Abstract
Precise monitoring of DNA replication and chromosome segregation ensures that there is accurate transmission of genetic information from a cell to its daughters. Eukaryotic cells have developed a complex network of checkpoint pathways that sense DNA lesions and defects in chromosome segregation, spindle assembly and the centrosome cycle, leading to an inhibition of cell-cycle progression for the time required to remove the defect and thus preventing genomic instability. The activation of checkpoints that are responsive to DNA damage or incomplete DNA replication ultimately results in the inhibition of cyclin-dependent kinases. This review focuses on our understanding of the biochemical mechanisms that specifically inactivate Cdc25 (cell division cycle 25) phosphatases to achieve this. The evidence for links between checkpoint deregulation and oncogenesis is discussed.
Introduction
Timely progression through the cell-division cycle requires phosphorylation events carried out by cyclin-dependent protein kinases (Cdks). Specific checkpoints that are activated by damaged or unreplicated DNA rapidly inhibit Cdk activity, delaying progression of the cell cycle to provide time to repair DNA or to complete replication (Zhou & Elledge, 2000). Cdks are initially activated by their association with cyclin subunits and by phosphorylation on a threonine residue that is located in a conserved amino-acid sequence, the T-loop. These events stabilize the Cdk subunit in its active form, allowing for optimal ATP and peptidesubstrate binding. Although inactivation of Cdk/cyclin complexes can be achieved by ubiquitin-mediated degradation of cyclins (Peters, 2002), a distinct mode of reversible inactivation can also be triggered by phosphorylating the crucial residues Tyr 15 and Thr 14, which are located within the Cdk ATP-binding loop (Nurse, 1997). The Wee1/Mik1/Myt1 protein kinases mediate this phosphorylation event, whereas dualspecificity phosphatases of the (cell division cycle 25) Cdc25 family are responsible for the dephosphorylation of Tyr 15 and Thr 14, which leads to Cdk activation (Fig. 1A; Pines, 1999). As dephosphorylation of these residues by Cdc25 proteins is the rate-limiting step for Cdk activation, which in turn leads to cell-cycle progression, elucidation of the mechanisms that govern the regulation of their phosphatase activity in response to checkpoints is of great interest. Here, we will summarize our view of how Cdc25 proteins are regulated during the cell cycle and how this might be linked to tumorigenesis.
Figure 1.
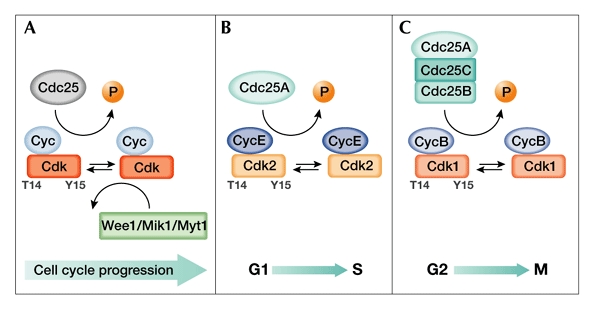
The activation of cyclin-dependent kinases by Cdc25 phosphatases. The coordinated action of kinases and phosphatases regulates cyclin-dependent kinase (Cdk) activity during cell-cycle progression. (A) Cdks are maintained in an inactive state through the phosphorylation of Thr 14 (T14) and Tyr 15 (Y15) in their amino-terminal ATP-binding regions by members of the Wee1/Mik1/Myt1 protein kinase family. The rate-limiting step in the activation of Cdks is dephosphorylation of these residues by Cdc25 dual-specificity phosphatases. (B) Cdc25A regulation of Cdk2/cyclin E during G1 and S phase. (C) Cdc25A, Cdc25B and Cdc25C regulation of Cdk1/cyclin B during G2 and M phase. Cdc, cell-division cycle; Cyc, cyclin.
Cdc25 phosphatases: key regulators of the cell cycle
The fission yeast cdc25 gene was first identified as a dose-dependent inducer of mitosis. Yeast cells that carry a temperature-sensitive allele (cdc25ts) arrest in G2 phase (Russell & Nurse, 1986). In mammals, three related genes (Cdc25A, Cdc25B and Cdc25C) have been identified and found to functionally complement the defective cdc25ts strain (Galaktionov & Beach, 1991). All of the Cdc25 proteins are dualspecificity phosphatases, and can dephosphorylate phosphotyrosine as well as phosphothreonine residues, therefore activating their physiological substrates, the Cdks (Fig. 1A; Dunphy & Kumagai, 1991). Despite sharing a common biochemical mechanism of action, Cdc25 family members have unique characteristics and specific roles in cell-cycle regulation.
Mammalian Cdc25A regulates both early and late cell-cycle transitions (Fig. 1B,C). Its role in the earlier cell-cycle phases was suggested by the observation that microinjection of Cdc25A antibodies prevents S-phase entry induced by serum stimulation and arrests cells in G1 phase (Hoffmann et al., 1994; Jinno et al., 1994). Conversely, overexpression of Cdc25A accelerates entry into S phase and prematurely activates Cdk2 (Blomberg & Hoffmann, 1999; Sexl et al., 1999). A later role in the cell cycle has been suggested by the more recent findings that Cdc25A steadystate levels increase from G1 to S phase, G2 phase and mitosis (Bernardi et al., 2000; Donzelli et al., 2002; Molinari et al., 2000) and that overexpression of the protein can induce mitotic events (Molinari et al., 2000). These and other data (Donzelli et al., 2002; Mailand et al., 2002) support the notion that Cdc25A has a comprehensive function in the cell cycle, regulating the G1/S transition, S phase and the G2/M transition.
