affected by γ-secretase inhibition. Immunohistochemical studies documented sufficient amounts of Tyr, Tyrp1, and DCT/Tyrp2, the three principal enzymes in the melanin synthesis pathway, in both control and DAPT-treated cells (data not shown).
PS Inactivation Leads to Abnormal Accumulation of Tyr-Containing Post-Golgi Vesicles. Ultrastructural analysis was performed to determine the effect of PS on melanosome structures (Fig. 3). Examination of RPE showed that, compared with the control in which melanosomes were mostly present in mature forms (Fig. 3Aa), the PS-null melanosomes were predominately at stages I and II (Fig. 3Ab). They were less pigmented and smaller in size. Nevertheless, the structure of the melanosomes appeared normal exhibiting melanofilament matrix formation. Similar to that of RPE, whereas vehicle-treated cultured melanocytes contained predominantly mature melanosomes (Fig. 3Ba), the γ-secretase inhibitor-treated melanocytes contained largely early stage (I and II) melanosomes with apparently normal morphology (Fig. 3Bb), the latter exhibited melanin reaction product upon DOPA histochemistry (Fig. 4Ba, solid arrow). Immunostaining and Western blot analysis of the melanosome structural protein Pmel 17 showed similar patterns and levels of expression in both control and DAPT-treated cells (data not shown), further supporting the notion that melanosome biogenesis was not affected by PS. Because the overall melanosomal protein levels were not significantly changed by γ-secretase inhibitor treatment (discussed in Fig. 5), these data indicate a possible role of PS in targeting these proteins to melanosomes.
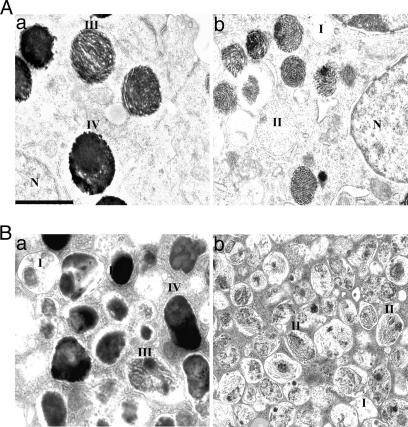
Fig. 3.
EM analysis of melanosome structures in RPE and melanocytes. (A) Representative EM images of control (a) and PS-null mutant (b) RPE. (B) Representative EM images of control (a) and DAPT-treated (b) melanocytes. I–IV denote melanosomes of various maturation stages. Genetic ablation and γ-secretase inhibitor treatment both resulted in the formation of predominately immature (stages I and II) melanosomes as opposed to mature (stages III and IV) melanosomes prevalent in control melanocytes. (Scale bar: 0.5 μm.)
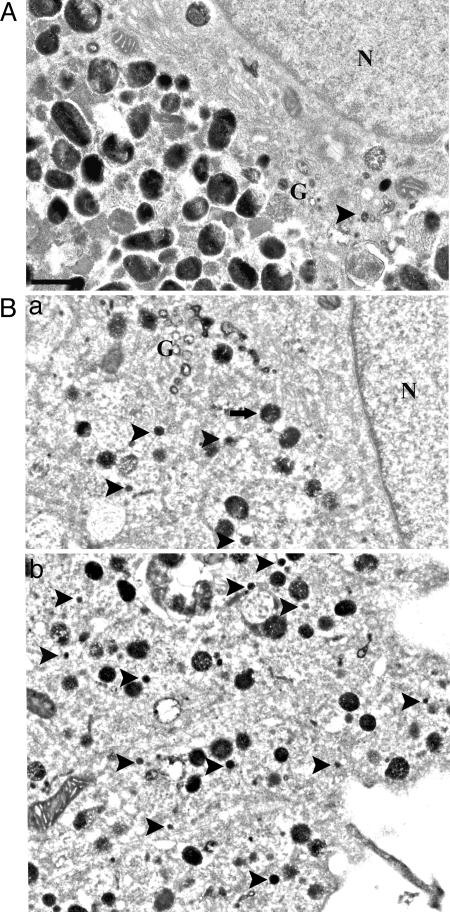
Fig. 4.
