Abstract
Dengue virus (DV) is a mosquito-borne flavivirus that causes haemorrhagic fever in humans. DV primarily targets immature dendritic cells (DCs) after a bite by an infected mosquito vector. Here, we analysed the interactions between DV and human-monocyte-derived DCs at the level of virus entry. We show that the DC-specific ICAM3-grabbing non-integrin (DC-SIGN) molecule, a cell-surface, mannose-specific, C-type lectin, binds mosquito-cell-derived DVs and allows viral replication. Conclusive evidence for the involvement of DC-SIGN in DV infection was obtained by the inhibition of viral infection by anti-DC-SIGN antibodies and by the soluble tetrameric ectodomain of DC-SIGN. Our data show that DC-SIGN functions as a DV-binding lectin by interacting with the DV envelope glycoprotein. Mosquito-cell-derived DVs may have differential infectivity for DC-SIGN-expressing cells. We suggest that the differential use of DC-SIGN by viral envelope glycoproteins may account for the immunopathogenesis of DVs.
Introduction
Dengue virus (DV) is an arthropod-borne flavivirus that belongs to the Flaviviridae family (Rice, 1996). Four antigenically distinct serotypes, DV types 1–4, are transmitted to humans through the mosquito vector, Aedes aegypti (Guzman & Kouri, 2002). DVs are lipid-enveloped viruses with a singlestranded, positive-sense RNA genome that encodes the structural proteins C (capsid), M (membrane) and E (envelope), and eight non-structural proteins, NS1 to NS5 (Rice, 1996). The E-glycoprotein, which is exposed on the surface of the viral membrane (Kuhn et al., 2002), mediates viral attachment to cells (Hung et al., 1999). The DV E-glycoprotein has two potential sites for N-linked glycosylation at positions Asn 67 and Asn 153, which are differentially used by the four DV serotypes (Johnson et al., 1994).
DV infection can cause self-limiting fever or severe haemorrhagic fever and shock-syndrome (Halstead, 1989; Rothman & Ennis, 1999; Lei et al., 2001). The pathogenesis of DV is still poorly understood. Immature dendritic cells (DCs), in contrast with macrophages and monocytes, are permissive for DV infection (Palucka, 2000; Wu et al., 2000; Ho et al., 2001; Marovich et al., 2001). The skin-epidermal Langerhans cells, which are considered to be immature DCs, have been proposed to be the primary target cells after the initial bite by an infected Aedes mosquito (Wu et al., 2000). Early interactions between mosquito-cell-derived DV and DCs may be crucial for transporting viral antigens to secondary lymphoid organs and for developing anti-viral immunity (Kelsa et al., 2002). Consistent with this, DV infection of immature DCs is considered to be a crucial step in the establishment of viral infection. However, DV interactions with DCs are poorly understood at the molecular level (Wu et al., 2000). The attachment sites for viral entry into leukocytes might contribute to the severity of the disease (Morens et al., 1991). Here, we show the importance of the lectin, DCspecific ICAM-3-grabbing nonintegrin (DC-SIGN; also known as CD209), for the productive infection of monocyte-derived DCs (MDDCs) by mosquito-cell-derived DVs.
Results and Discussion
The ability of immature human MDDCs that lack the monocytic CD14 marker and express DC-SIGN (Fig. 1A) to support DV type-1 (DV-1) virus infection was investigated. MDDCs were infected with the virulent DV-1 virus, FGA/NA d1d, which was grown in Aedes AP61 cells (Desprès et al., 1998; Duarte dos Santos et al., 2000). Inoculation with five AP61 focus-forming units (FFU) per cell was needed to infect 50% of MDDCs at 40 h post-infection, as determined by immunofluorescent staining of DV-1 antigens (Fig. 1B; using the anti-DV antibody) and of the DV-1 NS1 protein, a nonstructural protein that indicates active replication (Fig. 1B; using the anti-NS1 antibody).
Figure 1.