Microinjection studies with Cdc25B and Cdc25C indicate that these proteins may have more limited roles in promoting progression from G2 phase to mitosis (Fig. 1C); indeed, injection of antibodies against either protein causes cells to arrest only after they reach G2 (Lammer et al., 1998; Millar et al., 1991). Despite this similarity in the timing of Cdc25B- and Cdc25C-mediated arrest, the roles of these phosphatases do not seem to be identical. The activity of Cdc25B peaks before that of Cdc25C, and its overexpression induces premature mitosis more efficiently than the overexpression of Cdc25C. One possible mechanism that would account for these findings involves serial activation, with Cdc25B triggering mitosis by dephosphorylating Cdk1/cyclin B, and this modified complex, in turn, activating Cdc25C. As Cdk1/cyclin B is the main substrate of activated Cdc25C, this mechanism would create an amplification loop, leading to an irreversible commitment to mitosis (Hoffmann et al., 1993; Karlsson et al., 1999).
Cdc25 regulation
Given the structural and mechanistic similarities between the three mammalian Cdc25 homologues, it is likely that the differences in their biological roles are determined by the timing of activation, subcellular localization and substrate recognition of each of the phosphatases. Indeed, the regulation of Cdc25 phosphatases is intricate and occurs at many levels. Whereas Cdc25C protein levels remain constant throughout the cell cycle, those of both Cdc25A and Cdc25B fluctuate greatly. Furthermore, the activities of all three proteins seem to be modulated. The mechanisms that regulate the accumulation and the activation state of the Cdc25 phosphatase isoforms are discussed here, and are also summarized in Table 1.
Table 1.
Reported features of human Cdc25 phosphatases
| Cdc25A | Cdc25B | Cdc25C | |
|---|---|---|---|
| Subcellular localization | Nuclear | Cytoplasmic | Nuclear or cytoplasmic* |
| Transcriptional regulation | c-Myc and E2F | Unknown | Unknown |
| Cdk-mediated phosphorylation | Cdk2/cyclin E: stimulates activity | Cdk1/cyclin A: promotes | Cdk1/cyclinB: stimulates activity |
| Cdk1/cyclin B: prevents Ub-dependent degradation at mitosis through phosphorylation at Ser 17 and Ser 115 | Ub-dependent degradation | ||
| Checkpoint-mediated phosphorylation | Chk1: regulates Ub-dependent degradation in both unperturbed and perturbed cells (IR, UV irradiation and replication blocks) through phosphorylation at Ser 123, Ser 178, Ser 278 and Ser 292 Chk2: regulates Ub-mediated proteolysis on IR induced DNA damage, probably involving Ser123, Ser178 and Ser292 phosphorylation | p38: regulates protein activityon UV irradiation, probably through binding of 14-3-3proteins and protein delocalization | Chk1/Chk2: regulates protein localization by creating a binding site for 14-3-3 proteins at Ser 216 (regulates nuclearcytoplasmic shuttling) |
| Other phosphorylation events | c-Raf: increases activity on mitogenic stimulation | – | C-Tak1: mediates Ser 216 phosphorylation, creating a 14-3-3 protein binding site Plk1: promotes nuclear translocation during prophase |
| Stability and Ub-dependent degradation | Stabilized during mitosis through Ser 17 and Ser 115 phosphorylation events Degraded on mitosis exit and in G1 by APC/CCdh1 Unstable in interphase (SCF probably involved) Unstable on replication block and DNA damage (SCF probably involved) | Unstable protein | Stable protein |
*Still a matter of contention. See text for details.
APC/CCdh1, anaphase promoting complex or cyclosome; Cdc, cell division cycle; Cdk, cyclin-dependent kinase; Chk, checkpoint kinase; IR, ionizing radiation; Plk-1, polo-like kinase 1; SCF, Skp1/Cul1/F-box; Ub, ubiquitin; UV, ultraviolet.
In the case of Cdc25A, messenger RNA and protein levels begin to accumulate in late G1 after serum stimulation of quiescent fibroblasts (Jinno et al., 1994), and the expression of the protein from G1 to S is thought to depend on the transcription factors c-Myc and E2F (Bernardi et al., 2000; Galaktionov et al., 1996; Vigo et al., 1999). Cdc25A is phosphorylated and activated during S phase by its own substrate, Cdk2/cyclin E, allowing for amplification of the signal that triggers cell-cycle progression (Hoffmann et al., 1994). Cdc25A is rapidly turned over throughout interphase (Donzelli et al., 2002), yet it continues to accumulate through S phase and G2 and, in mitotic cells, it becomes stabilized by Cdk1/cyclinB-mediated phosphorylation on Ser 17 and Ser 115 (Mailand et al., 2002). As cells exit mitosis, rapid APC/CCdh1 (anaphase promoting complex or cyclosome)–ubiquitin-mediated degradation of Cdc25A occurs (Donzelli et al., 2002) Mailand and colleagues (2002) have suggested that Cdc25A exists in a stable mitotic form, as well as a labile interphase state, and that the latter allows for fine-tuning of the Cdc25A thresholds required for cell-cycle transitions and cell-cycle delays in response to checkpoint activation. The pathways that lead to this multi-tiered regulation of Cdc25A are probably controlled by the ubiquitin ligases classified as SCF (Skp1/Cul1/F-box) complexes (Donzelli et al., 2002).
