Abstract
The secreted protein sonic hedgehog (Shh) is crucial for the specification of neuronal subtype identity in the vertebrate neural tube. Zinc-finger proteins of the Gli family are known to be transcriptional mediators of Shh signalling, and to coordinately pattern the dorsal–ventral axis of the spinal cord. Recent studies indicate that additional signals may provide positional information in parallel to Shh to specify neuronal fate in this tissue. We review the role of Gli proteins in spinal-cord development and propose that various upstream patterning signals may be integrated by the Gli proteins to direct a coherent programme of neurogenesis.
Sonic hedgehog as a morphogen
The role of sonic hedgehog (Shh) in directing neuronal diversity in the vertebrate neural tube is well established (for reviews, see Jessell, 2000; Ingham & McMahon, 2001; and see Fig. 1). A long-range gradient of Shh signalling directs the emergence of multiple neuronal subtypes at precise positions along the dorsal–ventral axis of the neural tube. Each neuronal subtype is generated from a spatially discrete progenitor domain, and these distinct domains are defined by the expression of a characteristic combination of genes that encode homeodomain and basic helix–loop–helix (bHLH) transcription factors. These unique combinatorial expression patterns are determined by the negative or positive transcriptional regulation of each gene by the precise concentration of Shh to which the cells within the domain are exposed.
Figure 1.
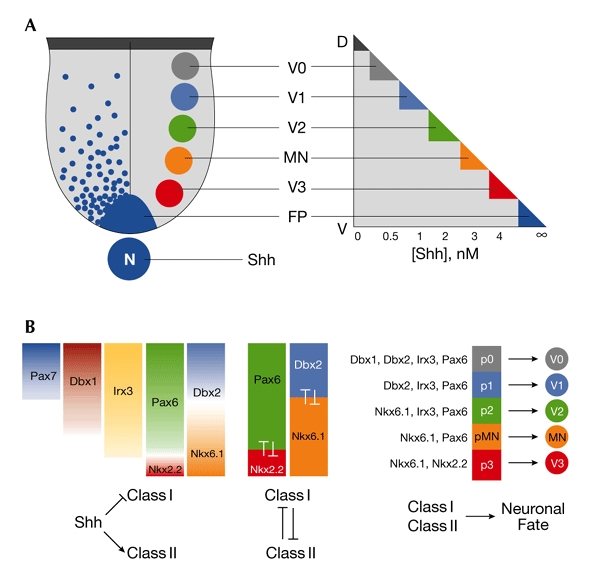
Ventral neural progenitor domains of the spinal cord are defined by sonic-hedgehog-regulated combinatorial expression of transcription factors. (A) Five distinct ventral neuronal subtypes arise from an equivalent number of progenitor domains in the ventricular zone of the ventral spinal cord. Progressively more dorsal progenitor domains are exposed to a decreasing concentration of sonic hedgehog (Shh) protein. (B) The concentration gradient of Shh regulates the ventral expression domains of a series of transcription factors in ventral progenitor (p) cells. Three aspects are crucial to this system: Shh either represses (class I genes) or induces (class II genes) expression at different concentration thresholds (left). Progenitor gene-expression domains are refined and maintained by negative cross-regulatory interactions between those proteins that share a boundary (centre). The combinatorial expression of homeodomain proteins in distinct progenitor domains determines the neuronal subtype that arises from each domain (right). D, dorsal; Dbx, developing brain homeobox transcription factor; FP, floor plate; Irx, iroquois homeodomain protein; MN, motor neuron; N, notochord; Nkx, Nkx homeodomain protein; Pax, Paired homeodomain protein; V, ventral; V0–V3, ventral interneurons 0–3.
Understanding graded sonic hedgehog signalling
The pivotal role of graded Shh signalling focuses attention on how differences in the extracellular concentration of Shh are interpreted by cells such that they can direct differential transcriptional responses. Details of this signalling pathway initially emerged from work on the homologous Hedgehog (Hh) pathway in Drosophila (Ingham & McMahon, 2001), and have recently been elaborated on by studies of Shh signal transduction in vertebrates. Here, we focus on the role of the Cubitus interruptus (Ci) transcription factor in Drosophila, and its vertebrate homologues Gli1, Gli2 and Gli3.