Ultrastructural analysis of positive DOPA reaction products. (A) DOPA histochemistry identified Golgi tubules (G) in control melanocytes. The 50-nm vesicles were infrequent and confined to the Golgi area. (B) In DAPT-treated melanocytes, numerous 50-nm vesicles (arrowheads) could be identified close to Golgi area (G), throughout the cell body (a), as well as in dendrites (b). (Scale bar: 0.5 μm.)
Fig. 5.
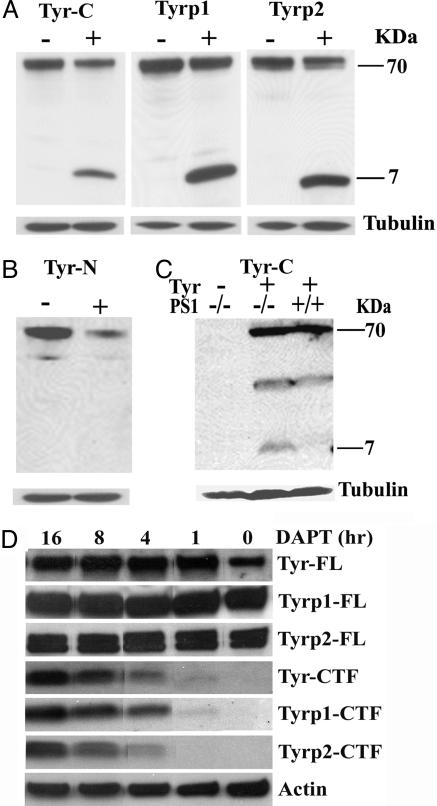
Western blot analysis of Tyr, Tyrp1, and DCT/Tyrp2 proteins. (A) Western blotting using anti-C-terminal Tyr (Tyr-C), Tyrp1, and DCT/Tyrp2 (Tyrp2) Abs in the presence (+) or absence (-) of DAPT. A 7-kDa CTF can be detected with each of the three C-terminal Abs in inhibitor-treated (+) samples but not in vehicle-treated controls (-). (B) Western blotting by using an anti-N-terminal Tyr Ab (Tyr-N). Only the 70-kDa full-length protein was present. (C) Transfection of a Tyr expression vector into WT (PS1+/+) mouse embryonic fibroblasts led to the expression of Tyr full-length protein at 70 kDa. However, transfection of the same vector into PS1-/- MEF resulted in the appearance of both the full-length protein and a 7-kDa CTF, demonstrating a direct processing of Tyr by PS1. Empty-vector transfection (Tyr-) was used as a negative control. Hybridization with an anti-tubulin Ab (Tubulin) was used as loading controls. (D) Time course analysis of Tyr, Tryp1, and DCT/Tryp2 full-length (FL) and CTF levels after DAPT treatment. Anti-actin Ab (Actin) was used as loading control.
Tyr is synthesized and matured through the secretory pathway and buds off the trans-Golgi network in 50-nm coated vesicles, presumably en route to melanosomes in the vicinity. DOPA reaction followed by EM analysis allows identification of these Tyr-positive cargo vesicles. Assessment of extra-melanosomal Tyr active organelles by DOPA histochemistry revealed that, in vehicle-treated primary melanocytes, DOPA histochemistry was restricted to Golgi areas and to post-Golgi vesicles in the vicinity of trans-Golgi network (Fig. 4A, arrowhead) and absent from lateral part of the cell body and dendrites (data not shown). In γ-secretase inhibitor-treated culture, however, there were numerous DOPA-positive, 50-nm vesicles present peripheral to the Golgi zone (Fig. 4Ba, arrowheads) and throughout the cell body and dendrites (Fig. 4Bb). These results demonstrate that mislocalization of Tyr in these 50-nm vesicles, which are normally destined to melanosomes, is likely the primary cause for the impaired pigmentation.