Cdc25B is also an unstable protein (Nishijima et al., 1997), although its expression pattern is less complex than that of Cdc25A. It accumulates during the late S and early G2 phases of the cell cycle and its activity peaks at the G2/M transition, at which point it becomes hyperphosphorylated by an as yet unidentified kinase (Gabrielli et al., 1997; Lammer et al., 1998). Cdc25B is ultimately targeted for proteasome-dependent degradation, which requires phosphorylation mediated by the Cdk1/cyclin A complex (Baldin et al., 1997).
Cdc25C remains inactive for most of the cell cycle. Indeed, Cdc25C phosphatase activity is undetectable during interphase and only becomes measurable as cells enter mitosis (Jessus & Beach, 1992). This activation correlates with Cdc25C hyperphosphorylation, which is triggered by Cdk1/cyclin B. Cdc25C phosphorylation and activation by its own substrate allows for signal amplification and contributes to the burst of Cdk1/cyclin B activity that drives mitosis (Hoffmann et al., 1993). This finding also indicated that a distinct Cdk1/cyclin B phosphatase (Cdc25A or Cdc25B) could trigger the initial activation of the complex. In addition to Cdk1/cyclin B, other protein kinases phosphorylate Cdc25C and regulate its function. C-Tak1 (Cdc25C-associated protein kinase) can bind and phosphorylate human Cdc25C on Ser 216 (Peng et al., 1998). The checkpoint kinases Chk1 and Chk2 can also catalyse this phosphorylation event (Matsuoka et al., 1998; Peng et al., 1997; Sanchez et al., 1997), as discussed in more detail below. In human cells, Plk1, which belongs to the polo-like kinase (PLK) family (Glover et al., 1998; Nigg, 1998), has also been shown to phosphorylate Cdc25C and to promote its nuclear translocation during prophase (Toyoshima-Morimoto et al., 2002).
In human cells, Cdc25A is localized to the nucleus (Hoffmann et al., 1994; Molinari et al., 2000), whereas Cdc25B has been shown to localize to the cytosol (Gabrielli et al., 1996). The localization of Cdc25C is still a matter of contention, as Cdc25C has been described as nuclear-based in some studies but cytoplasmic in others (Graves et al., 2001; Heald et al., 1993; Millar et al., 1991). The reasons for this discrepancy are unclear at present and might reflect differences in the genetic backgrounds of the cell lines used. Thus, the issue requires further work to be univocally resolved. In human cells, CDC25C is phosphorylated on Ser 216 throughout interphase, but not in mitosis. This creates a binding site for 14-3-3 proteins (Peng et al., 1997), which are known to specifically recognize phosphoserine-containing motifs in proteins involved in signal transduction and cell-cycle regulation (Muslin et al., 1996; Yaffe et al., 1997). It has been suggested that 14-3-3 protein binding is responsible for retaining Cdc25C in the cytoplasm during interphase, thereby contributing to the prevention of premature initiation of mitotic events (Dalal et al., 1999; for a review, see Takizawa & Morgan, 2000).
Cdc25 and DNA damage checkpoints
Cdc25 phosphatases have a key role in the checkpoint response to unreplicated or damaged DNA. This was first shown in fission yeast, where it was found that Cdc25 is phosphorylated by the Chk1 protein kinase in response to DNA damage (Furnari et al., 1997). Indeed, in all eukaryotes studied, with the exception of budding yeast, checkpoint-mediated phosphorylation of Cdc25 proteins results in their functional inactivation, in turn preventing Cdk dephosphorylation and activation. In fission yeast, Chk1-mediated phosphorylation of Cdc25 creates a binding site for 14-3-3 proteins. Mutations of canonical 14-3-3 binding sites that are created by both Chk1- and Cds1 (Chk2)-mediated phosphorylation, disrupt the checkpoint response to damaged or unreplicated DNA (Zeng & Piwnica-Worms, 1999). It has been proposed that 14-3-3 proteins provide a nuclear-export signal (NES) that enhances the export of Cdc25, thus separating Cdc25 from the nuclear pools of Cdk1/cyclinB and preventing the cell from entering mitosis until the damaged DNA is repaired (Lopez-Girona et al., 1999). More recent findings have shown, however, that yeast cells carrying a nuclear localization signal (NLS)-targeted Cdc25 have an intact DNA damage response (Lopez-Girona et al., 2001). Furthermore, Cdc25 phosphorylation and 14-3-3 binding do not require Chk1 and are not affected by DNA damage (Chen et al., 1999).
Chk1-mediated phosphorylation of Cdc25C has also been shown to occur in Xenopus and human cells (Kumagai et al., 1998a,b; Peng et al., 1997; Sanchez et al., 1997). Evidence from Xenopus suggests that 14-3-3 proteins might sequester Cdc25C in the cytoplasm by masking an NLS on the protein (Kumagai & Dunphy, 1999; Yang et al., 1999).
In mammalian cells, the activation of Chk2 and Chk1 after DNA damage and/or a replication block is dependent on the ataxia-telangiectasia mutated (ATM) and ATM-related (ATR) protein kinases. ATM and ATR become activated primarily in response to ionizing radiation (IR), or ultraviolet (UV) light and DNA replication inhibitors, respectively, although some level of crosstalk exists between the two pathways (Fig. 2A,C; Shiloh, 2001, 2003; Zhou & Elledge, 2000).
Figure 2.