Cubitus interruptus and Hedgehog signalling. In Drosophila, the zinc-finger-containing transcription factor Ci is the key regulator of Hh target genes (Methot & Basler, 2001). Ci functions as both a repressor and an activator of transcription. In the absence of Hh signalling, Ci is proteolytically processed into a repressor form that comprises the amino-terminal region and the zinc-finger DNA-binding domain but lacks the carboxy-terminal portion of the protein (Aza-Blanc et al., 1997; Robbins et al., 1997). This cleavage is inhibited in cells that respond to Hh, resulting in the generation of a full-length protein that functions as a transcriptional activator (Ohlmeyer & Kalderon, 1998; Methot & Basler, 1999; Jia et al., 2002; Price & Kalderon, 2002). Although the inhibition of cleavage is an essential step in the generation of a fully active Ci protein, Hh signalling also potentiates the transcriptional activity of Ci, possibly through a mechanism that increases its nuclear accumulation (Ohlmeyer & Kalderon, 1998; Chen et al., 1999; Wang & Holmgren, 2000).
The cleavage of Ci seems to take place in a large, multiprotein complex that is associated with cytoplasmic microtubules and is dependent on the proteasome (for a review, see Jiang, 2002). A prerequisite for Ci proteolysis is the integrity of a cluster of phosphorylatable serines C-terminal to the zinc-finger domain (Jia et al., 2002; Price & Kalderon, 2002). Corresponding sites are found in Gli3 and are important for Gli3 processing, indicating that this mechanism is likely to have been conserved in vertebrates (Wang et al., 2000).
Gli proteins. In vertebrates, all three Ci homologues, Gli1, Gli2 and Gli3, are expressed in the neural tube (Hui et al., 1994; Lee et al., 1997; Ruiz i Altaba, 1998). These bind to Ci-related consensus sequences that are present in several Shh-responsive genes (Sasaki et al., 1997, 1999; Yoon et al., 2002). The functional conservation of the role of vertebrate Gli proteins in transducing Shh signals was initially suggested by the ability of Gli1 to induce ventral cell types in the neural tube, mimicking the effect of Shh signalling (Hynes et al., 1997; Ruiz i Altaba, 1998). Understanding the functions of the individual Gli proteins has, however, been complicated by the existence of three closely related family members and the potential for post-translational regulation of these proteins (Aza-Blanc et al., 2000; Wang et al., 2000). To define the role of Gli proteins in spinal-cord development, three issues need to be addressed. First, is Gli-mediated transcription sufficient to account for the patterning of the ventral neural tube? Second, what contributions do individual Gli proteins make to the patterning activity of Shh, and are these unique or redundant activities? Third, what role does post-translational regulation have? Although complete answers to these questions are not yet available, biochemical studies in heterologous systems and cell lines have begun to examine the regulation of Gli activity, and in vivo gain-of-function and loss-of-function experiments have shed light on the roles of Gli proteins in the neural tube.
In mice, Gli1 is expressed in the ventral neural tube, and its expression is dependent on Shh signalling (Bai et al., 2002). Most evidence suggests that, in contrast with Ci, Gli1 is not proteolytically processed and functions only as a transcriptional activator (Lee et al., 1997; Ruiz i Altaba, 1998). These data have led to the suggestion that Gli1 is unlikely to mediate the immediate-early response of cells to Shh. Consistent with this, Gli1 mutant mice lack any developmental defects (Park et al., 2000; Bai & Joyner, 2001) and Gli2 rather than Gli1 seems to mediate the initial response of cells to Shh (Bai et al., 2002). A recent study, however, has shown that N-terminal regions of Gli1 recruit components of histone deacetylase complexes (Cheng & Bishop, 2002), raising the possibility that in some circumstances Gli1 may inhibit transcription. By contrast, zebrafish that lack gli1 have significant defects in the activation of Hh target genes in the neural tube (Karlstrom et al., 2003). This suggests that the roles of Gli proteins have diverged during vertebrate evolution, with Gli1 having the main role in activating Shh-responsive genes in the spinal cord of zebrafish, and this role having been taken on by other Gli family members in mice.