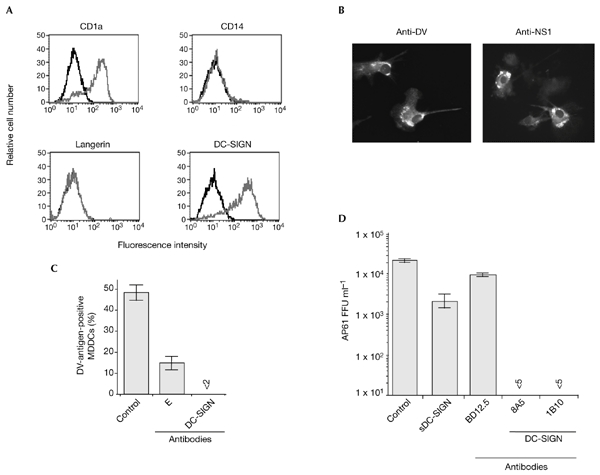
Anti-DC-SIGN antibodies and soluble DC-SIGN inhibit the ability of dengue virus type 1 to infect DC-SIGN-expressing monocyte-derived dendritic cells. (A) The expression levels of the cell-surface markers CD1a, CD14, langerin and DC-SIGN on immature human monocyte-derived dendritic cells (MDDCs) were assessed by direct fluorescence. DC-SIGN-expressing cells were detected using the anti-DC-SIGN monoclonal antibody, 1B10. The relative fluorescence intensity was measured by FACScan analysis and the histograms show the binding of the specific antibody (grey) and isotype-matched control antibody (black). (B) MDDCs were infected for 40 h with dengue virus (DV)-1 at a multiplicity of infection of 5 and were immunostained with anti-DV-1-specific hyperimmune mouse ascites fluids (HMAF) or anti-DV-1 NS1 monoclonal antibody (anti-NS1), and were observed using a fluorescent microscope. (C) Before infection, MDDCs were mock-treated (control) or incubated with 20 µg ml−1 monoclonal antibody 1B10 (DC-SIGN) or a 1:50 dilution of anti-DV E monoclonal antibody 9D12 (E) in RPMI, 0.2% BSA, for 20 min at 25 °C. Treated MDDCs were infected for 2 h at 37 °C in the continuous presence of antibodies. DV antigens were visualized by an immunofluorescence assay using anti-DV-1-specific HMAF. (D) Before infection, MDDCs were mock-treated (control) or were incubated with 20 µg ml−1 anti-LCMV (lymphocytic choriomeningitis virus) monoclonal antibody (BD12.5), anti-DC-SIGN monoclonal antibody (8A5) or anti-DC-SIGN monoclonal antibody (1B10). FGA/NA d1d virus (1 × 105 AP61 focus-forming units (FFU)) was mixed with 10 µg ml−1 sDC-SIGN in RPMI, 0.2% BSA, for 20 min at 25 °C. MDDCs were infected for 2 h at 37 °C in the continuous presence of inhibitors. Infectious virus particles produced in the supernatants were titred. Each experimental point represents the mean ± s.d. of results obtained from three chambers. Magnification in (B), ×100. DC-SIGN, dendritic-cell-specific ICAM3-grabbing non-integrin; sDC-SIGN, the soluble, tetrameric ectodomain of DC-SIGN.
It has been suggested that carbohydrates that are present on the DV virion glycoprotein contribute to both binding and to penetration of the virus into host cells (Hung et al., 1999). The FGA/NA d1d E-glycoprotein has two N-linked oligosaccharides (Courageot et al., 2000), and attached carbohydrates for mosquito-cell-derived DV are of the high-mannose type (Johnson et al., 1994). Therefore, we reasoned that DCsIGN, which is a type-II integral tetrameric protein (Geijtenbeek et al., 2000b; Steiman, 2000; Feinberg et al., 2001; Figdor et al., 2002), might interact with the DV-1 E-glycoprotein. Indeed, the DCsIGN carbohydrate-recognition domain (CRD) binds mannose residues within high-mannose oligosaccharides in a calcium-dependent manner (Feinberg et al., 2001). High-mannose oligosaccharides are present on envelope glycoproteins of human immunodeficiency virus (HIV; Lin et al., 2003), cytomegalovirus (CMV; Halary et al., 2002), Ebola virus (Alvarez et al., 2002; Lin et al., 2003) and hepatitis C virus (Lozach et al., 2003; Pöhlmann et al., 2003). Incubation with the DCsIGN CRD-specific monoclonal antibodies 1B10 or 8A5 (Halary et al., 2002), or with the soluble, tetrameric ectodomain of DCsIGN (sDC-SIGN; Halary et al., 2002), reduced infection by more than 90%, as assessed by immunodetection of DV antigens (Fig. 1C) or by titration of viral progeny (Fig. 1D). As a positive control, the neutralizing anti-DV E monoclonal antibody, 9D12, which recognizes an accessible epitope in the DV-1 virion (Desprès et al., 1993; Duarte dos Santos et al., 2000), reduced infection by 70% (Fig. 1B).