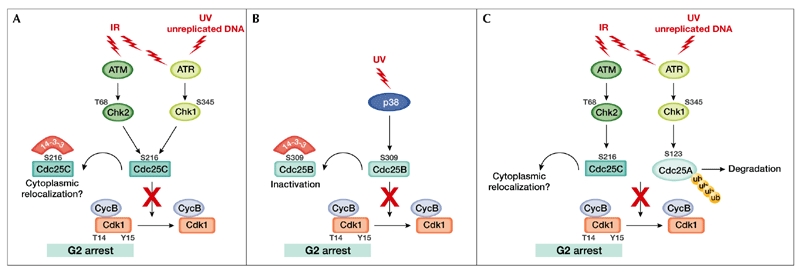
Proposed regulation of Cdc25 proteins at the G2/M checkpoint. (A) Cells exposed to ionizing radiation (IR) or ultraviolet (UV) light respond by activating kinases of the ataxia-telangiectasia mutated (ATM) protein family. ATM and ATM-related (ATR) phosphorylate and activate the checkpoint kinases Chk2 and Chk1, respectively. Activated checkpoint kinases phosphorylate Cdc25C on Ser 216, creating a binding site for 14-3-3 proteins, which then act by excluding Cdc25C from the nucleus. Cytoplasmic relocalization of Cdc25C would prevent cyclin-dependent kinase 1 (Cdk1)/cyclin B (CycB) from being dephosphorylated and cause an arrest in G2. (B) After UV irradiation, p38 phosphorylates Cdc25B on Ser 309, allowing binding of 14-3-3 proteins and resulting in Cdc25B inhibition, leading to G2 arrest. (C) Many levels of Cdc25 phosphatases at the G2/M transition allow for fine modulation of cell division in response to the cellular environment. Cdc, cell-division cycle; Ub, ubiquitin.
Activated Chk1 and Chk2 can phosphorylate Cdc25C on Ser 216 in vitro, thus creating a binding site for 14-3-3 proteins (Matsuoka et al., 1998; Peng et al., 1997; Sanchez et al., 1997). A mutation that prevents phosphorylation of Ser 216 abrogates 14-3-3 binding and expression of a Ser 216-Ala mutant causes cells to bypass a cell-cycle arrest induced by IR or DNA replication inhibitors (Peng et al., 1997). This is thought by some to reflect a requirement for Cdc25C to be retained in the cytoplasm in the presence of damaged DNA, and raises the possibility that binding to 14-3-3 proteins might prevent mitosis, allowing time for DNA repair (Dalal et al., 1999; Takizawa & Morgan, 2000). However, another appealing model that is consistent with data from yeast and from the reports of nuclear Cdc25C localization, is that 14-3-3 proteins bind and export Cdc25C from the nucleus in response to checkpoint activation (Fig. 2A; Pines, 1999). Although genetic and biochemical evidence in Xenopus and fission yeast supports both models, the finding that Cdc25C in interphase human cells is constitutively phosphorylated on Ser 216 and bound to 14-3-3 proteins challenges their universal validity. Furthermore, although it is evident that 14-3-3 proteins negatively regulate Cdc25C, it is unclear how the sequestration of a 14-3-3/Cdc25C complex in the cytoplasm would function as a checkpoint, as this places Cdc25C in the same subcellular compartment as Cdk1/cyclin B complexes. It is also unclear what the relative contributions of the C-Tak1, Chk1 and Chk2 kinases are in terms of determining the Cdc25C Ser 216 phosphorylation status in cycling cells and in cells treated with DNA-damaging agents or DNA-replication inhibitors.
Recently, an additional mechanism for regulating Cdc25C has been reported, whereby arsenite-induced G2 arrest might occur through ubiquitin-dependent, APC/C-mediated degradation of the phosphatase (Chen et al., 2002). The relevance of these findings remains to be clarified, given that Cdc25C was not clearly shown in this study to be a direct substrate of APC.
Like Cdc25C, Cdc25B seems to be targeted by DNA-damage checkpoints. Bulavin and colleagues (2001) showed that inhibition of p38 results in an abrogation of the G2/M checkpoint after UV irradiation. They also showed that p38 can phosphorylate Cdc25B in vitro, unmasking a putative 14-3-3 binding site (Ser 309), and that expression of a S309A mutant can overcome a G2 arrest induced by UV irradiation (Fig. 2B). Given that the serine residue targeted by p38 was found to be phosphorylated in unperturbed cells as well, and that most of the results are from in vitro studies, further work will be needed to understand the contribution of this mechanism to a checkpoint response.