Both Gli2 and Gli3 are expressed in neural tissue before neural-tube closure (Hui et al., 1994; Lee et al., 1997) and, as development proceeds, the expression pattern of Gli3 becomes confined to intermediate and dorsal spinal-cord regions, whereas that of Gli2 remains uniform. C-terminal transcriptional activator and N-terminal repressor regions have been identified in both Gli2 and Gli3 (Sasaki et al., 1999; Ruiz i Altaba, 1999; Dai et al., 1999). For Gli3, there is evidence that Shh-controlled processing regulates transcriptional activity in a manner equivalent to the regulation of Ci. In fly imaginal discs and cell lines that express Gli3, Hh inhibits its proteolytic processing to an N-terminal repressor form (Dai et al., 1999; Aza-Blanc et al., 2000). Moreover, although the cleavage of Gli3 has not been reported in the neural tube, in mouse and chick limb buds Shh signalling seems to establish a gradient of Gli3 processing, resulting in the ratio of full-length to cleaved Gli3 diminishing at increasing distance from the source of Shh (Wang et al., 2000; Litingtung et al., 2002). Whether processing also controls the activity of Gli2 remains unclear. Cleavage of Gli2 is not seen in vertebrate cell lines (Wang et al., 2000); however, ectopic expression of Gli2 in fly imaginal discs yields a cleaved, albeit Hh-independent, product (Aza-Blanc et al., 2000).
Mice that lack Gli2 function show defects in several tissues, including the lungs and skeleton (Ding et al., 1998; Matise et al., 1998). In the spinal cord, the development of the floor plate and adjacent V3 ventral interneurons is severely affected, and there is a concomitant ventral expansion of motor neurons (MNs; Fig. 2). Other ventral neurons are, however, generated at their appropriate dorsoventral positions. These data indicate that Gli2 functions downstream of Shh and contributes to the induction of the most ventral region of the neural tube. By contrast, zebrafish that lack gli2 have only minor defects in Shh signalling, emphasizing the functional divergence of Gli proteins during evolution (Karlstrom et al., 2003).
Figure 2.
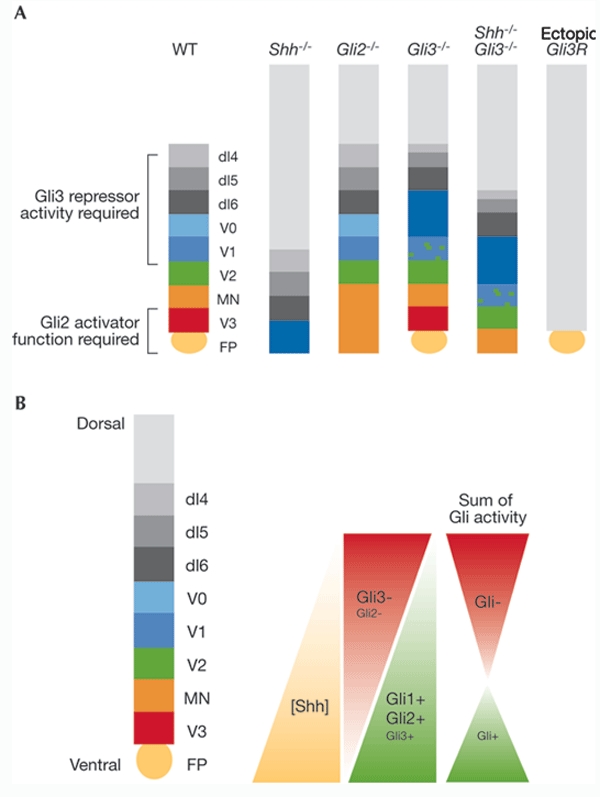
Dorsoventral spinal-cord patterning defects in Gli mutant embryos can be explained by a gradient model of Gli activator and repressor activity. (A) Effects of sonic hedgehog (Shh) and Gli loss-of-function mutations on patterning of the ventral and intermediate regions of the spinal cord. In Gli2−/− embryos, the most ventral cell types—the floorplate (FP) and ventral interneuron 3 (V3) neurons—are absent and there is a concomitant expansion of the motor neuron (MN) domain. By contrast, in Gli3−/− mutants, patterning of the intermediate region of the neural tube is disrupted (see text for details). In comparison with Shh-null mutants, embryos that lack both Shh and Gli3 function have substantially restored dorsoventral patterning. Inhibition of all Gli activity by the misexpression of the dominant-negative protein Gli3R completely suppresses the formation of all ventral and intermediate neuronal subtypes. (B) According to the model, Shh-induced, graded Gli activator and repressor transcriptional activities assign distinct cellular identities along the ventral neural tube. The ventral-to-dorsal gradient of Shh activity induces a parallel gradient of Gli activator function, that is, a gradient that is maximal in the ventral midline and declines in dorsal regions of the neural tube. The gradient of Gli repressor activity is orientated in the opposite direction to the Shh gradient because of the antagonistic effect of Shh signalling on Gli repressor activity. The model proposes partial redundancy of the transcriptional activity of each Gli protein by the other so that, for example, in the absence of Gli2, the transcriptional activation function of Gli3 partially compensates for this. The combined transcriptional activity of the Gli proteins results in a gradient of transcriptional activation (+) in ventral regions of the neural tube and repression (−) in intermediate and dorsal regions. dI, dorsal interneuron; WT, wild type.