To further evaluate the ability of DC-SIGN alone to render cells susceptible to DV infection, we compared the ability of parental THP-1 cells and DC-SIGN-expressing THP-1 cells (THP/DC-SIGN; Kwon et al., 2002) to support DV-1 virus replication. THP/DCsIGN cells internalize HIV and CMV in a DC-SIGN-dependent manner (Geijtenbeek et al., 2000a; Kwon et al., 2002; Halary et al., 2002). THP-1 and THP/DCsIGN cells were infected with various multiplicities of infection (m.o.i.) of FGA/NA d1d virus, and the efficiencies of infection were monitored by immunostaining for viral antigens at 40 h post-infection (Fig. 2A). Parental THP-1 cells were almost completely refractory to FGA/NA d1d virus infection (0.5% infected cells were infected at a m.o.i. of 10), whereas THP/DCsIGN cells were infected in a dose-dependent manner, reaching 60% infection at an m.o.i. of 10 (Fig. 2A). DV-1 that was generated from infected THP/DCsIGN cells (at a m.o.i. of 5) was ∼1 × 105 AP61 FFU ml−1 at 48 h post-infection, and cell death was detected after 72 h (data not shown). Thus, the infectivity shown by mosquito-cell-derived DV-1 was similar in MDDCs and DC-SIGN-expressing THP-1 cells. The ability of DC-SIGN that was expressed in otherwise refractory Jurkat cells (a lymphoblastoid T-cell line) to mediate DV-1 infection (30% cells were positive for DV antigens at a m.o.i. of 5; data not shown) further substantiates the important function of the C-lectin in the DV life cycle.
Figure 2.
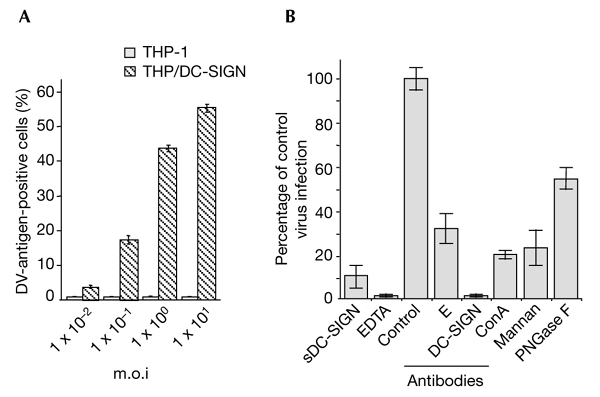
The lectin DC-SIGN is crucial for the entry of dengue virus into monocytic cells. THP-1 and TPH/DC-SIGN cells infected with dengue virus (DV)-1 FGA/NA d1d were analysed for viral protein production as described in the legend for Fig. 1. (A) Cells infected at various multiplicities of infection (m.o.i.s) were analysed using an immunofluorescence assay. (B) THP/DCsIGN cells were infected with FGA/NA d1d virus at m.o.i.s of 5 (columns labelled EDTA and antibodies) or 1 (columns labelled sDC-SIGN, mannan and ConA). Before infection, THP/DC-SIGN cells were incubated with either 20 µg ml−1 mannan, 5 mM EDTA, 20 µg ml−1 monoclonal antibody BD12.5 (control), a 1:50 dilution of monoclonal antibody 9D12 (E), or 20 µg ml−1 monoclonal antibody 1B10 (against DC-SIGN) as described in the legend to Fig. 1. FGA/NA d1d virus (1 × 105 AP61 focus-forming units) was mixed with 10 µg ml−1 sDCsIGN (20 min at 25 °C) or incubated with 25 µg ml−1 ConA (1 h at 37 °C) or N-glycosidase F (PGNase F; see the Methods section). Cells positive for DV antigens are expressed as a percentage of untreated, DV-infected THP/DC-SIGN cells (percentage of control virus infection). ConA, concanavalin A; DC-SIGN, dendritic-cell-specific ICAM 3-grabbing non-integrin; sDC-SIGN, the soluble, tetrameric ectodomain of DC-SIGN.
As is the case for MDDCs, the use of the anti-DC-SIGN monoclonal antibody 1B10, EDTA or sDC-SIGN abolished or markedly restricted FGA/NA d1d virus infection in THP/DC-SIGN cells (Fig. 2B). Furthermore, pre-incubation of FGA/NA d1d virus with concavalin A (ConA), which binds to N-linked high-mannose, or treatment of THP/DCsIGN cells with yeast mannan, reduced virus infectivity by ∼75% (Fig. 2B). The inhibition by ConA supports the theory that α-mannose carbohydrate residues participate in the attachment of DV to DCsIGN. Exposure of the FGA/NA d1d virus to enzymatic attack by N-glycosidase F (PNGase F) reduced virus infectivity by 50%, thus proving that carbohydrate moieties of the DV-1 E-glycoprotein are involved in DV/DC-SIGN interactions (Fig. 2B). These results suggest that DCsIGN functions as a DV-binding molecule that is required for the productive infection of MDDCs.