Cdc25A inactivation can contribute to an immediate block of the cell cycle, leading to an apparently p53-independent G1 arrest (Bartek & Lukas, 2001), an S-phase delay (Falck et al., 2002) or a G2 arrest (Mailand et al., 2002). Cdc25A is an unstable protein (Donzelli et al., 2002) and its degradation can be critically accelerated in the presence of unreplicated or damaged DNA (Falck et al., 2001; Mailand et al., 2000; Molinari et al., 2000). Accelerated Cdc25A degradation, as a result of phosphorylation by the Chk1 and/or Chk2 protein kinases, results in sustained inhibitory phosphorylation of Cdk2, leading to G1 arrest and a block in S-phase entry (Fig. 3A,B). Likewise, a failure to dephosphorylate and activate Cdk2 results in an S-phase delay, as it prevents the loading of the essential replication factor Cdc45 and, in turn, recruitment of DNA polymerase to replication origins (Fig. 3B; Falck et al., 2002). In human cells, the integrity of the ATM/Chk2/Cdc25A/Cdk2 pathway was found to be required to prevent radioresistant DNA synthesis (Falck et al., 2001). In the presence of doublestranded breaks (DSBs) caused by ionizing radiation, cells activate the ATM protein kinase, which has a broad range of target proteins, including Chk2. The activated Chk2 then phosphorylates Cdc25A on Ser 123, targeting the phosphatase for ubiquitin-dependent proteasomal degradation (Falck et al., 2001). It should be pointed out, however, that primary mouse embryonic fibroblasts (MEFs) derived from Chk2-deficient mice did not undergo radioresistant DNA synthesis in response to IR, a phenotype consistent with a functional intras-phase checkpoint (Hirao et al., 2002). This points to the existence of mechanisms in addition to Chk2-mediated inactivation of Cdc25A that prevent cell-cycle progression of damaged cells. Chk1 is likely to be one of the factors that compensates for a lack of Chk2. Indeed, Cdc25A is a versatile sensor of damage, as it can also be phosphorylated by Chk1 in response to exposure to both UV light (Mailand et al., 2000) and IR (Sørensen et al., 2003; Zhao et al., 2002). Sørensen and co-workers (2003) have shown that Chk1-mediated phosphorylation of Cdc25A induced by IR exposure occurs on Ser 123 and three additional serine residues, and that this event is ATM-dependent (Fig. 3B). The same authors reported that phosphorylation at these residues by Chk1 occurs even during an unperturbed cell cycle, consistent with the finding that Chk1 is active during S phase and G2 irrespective of checkpoint activation (Liu et al., 2000). They suggest that both Chk2 and Chk1 are involved in Cdc25A degradation induced by IR and that accelerated proteolysis results from increased phosphate incorporation on crucial residues by the combined actions of both kinases. The observation that Chk1 and Chk2 have analogous and significantly overlapping functions, the former being an essential kinase and the latter being a modulator of checkpoint responses, partially explains the unpredicted phenotype of Chk2 knockout mice.
Figure 3.
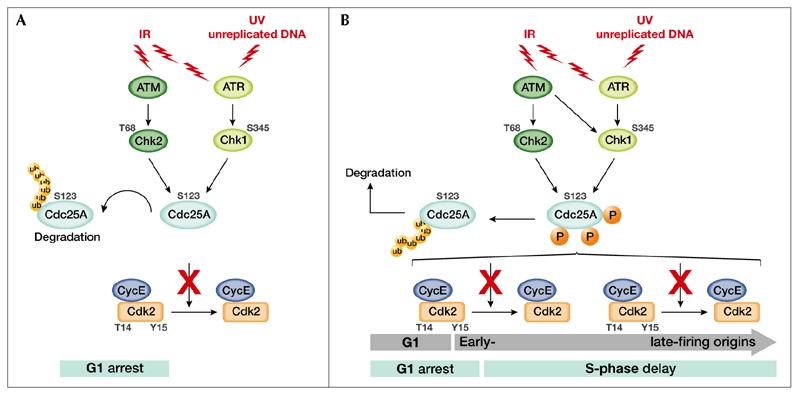
Proposed regulation of Cdc25A at the G1 and intra-S-phase checkpoints. (A) Cells exposed to ionizing radiation (IR) or ultraviolet light (UV) respond by activating kinases of the ataxia-telangiectasia mutated (ATM) protein family. ATM and ATM-related (ATR) phosphorylate and activate the checkpoint kinases Chk2 and Chk1, respectively. Activated checkpoint kinases phosphorylate Cdc25A on Ser 123, accelerating its degradation through the ubiquitin (Ub)–proteasome pathway. Inactivation of Cdc25A results in the sustained phosphorylation and inhibition of cyclin-dependent kinase 2 (Cdk2), thus leading to G1 arrest. (B) A unified view of how Cdc25A degradation brings about G1 arrest and an S-phase delay (blue bars) by inhibiting both early- and late-firing origins of replication (grey arrows). Cdc25A, cell-division cycle 25A; Cyc, cyclin.
Finally, a requirement for Cdc25A-induced degradation during G2-phase checkpoint activation has been postulated, consistent with its apparent role in mitosis (Mailand et al., 2002). A tentative model is represented in Fig. 2C, although biochemical validation is still missing.
The regulation of Cdc25 phosphatases at many levels in response to the activation of intrinsically (for example, by replication errors) or extrinsically (for example, by DNA damage) induced checkpoints allows for close monitoring of cell-cycle progression. In organisms such as fission yeast, a single Cdc25 phosphatase is targeted by distinct checkpoint mechanisms. In higher eukaryotes, the existence of several Cdc25 proteins, which show some redundancy in their ability to control the cell cycle and the response to checkpoint activation, might be explained by their localization to specific cellular compartments and their distinct modes of temporal activation. Although earlier studies focused on elucidating the role of the Cdc25C phosphatase in checkpoint control, with an attempt to describe an evolutionarily conserved mode of checkpoint activation, more recent work has highlighted the function of Cdc25A as a master Cdk phosphatase. Rapid inactivation of this phosphatase by ubiquitin-mediated proteolysis can achieve a sudden inhibition of Cdk dephosphorylation and contribute to an immediate arrest in cell-cycle progression. The relative contribution of Cdc25A, Cdc25B and Cdc25C to checkpoint activation in mammalian cells remains to be clarified. In this regard, it should be noted that Cdc25C Ser 216 phosphorylation does not occur in mouse cells, as mouse Cdc25C does not have an equivalent to this residue. Furthermore, mice that lack Cdc25C are viable and fertile, and their cells seem to have a normal checkpoint response to DNA damage (Chen et al., 2001). This unexpected phenotype can be partially explained by data reported by Mailand and colleagues (2002), who have proposed a model in which all three Cdc25 proteins are involved in mitotic regulation. According to this model, Cdc25A and Cdc25B would compensate for the lack of Cdc25C in the knockout mice by performing redundant functions. Alternatively, the compensation for Cdc25C loss might be ascribed to the role of the Wee1/Mik1/Myt1 protein kinases in the G2/M checkpoint, as has been suggested by Raleigh & O'Connell (2000).