Inactivation of PS Leads to the Accumulation of Tyr, Tyrp1, and DCT/Tyrp2 C-Terminal Fragments (CTFs). PS have been shown to cleave a large number of type I transmembrane proteins based on the evidence that loss of PS results in the accumulation of their CTFs. Western blot analysis using Abs against the C-terminal end of Tyr, Tyrp1, and DCT/Tyrp2 documented that, although the 70-kDa full-length Tyr, Tyrp1, and DCT/Tyrp2 were expressed in vehicle- and γ-secretase inhibitor-treated cultures, an additional CTF of 7 kDa was detected for all three proteins only in inhibitor-treated samples (Fig. 5A). The fact that (i) this length represents a combination of the intracellular domain (≈28 aa), the transmembrane domain (≈22 aa), and 15–20 aa beyond the transmembrane domain of each of these proteins (27), (ii) an Ab against the N-terminal sequence of Tyr failed to recognize this fragment (Fig. 5B), and (iii) transfection of a Tyr expression vector resulted in the production of the 7-kDa fragment only in PS1-/- cells, but not in PS1+/+ cells (Fig. 5C), support the notion that these fragments are bona fide CTFs analogous to those of APP and other putative PS targets, and that an enzyme(s) with the characteristic of membrane shedding is responsible for extracellular processing of these molecules. Time course studies showed that, while the full-length proteins remained constant, the CTFs of Tyr, Tyrp1, and DCT/Tyrp2 could be detected after 1 h of DAPT treatment and accumulate rapidly overtime (Fig. 5D).
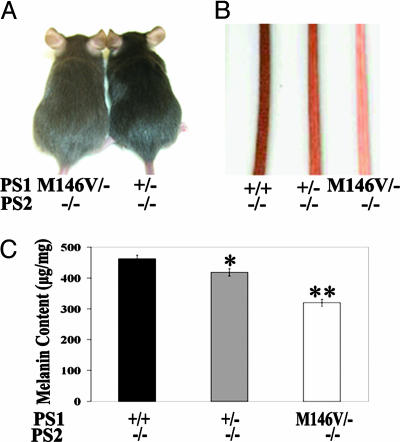
PS1 FAD Mutation Exhibits Partial Loss of Function in Melanin Synthesis. Having established a definitive role of PS in regulating melanosomal protein and pigment processes, we next investigated the effect of PS1 FAD mutations by using the PS1M146V knock-in mice as a model system (32, 37). In these mice, the PS1M146V FAD mutation was introduced into the endogenous mouse PS1 gene and expressed in a completely physiological environment. Our previous studies documented impaired contextual fear learning and adult neurogenesis by the FAD mutation (37). However, these effects can only be detected on a sensitized genetic background, i.e., when endogenous WT PS1 allele was removed (37). Based on this observation, we created mice of various combinations of PS1 alleles on PS2-null (PS2-/-) background. Specifically, crossing of PS1M146V heterozygous (PS1M146V/+) with PS1+/- mice generated animals that were PS1+/+ (+/+), PS1+/- (+/-), or PS1M146V/- (M146V/-). Replacing the WT allele with the PS1M146V mutant allele led to a dramatic reduction in the coat color (Fig. 6A; compare M146V/- to +/-). Although a difference between PS1+/+ and PS1+/- mice could not be readily recognized on the coat (data not shown), mild hypopigmentation could be observed in PS1+/- (+/-) tail skin when compared with that of PS1+/+ (+/+), and it was further reduced in PS1M146V/- (M146V/-) samples (Fig. 6B). This assessment was corroborated by measuring the melanin content in melanocyte cultures derived from these animals (Fig. 6C), which revealed lowered melanin production by either removing the WT allele (compare +/+ with +/-) or replacing the WT allele with the M146V mutant (compare +/- with M146V/-). This result is consistent with our inhibitor studies documenting a dose-dependent inhibition of melanin synthesis by the γ-secretase inhibitor (Fig. 2B). This finding thus establishes a partial loss-of-function activity of the PS1M146V mutation in melanin synthesis in vivo.
Fig. 6.
Reduced pigmentation by PS1M146V FAD mutation. All PS1 alleles were expressed on PS2-null background. (A) Reduced coat color in a representative 2-mo-old PS1M146V/- (M146V/-) mouse as compared with a littermate PS1+/- (+/-) animal. (B and C) Pigment colors of the tail skin of +/+, +/-, and M146V/- mice (B), which correlates with the melanin levels measured from melanocyte cultures derived from these animals (C). *, P < 0.001; **, P < 0.0001 (Student's t test).