We investigated whether the extent of E-glycoprotein glycosylation correlates with the effective use of DC-SIGN for viral entry. DV-1 and DV-3 E-glycoproteins are glycosylated at Asn 67 and Asn 153, whereas DV-2 and DV-4 E-glycoproteins are glycosylated only at Asn 67 (Johnson et al., 1994). The potential glycosylation site at Asn 67 is unique among flaviviruses. THP-1 cells are refractory to infection by the virulent, wild-type strain, Jam, of DV-2 virus that was grown in AP61 cells (Johnson et al., 1994; and data not shown). Immunofluorescent staining of infected cells showed marked differences in infectivity between DV-1 and DV-2 viruses at 40 h post-infection (Fig. 3A). At an m.o.i. of 1, 50% and 5% of THP/DCsIGN cells were infected by DV-1 and DV-2, respectively. Similar results were seen with mosquito-cell-derived DV-3 and DV-4, with respect to the predicted number of carbohydrate moieties attached (Fig. 3A). Inoculation with 50 AP61 FFU per cell was required to infect ∼10–20% of THP/DCsIGN cells with DV-4 or DV-2 (data not shown). Because DVs show a variation in their ability to infect DC-SIGN-expressing cells, immature MDDCs were exposed to highly purified DV-1 FGA/NA d1d and DV-2 Jam viruses that were grown in mosquito cells (Fig. 3B). On average, ∼25% of MDDCs were infected by DV-2 and ∼70% by DV-1 at the highest m.o.i. tested (p < 0.001, from t-tests carried out in accordance with the method of Fisher and Yates). Thus, mosquito-cell-derived DVs may have differential infectivities for MDDCs. Notably, monoclonal antibody 1B10 can block DV-2 infection of MDDCs, as is the case for DV-1 virus (Fig. 3B, +1B10). These findings support the theory that the high-mannose N-oligosaccharide at Asn 153 might account for the higher affinity of the E-glycoprotein binding to the oligomeric DCsIGN CRD (Feinberg et al., 2001) and, ultimately, for the efficient infection of MDDCs.
Figure 3.
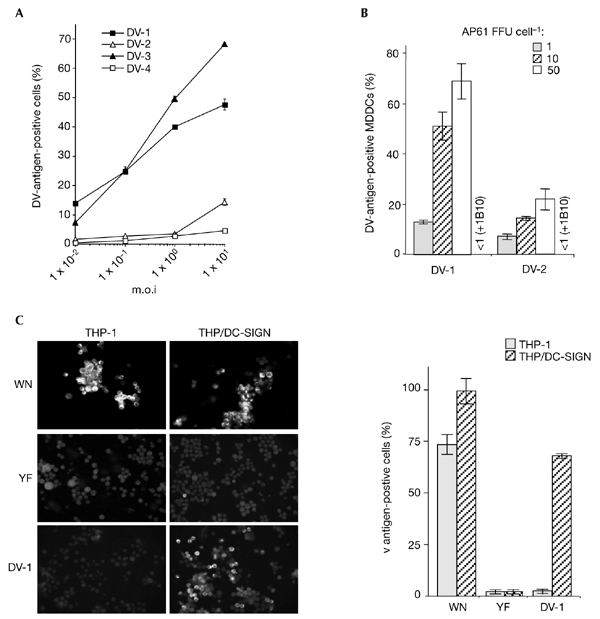
Flavivirus infectivity in DC-SIGN-expressing monocytic cells. Cells infected with flaviviruses were assayed 25 h (monocyte-derived dendritic cells (MDDCs)) or 40 h (THP/DC-SIGN) post-infection. for the presence of viral antigens, using the immunofluorescence assay described in the legend of Fig. 1. (A) THP/DCsIGN cells were infected with strain FGA/NA d1d of dengue virus (DV)-1, the Jam strain of DV-2, strain H-87 of DV-3 or strain H-241 of DV-4, at various multiplicities of infection (m.o.i.s) (B) MDDCs were infected with highly purified DV-1 FGA/NA d1d and DV-2 Jam viruses at various m.o.i.s. Before infection, MDDCs infected at an m.o.i. of 10 were incubated with 20 mg ml−1 monoclonal antibody 1B10 (+1B10), as described in the legend for Fig. 1. Experiments were performed twice using two MDCC donors. The results of one representative experiment are shown. (C) THP-1 and THP/DCsIGN cells were infected with strain IS-98-ST1 of West Nile (WN) virus (m.o.i. of 5), vaccine strain 17D of yellow fever (YF) virus (m.o.i. of 50) or DV-1 (m.o.i. of 5) and viral antigens were visualized by immunofluorescence assays with specific hyperimmune mouse ascites fluids. Each experimental point represents the mean ± s.d. of results obtained from three chambers. Magnification in left panel of (C), x100. DC-SIGN, dendritic-cell-specific ICAM3-grabbing non-integrin.