Cdc25 deregulation in cancer
Cdc25A and Cdc25B, but not Cdc25C, can induce the transformation of primary rodent cells in the presence of either an activated Ha-RAS mutant or a retinoblastoma (pRb) deletion (Galaktionov et al., 1995). Deregulated expression of Cdc25B in transgenic mice induces mammary gland hyperplasia and increases susceptibility to mammary tumours (Yao et al., 1999), and the overexpression of Cdc25A and Cdc25B has been described in cancer cell lines and human primary tumour samples (Takemasa et al., 2000). Overexpression of Cdc25 proteins in tumours could also result from a reduced rate of degradation. Mutations occurring in both the ATM and Chk2 genes, or upstream components of these pathways, are expected to result in a failure to activate Cdc25 degradation in response to DNA damage, thus contributing to the genomic instability and cancer predisposition described in syndromes in which these genes are affected (Bell et al., 1999; Painter & Young, 1980). Mutations in the ubiquitin ligases that mediate the degradation of Cdc25A could also cause an increase in its abundance. It has indeed been shown that Cdc4, an F-box protein that targets cyclin E in human cells, is mutated in a subset of human tumours, leading to an upregulation of cyclin E (Strohmaier et al., 2001). The identification of the Cdc25A ubiquitin ligase will allow this hypothesis to be tested.

Acknowledgments
We thank M. Squatrito and M. Melixetian for critically reading the manuscript. Work in the authors' laboratory is supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC), the Consiglio Nazionale delle Ricerche (CNR), the Fondazione Italiana per la Ricerca sul Cancro (FIRC) and Telethon. M.D. was supported by a FIRC fellowship.
References
- Baldin V., Cans C., Knibiehler M. & Ducommun B. (1997) Phosphorylation of human CDC25B phosphatase by CDK1–cyclin A triggers its proteasome-dependent degradation. J. Biol. Chem., 272, 32731–32734. [DOI] [PubMed] [Google Scholar]
- Bartek J. & Lukas J. (2001) Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr. Opin. Cell Biol., 13, 738–747. [DOI] [PubMed] [Google Scholar]
- Bell D.W. et al. (1999) Heterozygous germ line hCHK2 mutations in Li–Fraumeni syndrome. Science, 286, 2528–2531. [DOI] [PubMed] [Google Scholar]
- Bernardi R., Liebermann D.A. & Hoffman B. (2000) Cdc25A stability is controlled by the ubiquitin-proteasome pathway during cell cycle progression and terminal differentiation. Oncogene, 19, 2447–2454. [DOI] [PubMed] [Google Scholar]
- Blomberg I. & Hoffmann I. (1999) Ectopic expression of Cdc25A accelerates the G(1)/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases. Mol. Cell. Biol., 19, 6183–6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulavin D.V., Higashimoto Y., Popoff I.J., Gaarde W.A., Basrur V., Potapova O., Appella E. & Fornace A.J. Jr. (2001) Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature, 411, 102–107. [DOI] [PubMed] [Google Scholar]
- Chen L., Liu T.H. & Walworth N.C. (1999) Association of Chk1 with 14-3-3 proteins is stimulated by DNA damage. Genes Dev., 13, 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.S., Hurov J., White L.S., Woodford-Thomas T. & Piwnica-Worms H. (2001) Absence of apparent phenotype in mice lacking Cdc25C protein phosphatase. Mol. Cell. Biol., 21, 3853–3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Zhang Z., Bower J., Lu Y., Leonard S.S., Ding M., Castranova V., Piwnica-Worms H. & Shi X. (2002) Arsenite-induced Cdc25C degradation is through the KEN-box and ubiquitin-proteasome pathway. Proc. Natl Acad. Sci. USA, 99, 1990–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal S.N., Schweitzer C.M., Gan J. & DeCaprio J.A. (1999) Cytoplasmic localization of human cdc25C during interphase requires an intact 14-3-3 binding site. Mol. Cell. Biol., 19, 4465–4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzelli M., Squatrito M., Ganoth D., Hershko A., Pagano M. & Draetta G.F. (2002) Dual mode of degradation of Cdc25 A phosphatase. EMBO J., 21, 4875–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W.G. & Kumagai A. (1991) The cdc25 protein contains an intrinsic phosphatase activity. Cell, 67, 189–196. [DOI] [PubMed] [Google Scholar]
- Falck J., Mailand N., Syljuasen R.G., Bartek J. & Lukas J. (2001) The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature, 410, 842–847. [DOI] [PubMed] [Google Scholar]
- Falck J., Petrini J.H., Williams B.R., Lukas J. & Bartek J. (2002) The DNA damage-dependent intras phase checkpoint is regulated by parallel pathways. Nature Genet., 30, 290–294. [DOI] [PubMed] [Google Scholar]
- Furnari B., Rhind N. & Russell P. (1997) Cdc25 mitotic inducer targeted by chk1 DNA damage checkpoint kinase. Science, 277, 1495–1497. [DOI] [PubMed] [Google Scholar]
- Gabrielli B.G., De Souza C.P., Tonks I.D., Clark J.M., Hayward N.K. & Ellem K.A. (1996) Cytoplasmic accumulation of cdc25B phosphatase in mitosis triggers centrosomal microtubule nucleation in HeLa cells. J. Cell Sci., 109, 1081–1093. [DOI] [PubMed] [Google Scholar]