Discussion
The role of PS in regulated intramembrane proteolysis has been extensively studied and well recognized. In contrast, the significance of PS in protein trafficking remains speculative. The melanosomes offer an attractive system to study intracellular protein transport because all organelles with catalytically active Tyr can be marked through the DOPA reaction and visualized by EM. Using this system and taking advantage of our PS rescue model and the availability of highly potent PS γ-secretase inhibitors, we report here a unique function of PS in pigment synthesis in both RPE and cutaneous melanocytes. We demonstrate that absence of PS or application of PS inhibitor leads to mislocalization of post-Golgi Tyr-containing vesicles, thus establishing a functional role of PS in mediating Tyr trafficking in vivo. Our work identifies Tyr, Tyrp1, and DCT/Tyrp2 as physiological substrates for PS and links the intracellular transport with γ-secretase activity because γ-secretase inhibition results in simultaneous mistrafficking of Tyr and related proteins and accumulation of their CTFs. Although current studies do not address the cause–effect relationship between the secretase activity and Tyr transport, mislocalization of Tyr in post-Golgi vesicles most likely contributes directly to the pigment defect. We further document that the PS1M146V FAD mutation exhibits a partial loss of function in melanin synthesis and, by extension, Tyr trafficking, in vivo.
Tyr, Tyrp1, and Dct/Tyrp2 are type I membrane glycoproteins. They undergo synthesis and maturation through endoplasmic reticulum and Golgi and are ultimately transported to premelanosomes via 50-nm post-Golgi vesicles. Although the initial processes are similar, they exhibit distinct differences in subsequent intracellular regulations, likely due to the differences in their targeting signals in the carboxyl domains of these molecules. Adaptor proteins have been linked to these processes (reviewed in ref. 38). Specifically, adaptor protein-3 (AP3) deletion leads to hypopigment phenotypes in Drosophila, mouse, and human, and interaction of Tyr with AP3 has been shown to be required for its targeting to melanosomes (38). On the contrary, Tyrp1 may be transported by an AP3-independent but AP1-dependent mechanism (39, 40). Although both Tyr and Tyrp1 contain the dileucine motif, which is required for correct routing of the two molecules (41, 42), there is no dileucine sequence in DCT/Tyrp2 C terminus, and a tyrosine-based signal may be used for its trafficking to melanosomes instead (43). Because all three molecules are affected in PS-null melanocytes, PS appears to act on these substrates at a common step independent of adapter proteins. Indeed, Western blot analysis showed normal expression of AP3 subunits in PS-null melanocytes (data not shown). The fact that the APP C terminus does not have the dileucine motif and that PS was reported to affect APP trafficking to plasma membrane or endocytosis not known to mediate Tyr routing further corroborate with this notion (10, 11). Further work is required to identify the mechanism where by PS regulates the trafficking of these diverse type I membrane proteins.
Of interest, PS-null melanocytes resemble the pathology of melanocytes in Hermansky–Pudlak syndrome-type 3 (HPS3), where in the absence of the HPS3 gene product, 50-nm vesicles carrying Tyr cargo are aberrantly distributed throughout the entire cytoplasm and that all three Tyr-related enzymes are affected (44). However, HPS3 protein was found to be normally expressed in PS-null melanocytes, and immunohistochemical staining failed to detect changes of PS in HPS3 melanocytes (not shown). Thus, PS-mediated Tyr trafficking is likely independent of that of the HPS3 gene product.
Our finding that PS inhibition leads to the accumulation of Tyr, Tyrp1, and DCT/Tyrp2 CTFs identifies these molecules as endogenous substrates for PS. The question arises as to what could be the physiological function of proteolytic processing of these enzymes. This question becomes increasingly important in light of the large number of putative PS substrates that have been identified. In this regard, two competing views have been put forward for the PS-dependent γ-secretase activity: Extending from Notch receptor studies in which γ-secretase cleavage of Notch has been shown to be required to liberate the Notch intracellular domain and to activate its downstream signaling, other type I membranes have been implicated to subject to similar PS-dependent intracellular domain production and cellular signaling (reviewed in ref. 45). Alternately, γ-secretase cleavage has been proposed to degrade membrane proteins and possibly attenuate signaling pathways (8, 46). The well-established enzymatic activities of Tyr and related proteins make them unlikely cell signaling molecules. Because the C-terminal sequences of these proteins contain melanosomal targeting signals (41–43), breakage of the CTFs by γ-secretase is expected to disrupt their melanosomal localization and melanin synthesis activity. Thus, we favor the idea that γ-secretase functions here to degrade these membrane proteins. As such, the accumulation of CTFs of Tyr family of proteins in the absence of PS would represent degradation intermediates, which otherwise may escape detection due to PS-mediated proteolysis. Future studies are required to identify the cellular sites of CTF accumulation and to determine the enzymes responsible for their extracellular cleavages.