We examined whether the wild-type strain IS-98-ST1 of West Nile (WN) virus and the 17D vaccine strain of yellow fever (YF) virus use DC-SIGN for the infection of monocytic cells. Asn 153 in WN IS-98-ST1 E-glycoprotein (Mashimo et al., 2002) carries a carbohydrate (P.D., unpublished data) and the YF 17D E-protein has an N-glycosylation site that is not used (Desprès et al., 1991). Neither THP-1 nor THP/DCsIGN cells were infected by YF virus at an m.o.i. of 50 vero-FFU per cell (Fig. 3C). From these results, it is reasonable to predict that carbohydrate moieties attached to the flavivirus E-glycoprotein are crucial for efficient and productive infection, which requires host-cell expression of DCsIGN. Interestingly, the replication of WN virus in THP-1 cells was not dependent on DC-SIGN (Fig. 3C). This suggests that alternative receptor molecules that allow the infection of monocytic cells by flaviviruses, such as WN virus, must exist.
Heparan sulphate (HS) glycosaminoglycans have been found to function in the cell binding of DVs and have been suggested to be putative receptors for the infection of susceptible cells by DV (Chen et al., 1997; Hung et al., 1999). Consistent with a recent report by Germi et al. (2002), we found that heparin-lyase I, which specifically removes HS, partially inhibited YF 17D virus infection in VERO cells (data not shown). Treatment of THP/DCsIGN cells with heparin lyase I or pre-incubation of DV-1 with heparin 6,000 (an HS analogue) had no effect on virus infection (data not shown), indicating that HS did not influence the interactions between DV and DC-SIGN. Flavivirus uptake involves the endocytic pathway, in which acidification and endosomal vesicles are required (Heinz & Allison, 2000). Treatment with the pH-interfering drugs bafilomycin A1 (Fig. 4A) and chloroquine (Fig. 4B) caused a dose-dependent reduction of DV-1 infectivity in THP/DCsIGN cells. This suggests that DC-SIGN-mediated DV entry is a pH-dependent process that probably requires the endocytosis of incoming virions.
Figure 4.
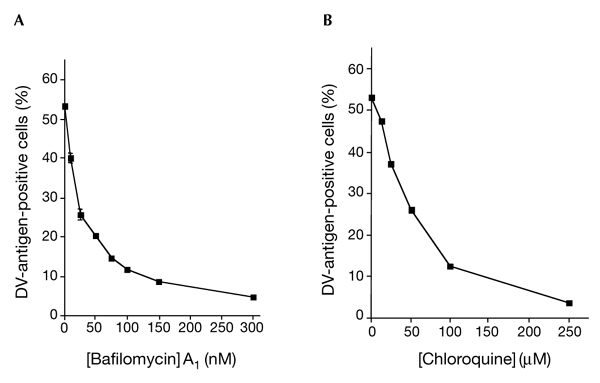
DC-SIGN-mediated dengue virus entry requires acidification. THP/DC-SIGN cells were infected with dengue virus (DV)-1 FGA/NA d1d (multiplicity of infection of 1) in the presence of increasing doses of bafilomycin A1 (A) or chloroquine (B), as described in the Methods section. Treated cells were assayed 40 h post-infection for the presence of viral antigens using an immunofluorescence assay, as described in the legend for Fig. 1. Each data point represents the mean ± s.d. of results from two chambers. DCsIGN, dendritic-cell-specific ICAM3-grabbing non-integrin.
Our data suggest that DC-SIGN is crucial for DV binding to MDDCs, the putative primary host cells in humans. However, they do not show whether DC-SIGN functions in both attachment and penetration of DV or constitutes part of a putative viral receptor complex. The dependence of the viral entry of prototype strain New Guinea C of DV on HS proteoglycan interactions has been reported in cells that lack DC-SIGN, such as epithelial cells (Chen et al., 1997; Germi et al., 2002). Although the physiological relevance of this observation needs to be elucidated, it underlines the existence of molecules other than DCsIGN that show viral attachment properties for DV (Bielefeldt-Ohmann et al., 2001). The blocking effect of pH-interfering drugs suggests that the endocytic pathway contributes to the penetration of DV. Two sorting signals for the endocytic pathway are located in the cytoplasmic tail of DCsIGN, and they could account for the DV entry process (Soilleux et al., 2000). However, we cannot formally rule out the possibility that an unidentified protein that shares the ability to promote DV uptake with DCsIGN is the target of the blocking effect of bafilomycin A1.