- Gabrielli B.G., Clark J.M., McCormack A.K. & Ellem K.A. (1997) Hyperphosphorylation of the N-terminal domain of Cdc25 regulates activity toward cyclin B1/Cdc2 but not cyclin A/Cdk2. J. Biol. Chem., 272, 28607–28614. [DOI] [PubMed] [Google Scholar]
- Galaktionov K. & Beach D. (1991) Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell, 67, 1181–1194. [DOI] [PubMed] [Google Scholar]
- Galaktionov K., Lee A.K., Eckstein J., Draetta G., Meckler J., Loda M. & Beach D. (1995) CDC25 phosphatases as potential human oncogenes. Science, 269, 1575–1577. [DOI] [PubMed] [Google Scholar]
- Galaktionov K., Chen X. & Beach D. (1996) Cdc25 cell-cycle phosphatase as a target of c-myc. Nature, 382, 511–517. [DOI] [PubMed] [Google Scholar]
- Glover D.M., Hagan I.M. & Tavares A.A. (1998) Polo-like kinases: a team that plays throughout mitosis. Genes Dev., 12, 3777–3787. [DOI] [PubMed] [Google Scholar]
- Graves P.R., Lovly C.M., Uy G.L. & Piwnica-Worms H. (2001) Localization of human Cdc25C is regulated both by nuclear export and 14-3-3 protein binding. Oncogene, 20, 1839–1851. [DOI] [PubMed] [Google Scholar]
- Heald R., McLoughlin M. & McKeon F. (1993) Human wee1 maintains mitotic timing by protecting the nucleus from cytoplasmically activated Cdc2 kinase. Cell, 74, 463–474. [DOI] [PubMed] [Google Scholar]
- Hirao A. et al. (2002) Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol. Cell. Biol., 22, 6521–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann I., Clarke P.R., Marcote M.J., Karsenti E. & Draetta G. (1993) Phosphorylation and activation of human cdc25-C by cdc2–cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J., 12, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann I., Draetta G. & Karsenti E. (1994) Activation of the phosphatase activity of human cdc25A by a Cdk2-cyclin E dependent phosphorylation at the G1/S transition. EMBO J., 13, 4302–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessus C. & Beach D. (1992) Oscillation of MPF is accompanied by periodic association between cdc25 and cdc2–cyclin B. Cell, 68, 323–332. [DOI] [PubMed] [Google Scholar]
- Jinno S., Suto K., Nagata A., Igarashi M., Kanaoka Y., Nojima H. & Okayama H. (1994) Cdc25A is a novel phosphatase functioning early in the cell cycle. EMBO J., 13, 1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson C., Katich S., Hagting A., Hoffmann I. & Pines J. (1999) Cdc25B and Cdc25C differ markedly in their properties as initiators of mitosis. J. Cell Biol., 146, 573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A. & Dunphy W.G. (1999) Binding of 14-3-3 proteins and nuclear export control the intracellular localization of the mitotic inducer Cdc25. Genes Dev., 13, 1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A., Guo Z., Emami K.H., Wang S.X. & Dunphy W.G. (1998a) The Xenopus Chk1 protein kinase mediates a caffeinesensitive pathway of checkpoint control in cell-free extracts. J. Cell Biol., 142, 1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A., Yakowec P.S. & Dunphy W.G. (1998b) 14-3-3 proteins act as negative regulators of the mitotic inducer Cdc25 in Xenopus egg extracts. Mol. Biol. Cell, 9, 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammer C., Wagerer S., Saffrich R., Mertens D., Ansorge W. & Hoffmann I. (1998) The cdc25B phosphatase is essential for the G2/M phase transition in human cells. J. Cell Sci., 111, 2445–2453. [DOI] [PubMed] [Google Scholar]
- Liu Q. et al. (2000) Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev., 14, 1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Lopez-Girona A., Furnari B., Mondesert O. & Russell P. (1999) Nuclear localization of Cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature, 397, 172–175. [DOI] [PubMed] [Google Scholar]
- Lopez-Girona A., Kanoh J. & Russell P. (2001) Nuclear exclusion of Cdc25 is not required for the DNA damage checkpoint in fission yeast. Curr. Biol., 11, 50–54. [DOI] [PubMed] [Google Scholar]
- Mailand N., Falck J., Lukas C., Syljuasen R.G., Welcker M., Bartek J. & Lukas J. (2000) Rapid destruction of human Cdc25A in response to DNA damage. Science, 288, 1425–1429. [DOI] [PubMed] [Google Scholar]
- Mailand N., Podtelejnikov A.V., Groth A., Mann M., Bartek J. & Lukas J. (2002) Regulation of G(2)/M events by Cdc25A through phosphorylation-dependent modulation of its stability. EMBO J., 21, 5911–5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S., Huang M. & Elledge S.J. (1998) Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science, 282, 1893–1897. [DOI] [PubMed] [Google Scholar]
- Millar J.B., Blevitt J., Gerace L., Sadhu K., Featherstone C. & Russell P. (1991) p55CDC25 is a nuclear protein required for the initiation of mitosis in human cells. Proc. Natl Acad. Sci. USA, 88, 10500–10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M., Mercurio C., Dominguez J., Goubin F. & Draetta G.F. (2000) Human Cdc25 A inactivation in response to S phase inhibition and its role in preventing premature mitosis. EMBO Rep., 1, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslin A.J., Tanner J.W., Allen P.M. & Shaw A.S. (1996) Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell, 84, 889–897. [DOI] [PubMed] [Google Scholar]