Support for a function of PS in intracellular protein degradation also came from recent studies by Wilson et al. (21) and by Esselens et al. (20), which documented delayed turnover and subsequent accumulation of α- and β-synucleins and telencephalin, respectively, in degradative organelles in PS1-null cells. However, because these effects are not seen by γ-secretase inhibitor treatment and because full-length proteins, not the CTFs, accumulate, these are likely mediated through independent pathways.
In summary, we identify here a unique function of PS in melanin synthesis and pigmentation. We present data that it is mediated by its intracellular transport of post-Golgi Tyr-containing vesicles and that this effect is γ-secretase dependent. We document a partial loss-of-function activity by the PS1 M146V FAD mutation. These findings raise the intriguing possibility that a compromised post-Golgi vesicle transport may contribute to Alzheimer's disease pathogenesis.
Acknowledgments
We thank G. Martin and B. Sopher (University of Washington, Seattle) for PS1M146V knock-in mice, R. Swank (Roswell Park Cancer Institute, Buffalo, NY) for HPS mouse tissues, and A. Houghton (Memorial Sloan–Kettering Cancer Center, New York) for the Tyr expression vector. We thank V. J. Hearing (National Institutes of Health, Bethesda) for the generous gifts of anti-Tyr, Tyrp1, and DCT/Tyrp2 and Pmel17 Abs; and H. Xu and P. Greenguard (The Rockefeller University, New York) for the AB14 Ab. We thank Claire Haueter for expert EM assistance. This work was supported by National Institutes of Health Grants NS40039 (to H.Z.), AG20670 (to H.Z.), and AR45429 (to R.E.B.) and by the Ellison Medical Foundation (H.Z.). R.W. and P.T. are trainees of National Institutes of Health Training Grant T32 AG000183. H.Z. is a Zenith Award recipient from the Alzheimer's Association.
Author contributions: R.W., P.T., P.W., and H.Z. designed research; R.W., P.T., and P.W. performed research; R.W. contributed new reagents/analytic tools; R.W., P.T., P.W., R.E.B., and H.Z. analyzed data; and R.W., R.E.B., and H.Z. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: PS, presenilin; APP, amyloid precursor protein; FAD, familial Alzheimer's disease; RPE, retinal pigment epithelium; DOPA, 3,4-dihydroxyphenylalanine; Tyr, tyrosinase; Tyrp1 or -2: Tyr-related protein-1 or -2; DCT/Tyrp, DOPA chrome tautomerase/Tyrp2; AP3, adaptor protein-3; HPS, Hermansky–Pudlak syndrome; CTF, C-terminal fragment; DAPT, N-[N-(3,5-difluorophenacetyl-l-alanyl)]-S-phenylglycine t-butyl ester.