Conclusions
Our findings identify DC-SIGN as an attachment factor for DV, which enters the blood after an initial bite by an infected mosquito. Although the relevance of these observations remains to be ascertained in vivo, we propose that the immunopathogenesis of DV infection might depend on both the expression of DC-SIGN in human DC subpopulations and the ability of viral envelope glycoproteins to interact with DC-SIGN.
Methods
Cells.
MDDCs were generated from human blood monocytes using granulocyte macrophage colony stimulating factor and interleukin-4, as described in Halary et al. (2002). The human monocytic cell line, THP-1, was grown in RPMI 1640 containing 10% FCS, 2 mM L-glutamine and antibiotics. THP/DCsIGN cells were provided by D.R. Littman (Kwon et al., 2002).
Antibodies and soluble DC-SIGN.
Anti-DC-SIGN monoclonal antibodies 1B10 and 8A5 have been described previously (Halary et al., 2002). Anti-DV E monoclonal antibody 9D12 was a gift from M.K. Gentry and R. Putnak. Anti-LCMV (lymphocytic choriomeningitis virus) monoclonal antibody BD12.5 (IgG γ2a) was used as a negative control. sDCsIGN has been described previously (Halary et al., 2002).
Viruses.
The production of DV-1 strain FGA/NA d1d (GenBank accession number AF226686), DV-2 strain Jam (M20558), DV-3 strain H-87 (NC001475), DV-4 strain H-241 (NC002640), and WN virus strain IS-98-ST1 (AF481864) from Aedes pseudoscutellaris AP61 cell monolayers and virus titration on AP61 cells by focus immunodetection assays (FIAs) were performed as described previously (Desprès et al., 1993). DV-1, DV-2 and WN viruses were highly purified on sucrose gradients, as described previously (Desprès et al., 1993). Infectivity titres were expressed as FFUs in AP61 cells. Vaccine strain 17D-204 of YF virus (STAMARIL; Aventis Pasteur Vaccins; GenBank accession number X15062) was propagated twice in African green monkey kidney VERO cell monolayers and purified using sucrose gradients. Infectivity titres were expressed as FFU in VERO cells.
Virus infection.
Cells were adhered to glass Lab-tek chambers (Nalge Nunc International) coated with poly-L-lysine (Sigma; 5 × 104 cells cm−2). Adherent cells were washed once with RPMI 1640, infected with flavivirus in RPMI 1640 supplemented with 0.2% BSA, pH 7.5, for 2 h at 37 °C, and incubated with RPMI, 2% FCS for 40 h at 37 °C.
Immunofluorescence assays.
Cells were with fixed with 3.2% paraformaldehyde (PFA) in PBS for 20 min, treated with 50 mM NH4Cl in PBS for 10 min, and permeabilized with 0.1% Triton X-100 for 5 min. Intracellular viral antigens were stained with anti-DV-specific hyperimmune mouse ascites fluids (HMAF), anti-YF-virus-specific HMAF or anti-WN-virus-specific HMAF, and the secondary antibody used was a FITC-conjugated goat-anti-mouse IgG (Sigma). Cells were examined using an AXIOPLAN 2 fluorescence microscope (Zeiss). Images were processed using RS Image 1.07, Adobe Photoshop and Powerpoint software.
Deglycosylation of dengue virus virions.
Highly purified FGA/NA d1d virus (1 × 108 AP61 FFU) was incubated with 1 unit of PNGase F (Roche Applied Science) in 20 mM sodium phosphate (pH 7.6) for 7 h at 37 °C. PNGase-F-treated virus and mock-treated virus were used to infect THP/DC-SIGN cells for 48 h. Infected cells were then fixed with 3.2% PFA, washed, and incubated sequentially with anti-DV-1 HMAF and phycoerythrin-conjugated anti-mouse IgG antibody (Sigma). Cells were analysed using a FACScan machine (Becton-Dickinson) and data were processed using CellQuest 3.3 software.
pH-interfering drug treatments.
Bafilomycin A1 and chloroquine were obtained from Sigma. Cells were infected with DV as described above. Infections were performed in the presence of the bafilomycin A1 or chloroquine, followed by washing to remove unbound virus, and cells were further incubated for 3 h with the same drugs. Cells were then washed, and were incubated at 37 °C until 40 h after infection.