- Nigg E.A. (1998) Polo-like kinases: positive regulators of cell division from start to finish. Curr. Opin. Cell Biol., 10, 776–783. [DOI] [PubMed] [Google Scholar]
- Nishijima H., Nishitani H., Seki T. & Nishimoto T. (1997) A dualspecificity phosphatase Cdc25B is an unstable protein and triggers p34(cdc2)/cyclin B activation in hamster BHK21 cells arrested with hydroxyurea. J. Cell Biol., 138, 1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. (1997) Checkpoint pathways come of age. Cell, 91, 865–867. [DOI] [PubMed] [Google Scholar]
- Painter R.B. & Young B.R. (1980) Radiosensitivity in ataxia-telangiectasia: a new explanation. Proc. Natl Acad. Sci. USA, 77, 7315–7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C.Y., Graves P.R., Thoma R.S., Wu Z., Shaw A.S. & Piwnica-Worms H. (1997) Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science, 277, 1501–1505. [DOI] [PubMed] [Google Scholar]
- Peng C.Y., Graves P.R., Ogg S., Thoma R.S., Byrnes M.J., Wu Z., Stephenson M.T. & Piwnica-Worms H. (1998) C-TAK1 protein kinase phosphorylates human Cdc25C on serine 216 and promotes 14-3-3 protein binding. Cell Growth Differ., 9, 197–208. [PubMed] [Google Scholar]
- Peters J.M. (2002) The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell, 9, 931–943. [DOI] [PubMed] [Google Scholar]
- Pines J. (1999) Four-dimensional control of the cell cycle. Nature Cell Biol., 1, E73–E79. [DOI] [PubMed] [Google Scholar]
- Raleigh J.M. & O'Connell M.J. (2000) The G(2) DNA damage checkpoint targets both Wee1 and Cdc25. J. Cell Sci., 113, 1727–1736. [DOI] [PubMed] [Google Scholar]
- Russell P. & Nurse P. (1986) cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell, 45, 145–153. [DOI] [PubMed] [Google Scholar]
- Sanchez Y., Wong C., Thoma R.S., Richman R., Wu Z., Piwnica-Worms H. & Elledge S.J. (1997) Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science, 277, 1497–1501. [DOI] [PubMed] [Google Scholar]
- Sexl V., Diehl J.A., Sherr C.J., Ashmun R., Beach D. & Roussel M.F. (1999) A rate limiting function of cdc25A for S phase entry inversely correlates with tyrosine dephosphorylation of Cdk2. Oncogene, 18, 573–582. [DOI] [PubMed] [Google Scholar]
- Shiloh Y. (2001) ATM and ATR: networking cellular responses to DNA damage. Curr. Opin. Genet. Dev., 11, 71–77. [DOI] [PubMed] [Google Scholar]
- Shiloh Y. (2003) ATM and related protein kinases: safeguarding genome integrity. Nature Rev. Cancer, 3, 155–168. [DOI] [PubMed] [Google Scholar]
- Sørensen C., Syljuåsen R.G., Falck J., Schroeder T., Rönnstrand L., Khanna K.K., Zhou B.-B., Bartek J. & Lukas J. (2003) Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell, 3, 247–258. [DOI] [PubMed] [Google Scholar]
- Strohmaier H., Spruck C.H., Kaiser P., Won K.A., Sangfelt O. & Reed S.I. (2001) Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature, 413, 316–322. [DOI] [PubMed] [Google Scholar]
- Takemasa I. et al. (2000) Overexpression of CDC25B phosphatase as a novel marker of poor prognosis of human colorectal carcinoma. Cancer Res., 60, 3043–3050. [PubMed] [Google Scholar]
- Takizawa C.G. & Morgan D.O. (2000) Control of mitosis by changes in the subcellular location of cyclin-B1- Cdk1 and Cdc25C. Curr. Opin. Cell Biol., 12, 658–665. [DOI] [PubMed] [Google Scholar]
- Toyoshima-Morimoto F., Taniguchi E. & Nishida E. (2002) Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep., 3, 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigo E., Muller H., Prosperini E., Hateboer G., Cartwright P., Moroni M.C. & Helin K. (1999) CDC25A phosphatase is a target of E2F and is required for efficient E2F- induced S phase. Mol. Cell. Biol., 19, 6379–6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe M.B., Rittinger K., Volinia S., Caron P.R., Aitken A., Leffers H., Gamblin S.J., Smerdon S.J. & Cantley L.C. (1997) The structural basis for 14-3-3:phosphopeptide binding specificity. Cell, 91, 961–971. [DOI] [PubMed] [Google Scholar]
- Yang J., Winkler K., Yoshida M. & Kornbluth S. (1999) Maintenance of G2 arrest in the Xenopus oocyte: a role for 14-3-3-mediated inhibition of Cdc25 nuclear import. EMBO J., 18, 2174–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Slosberg E.D., Wang L., Hibshoosh H., Zhang Y.J., Xing W.Q., Santella R.M. & Weinstein I.B. (1999) Increased susceptibility to carcinogen-induced mammary tumors in MMTV- Cdc25B transgenic mice. Oncogene, 18, 5159–5166. [DOI] [PubMed] [Google Scholar]
- Zeng Y. & Piwnica-Worms H. (1999) DNA damage and replication checkpoints in fission yeast require nuclear exclusion of the Cdc25 phosphatase via 14-3-3 binding. Mol. Cell. Biol., 19, 7410–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Watkins J.L. & Piwnica-Worms H. (2002) Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc. Natl Acad. Sci. USA, 99, 14795–14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B.B. & Elledge S.J. (2000) The DNA damage response: putting checkpoints in perspective. Nature, 408, 433–439. [DOI] [PubMed] [Google Scholar]