References
- 1.Annaert, W. & De Strooper, B. (2002) Annu. Rev. Cell Dev. Biol. 18, 25-51. [DOI] [PubMed] [Google Scholar]
- 2.Siman, R., Reaume, A. G., Savage, M. J., Trusko, S., Lin, Y. G., Scott, R. W. & Flood, D. G. (2000) J. Neurosci. 20, 8717-8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moehlmann, T., Winkler, E., Xia, X., Edbauer, D., Murrell, J., Capell, A., Kaether, C., Zheng, H., Ghetti, B., Haass, C. & Steiner, H. (2002) Proc. Natl. Acad. Sci. USA 99, 8025-8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, M. S., Ye, J., Rawson, R. B. & Goldstein, J. L. (2000) Cell 100, 391-398. [DOI] [PubMed] [Google Scholar]
- 5.De Strooper, B., Annaert, W., Cupers, P., Saftig, P., Craessaerts, K., Mumm, J. S., Schroeter, E. H., Schrijvers, V., Wolfe, M. S., Ray, W. J., et al. (1999) Nature 398, 518-522. [DOI] [PubMed] [Google Scholar]
- 6.Selkoe, D. & Kopan, R. (2003) Annu. Rev. Neurosci. 26, 565-597. [DOI] [PubMed] [Google Scholar]
- 7.Shah, S., Lee, S. F., Tabuchi, K., Hao, Y. H., Yu, C., LaPlant, Q., Ball, H., Dann, C. E., III, Sudhof, T. & Yu, G. (2005) Cell 122, 435-447. [DOI] [PubMed] [Google Scholar]
- 8.Kopan, R. & Ilagan, M. X. (2004) Nat. Rev. Mol. Cell Biol. 5, 499-504. [DOI] [PubMed] [Google Scholar]
- 9.Sisodia, S. S. & St. George-Hyslop, P. H. (2002) Nat. Rev. Neurosci. 3, 281-290. [DOI] [PubMed] [Google Scholar]
- 10.Kaether, C., Lammich, S., Edbauer, D., Ertl, M., Rietdorf, J., Capell, A., Steiner, H. & Haass, C. (2002) J. Cell Biol. 158, 551-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai, D., Leem, J. Y., Greenfield, J. P., Wang, P., Kim, B. S., Wang, R., Lopes, K. O., Kim, S. H., Zheng, H., Greengard, P., et al. (2003) J. Biol. Chem. 278, 3446-3454. [DOI] [PubMed] [Google Scholar]
- 12.Levitan, D. & Greenwald, I. (1998) Development (Cambridge, U.K.) 125, 3599-3606. [DOI] [PubMed] [Google Scholar]
- 13.Guo, Y., Livne-Bar, I., Zhou, L. & Boulianne, G. L. (1999) J. Neurosci. 19, 8435-8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naruse, S., Thinakaran, G., Luo, J. J., Kusiak, J. W., Tomita, T., Iwatsubo, T., Qian, X., Ginty, D. D., Price, D. L., Borchelt, D. R., et al. (1998) Neuron 21, 1213-1221. [DOI] [PubMed] [Google Scholar]
- 15.Noll, E., Medina, M., Hartley, D., Zhou, J., Perrimon, N. & Kosik, K. S. (2000) Dev. Biol. 227, 450-464. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura, M., Yu, G., Levesque, G., Zhang, D. M., Ruel, L., Chen, F., Milman, P., Holmes, E., Liang, Y., Kawarai, T., et al. (1999) Nat. Med. 5, 164-169. [DOI] [PubMed] [Google Scholar]
- 17.Chen, F., Tandon, A., Sanjo, N., Gu, Y. J., Hasegawa, H., Arawaka, S., Lee, F. J., Ruan, X., Mastrangelo, P., Erdebil, S., et al. (2003) J. Biol. Chem. 278, 19974-19979. [DOI] [PubMed] [Google Scholar]
- 18.Herreman, A., Van Gassen, G., Bentahir, M., Nyabi, O., Craessaerts, K., Mueller, U., Annaert, W. & De Strooper, B. (2003) J. Cell Sci. 116, 1127-1136. [DOI] [PubMed] [Google Scholar]
- 19.Leem, J. Y., Vijayan, S., Han, P., Cai, D., Machura, M., Lopes, K. O., Veselits, M. L., Xu, H. & Thinakaran, G. (2002) J. Biol. Chem. 277, 19236-19240. [DOI] [PubMed] [Google Scholar]
- 20.Esselens, C., Oorschot, V., Baert, V., Raemaekers, T., Spittaels, K., Serneels, L., Zheng, H., Saftig, P., De Strooper, B., Klumperman, J., et al. (2004) J. Cell Biol. 166, 1041-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson, C. A., Murphy, D. D., Giasson, B. I., Zhang, B., Trojanowski, J. Q. & Lee, V. M. (2004) J. Cell Biol. 165, 335-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.del Marmol, V. & Beermann, F. (1996) FEBS Lett. 381, 165-168. [DOI] [PubMed] [Google Scholar]