Acknowledgments
The authors thank M. Flamand for providing the anti-DV NS1 monoclonal antibody. We acknowledge the assistance provided by P.-E. Lozach, M.-T. Drouet, I. Staropoli and C. Houlès. This work was supported by grants from Direction de la Valorisation et des Partenariats Industriels (Pasteur Institute) and the French National AIDS Research agency (ANRS). E.N.-S. is funded by scholarship funds from the SFERE-CONACYT.
References
- Alvarez C.P., Lasala F., Carillo J., Muniz O., Corbi A.L. & Delgado R. (2002) C-type lectins DCsIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J.Virol., 76, 6841–6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeldt-Ohmann H., Meyer M., Fitzpatrick D.R. & Mackenzie J.S. (2001) Dengue virus binding to human leukocyte cell lines: receptor usage differs between cell types and virus strains. Virus Res., 73, 81–89. [DOI] [PubMed] [Google Scholar]
- Chen Y., Maguire T., Hileman R.E., Fromm J.R., Esko J.D., Linhardt R.J. & Marks R.M. (1997) Dengue virus infectivity depends on envelope protein binding to target cell heparane sulfate. Nature Med., 3, 866–871. [DOI] [PubMed] [Google Scholar]
- Courageot M.-P., Frenkiel M.-P., Duarte Dos Santos C., Deubel V. & Desprès P. (2000) α-Glucosidase inhibitors reduce dengue virus production by affecting the initial steps of virion morphogenesis in the endoplasmic reticulum. J. Virol., 74, 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprès P., Girard M. & Bouloy M. (1991) Characterization of yellow fever virus E and NS1 proteins expressed in Vero and Spodoptera frugiperda cells. J. Gen. Virol., 72, 1331–1342. [DOI] [PubMed] [Google Scholar]
- Desprès P., Frenkiel M.-P. & Deubel V. (1993). Difference between cell membrane fusion activities of two dengue type-1 isolates reflect modifications of viral structure. Virology, 196, 209–219. [DOI] [PubMed] [Google Scholar]
- Desprès P., Frenkiel M.-P., Ceccaldi P.-E., Duarte dos Santos C.N. & Deubel V. (1998) Apoptosis in the mouse central nervous system in response to infection with mouse-neurovirulent dengue virus. J. Virol., 72, 823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte dos Santos C.N., Frenkiel M.-P., Courageot M.-P., Rocha C.F.S., Vazeille-Falcoz M.C., Wien M.W., Rey F.A., Deubel V. & Desprès P. (2000) Determinants in the envelope E protein and viral RNA helicase NS3 that influence the induction of apoptosis in response to infection with dengue type-1 virus. Virology, 274, 292–308. [DOI] [PubMed] [Google Scholar]
- Feinberg H., Mitchell D.A., Drickamer K. & Wesi W.I. (2001) Structural analysis for selective recognition of oligosaccharides by DCsIGN and DC-SIGNR. Science, 294, 2163–2166. [DOI] [PubMed] [Google Scholar]
- Figdor C.G., van Kooyk Y. & Adema G.J. (2002) C-type lectin receptors on dendritic cells and Langerhans cells. Nature Rev. Immunol., 2, 77–84. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T.B.H., Krooshoop D.J.E.B., Bleijs D.A., van Vliet S.J, van Duijnoven G.C.F., Grabovski V., Alon R., Figdor C.J. & van Kooyk Y. (2000a) DCsIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nature Immunol., 1, 353–357. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T.B.H. et al. (2000b) DCsIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell, 100, 587–597. [DOI] [PubMed] [Google Scholar]
- Germi R., Crance J.-M., Garin D., Guimet J., Lortat-Jacob H., Ruigrok R.W.H., Zarski J.P. & Drouet E. (2002) Heparan sulfate-mediated binding of infectious dengue virus type-2 and yellow fever virus. Virology, 292, 162–168. [DOI] [PubMed] [Google Scholar]
- Guzman M.G. & Kouri G. (2002) Dengue: an update. Lancet Infect. Dis., 2, 33–42. [DOI] [PubMed] [Google Scholar]
- Halary F., Amara A., Lortat-Jacob H., Messerle M., Delaunay T., Houles C., Fieschi F., Arenzanaseisdedos F., Moreau J.F. & Dechanet-Merville J. (2002) Human cytomegalovirus binding to DC-SIGN is required for dendritic infection and target cell trans-infection. Immunity, 17, 653–664. [DOI] [PubMed] [Google Scholar]