- 23.Sturm, R. A., Teasdale, R. D. & Box, N. F. (2001) Gene 277, 49-62. [DOI] [PubMed] [Google Scholar]
- 24.Marks, M. S. & Seabra, M. C. (2001) Nat. Rev. Mol. Cell Biol. 2, 738-748. [DOI] [PubMed] [Google Scholar]
- 25.Maul, G. G. (1969) J. Ultrastruct. Res. 26, 163-176. [DOI] [PubMed] [Google Scholar]
- 26.Maul, G. G. & Brumbaugh, J. A. (1971) J. Cell Biol. 48, 41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oetting, W. S. & King, R. A. (1999) Hum. Mutat. 13, 99-115. [DOI] [PubMed] [Google Scholar]
- 28.Huizing, M., Boissy, R. E. & Gahl, W. A. (2002) Pigm. Cell. Res. 15, 405-419. [DOI] [PubMed] [Google Scholar]
- 29.Xia, X., Qian, S., Soriano, S., Wu, Y., Fletcher, A. M., Wang, X.-J., Koo, E. H., Wu, X. & Zheng, H. (2001) Proc. Natl. Acad. Sci. USA 98, 10863-10868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong, P. C., Zheng, H., Chen, H., Becher, M. W., Sirinathsinghji, D. J., Trumbauer, M. E., Chen, H. Y., Price, D. L., Van der Ploeg, L. H. & Sisodia, S. S. (1997) Nature 387, 288-292. [DOI] [PubMed] [Google Scholar]
- 31.Donoviel, D. B., Hadjantonakis, A. K., Ikeda, M., Zheng, H., St. George-Hyslop, P. & Bernstein, A. (1999) Genes Dev. 13, 2801-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo, Q., Fu, W., Sopher, B. L., Miller, M. W., Ware, C. B., Martin, G. M. & Mattson, M. P. (1999) Nat. Med. 5, 101-106. [DOI] [PubMed] [Google Scholar]
- 33.Wang, P., Pereira, F. A., Beasley, D. & Zheng, H. (2003) Development (Cambridge, U.K.) 130, 5019-5029. [DOI] [PubMed] [Google Scholar]
- 34.Li, Y. M., Xu, M., Lai, M. T., Huang, Q., Castro, J. L., DiMuzio-Mower, J., Harrison, T., Lellis, C., Nadin, A., Neduvelil, J. G., et al. (2000) Nature 405, 689-694. [DOI] [PubMed] [Google Scholar]
- 35.Cheng, H. T., Miner, J. H., Lin, M., Tansey, M. G., Roth, K. & Kopan, R. (2003) Development (Cambridge, U.K.) 130, 5031-5042. [DOI] [PubMed] [Google Scholar]
- 36.Qyang, Y., Chambers, S. M., Wang, P., Xia, X., Chen, X., Goodell, M. A. & Zheng, H. (2004) Biochemistry 43, 5352-5359. [DOI] [PubMed] [Google Scholar]
- 37.Wang, R., Dineley, K. T., Sweatt, J. D. & Zheng, H. (2004) Neuroscience 126, 305-312. [DOI] [PubMed] [Google Scholar]
- 38.Robinson, M. S. & Bonifacino, J. S. (2001) Curr. Opin. Cell Biol. 13, 444-453. [DOI] [PubMed] [Google Scholar]
- 39.Huizing, M., Sarangarajan, R., Strovel, E., Zhao, Y., Gahl, W. A. & Boissy, R. E. (2001) Mol. Biol. Cell 12, 2075-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raposo, G., Tenza, D., Murphy, D. M., Berson, J. F. & Marks, M. S. (2001) J. Cell Biol. 152, 809-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vijayasaradhi, S., Xu, Y., Bouchard, B. & Houghton, A. N. (1995) J. Cell Biol. 130, 807-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calvo, P. A., Frank, D. W., Bieler, B. M., Berson, J. F. & Marks, M. S. (1999) J. Biol. Chem. 274, 12780-12789. [DOI] [PubMed] [Google Scholar]
- 43.Simmen, T., Schmidt, A., Hunziker, W. & Beermann, F. (1999) J. Cell Sci. 112, 45-53. [DOI] [PubMed] [Google Scholar]
- 44.Boissy, R. E., Richmond, B., Huizing, M., Helip-Wooley, A., Zhao, Y., Koshoffer, A. & Gahl, W. A. (2005) Am. J. Pathol. 166, 231-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fortini, M. E. (2002) Nat. Rev. Mol. Cell Biol. 3, 673-684. [DOI] [PubMed] [Google Scholar]
- 46.Parent, A. T., Barnes, N. Y., Taniguchi, Y., Thinakaran, G. & Sisodia, S. S. (2005) J. Neurosci. 25, 1540-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]