- Halstead S.B. (1989) Antibody, macrophages, dengue virus infection, shock, and hemorrhage: a pathogenic cascade. Rev. Infect. Dis., 11, S830–S839. [DOI] [PubMed] [Google Scholar]
- Heinz F.X. & Allison S.L. (2000) Structures and mechanisms in flavivirus fusion. Adv.Virus Res., 55, 231–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L.-J., Wang J.-J., Shiao M.-F., Kao C.-L., Chang D.-M., Han S.-W. & Lai J.H. (2001) Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. Immunology, 166, 1499–1506. [DOI] [PubMed] [Google Scholar]
- Hung S.-L., Lee P.-L., Chen H.-W., Chen L.-K., Kao C.-L. & King C.-C. (1999) Analysis of the steps in dengue virus entry into host cells. Virology, 257, 156–167. [DOI] [PubMed] [Google Scholar]
- Johnson A.J., Guirakhoo F. & Roehrig J.T. (1994) The envelope glycoproteins of dengue 1 and dengue 2 grown in mosquito cells differ in their utilization of potential glycosylation sites. Virology, 203, 241–249. [DOI] [PubMed] [Google Scholar]
- Kelsall B.L., Biron C.A., Sharma O. & Kaye P.M. (2002) Dendritic cells at the host–pathogen interface. Nature Immunol., 3, 699–702. [DOI] [PubMed] [Google Scholar]
- Kuhn R.J. et al. (2002) Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell, 108, 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon D.S., Gregorio G., Bitton N., Hendrickson W.A. & Littman D.R. (2002) DCsIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity, 16, 135–144. [DOI] [PubMed] [Google Scholar]
- Lei H.Y., Yeh T.M., Liu H.S., Lin Y.S., Chen S.H. & Liu C.C. (2001) Immunopathogenesis of dengue virus infection. J. Biomed. Sci., 8, 377–388. [DOI] [PubMed] [Google Scholar]
- Lin G. et al. (2003) Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DCsIGN and DC-SIGNR. J. Virol., 77, 1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozach P.Y. et al. (2003) DCsIGN and L-SIGN are high affinity binding receptors for hepatitis C virus glycoprotein E2. J. Biol. Chem., 278, 20358–20366. [DOI] [PubMed] [Google Scholar]
- Marovich M. et al. (2001) Human dendritic cells as targets of dengue virus infection. JID Symp. Proc., 6, 219–224. [DOI] [PubMed] [Google Scholar]
- Mashimo T., Lucas M., Simon-Chazottes D., Frenkiel M.-P., Montagutelli X., Ceccaldi P.-E., Deubel V., Guenet J.L. & Desprès P. (2002) A nonsense mutation in the gene encoding 2'-5'-oligoadenylate synthetase/L-1 isoform is associated with West Nile virus susceptibility in laboratory mice. Proc. Natl Acad. Sci. USA, 99, 11311–11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens D.M., Marchette N.J., Chu M.C. & Halstead S.B. (1991) Growth of dengue type 2 virus isolates in human peripheral leukocytes correlates with severe and mild dengue diesease. Am. J. Trop. Med. Hyg., 45, 644–651. [DOI] [PubMed] [Google Scholar]
- Palucka A.K. (2000) Dengue virus and dendritic cells. Nature Med., 6, 748–749. [DOI] [PubMed] [Google Scholar]
- Pöhlmann S., Zhang J., Baribaud F., Chen Z., Leslie G.J., Lin G., Granelli-Piperno A., Doms R.W., Rice C.M. & McKeating J.A. (2003) Hepatitis C virus glycoproteins interact with DCsIGN and DC-SIGNR. J. Virol., 77, 4070–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C.M. (1996) in Fields Virology 3rd edn. (eds Fields, B.N., Knipe, D.M. & Howley, P.M.), 931–959. Lippincott–Raven, Philadelphia, Pennsylvania, USA. [Google Scholar]
- Rothman A. & Ennis F. (1999) Immunopathogenesis of dengue hemorrhagic fever. Virology, 257, 1–6. [DOI] [PubMed] [Google Scholar]
- Soilleux E.J., Barten R. & Trowsdale J. (2000) DCsIGN; a related gene, DC-SIGNR; and CD23 form a cluster on 19p13. J. Immunol., 165, 2937–2942. [DOI] [PubMed] [Google Scholar]
- Steiman R.M. (2000) DCsIGN: a guide to some mysteries of dendritic cells. Cell, 100, 491–494. [DOI] [PubMed] [Google Scholar]
- Wu S.J. et al. (2000) Human skin Langerhans are targets of dengue virus infection. Nature Med., 6, 816–820. [DOI] [PubMed] [Google Scholar]