Gene-targeting experiments in mice indicate that the neural-tube defects seen in Gli2 mutant embryos can be rescued by replacing Gli2 with Gli1. These data are consistent with the view that the inductive effects of Gli2 are mediated through transcriptional activation (Bai & Joyner, 2001). Moreover, these results support the idea that although the functions carried out by individual Gli proteins differ between species, within a species significant functional equivalency between Gli family members has been retained.
Gli3 has been proposed to function primarily as an inhibitor of Shh signalling. In Xenopus, Gli3 antagonizes floor-plate induction by Gli1 and blocks Gli2-induced MN differentiation (Ruiz i Altaba, 1998, 1999). In addition, the loss of MNs and certain classes of ventral interneurons in Shh mutant mice can, to a large extent, be alleviated by abrogating Gli3 function in Shh/Gli3 double-mutant mice (Litingtung & Chiang, 2000). Moreover, in Gli3 mutant embryos, although patterning is normal in ventral regions, progenitor transcription factors in the intermediate neural tube expand dorsally, and there is a concomitant switch in the identity of the neurons generated in this region; overall, cells respond as though they were exposed to a higher concentration of Shh (Persson et al., 2002; Fig. 2). These neural defects are rescued by a truncated allele of Gli3 that encodes only the N-terminus of the protein—and is therefore equivalent to proteolytically processed Gli3—suggesting that only the repressor activity of Gli3 is required in the spinal cord (Persson et al., 2002). In other tissues, however, unregulated Gli3 repressor activity alone is not sufficient to substitute for the lack of full-length Gli3, possibly reflecting a differential requirement for activator and repressor functions in different tissues (Böse et al., 2002).
The inhibitory effect of Gli3 on Shh signalling leaves open the question of whether Gli3 has any role as a transcriptional activator in the neural tube. Blocking all Gli transcriptional activation using a dominant inhibitory Gli protein (Gli3R; Fig. 2A) results in a ventral-to-dorsal shift in progenitor-cell identity and a concomitant failure to generate MNs and ventral interneurons (Persson et al., 2002). These data, together with the observation that only the most ventral regions of the neural tube are disrupted in embryos lacking Gli2 (Ding et al., 1998; Matise et al., 1998), lead us to favour a context-dependent, bipotential function of Gli3, with Gli3 repressor activity dominating in the absence of Shh activity, whereas activator function is revealed in response to Shh signalling.
A model of Gli-induced patterning of the ventral spinal cord
If the positional information encoded by the Shh gradient is transduced only by Gli proteins, is it possible to reconcile the relatively minor defects that result from loss-of-function mutations of individual Gli genes with the dramatic patterning defects in embryos that lack Shh signalling? For instance, MN and V2 neuron differentiation is preserved in each of the Gli mutant embryos.
The finding that ventral cell types fail to differentiate when Gli function is globally blocked argues for a central role for Gli-mediated transcription in normal dorsoventral spinal-cord patterning. An attractive model, in which the role of the Gli proteins is comparable to that proposed for Ci during Drosophila development (Methot & Basler, 2001), is that Shh establishes a gradient of Gli activity in the neural tube by inhibiting Gli repressor activity and potentiating Gli activator function (Fig. 2; Ruiz i Altaba, 1997). In this model, the Gli2 and Gli3 proteins can be viewed as being functionally equivalent, and the distinct neural-tube defects in Gli2 and Gli3 mutants may reflect differences in the expression patterns of these genes rather than intrinsic differences in their activity. Thus, loss of the floor plate and V3 neurons in Gli2 mutants could be a consequence of the lack of Gli3 expression in the most ventral regions of the neural tube. To test this, Gli3 could be transgenically expressed from the Gli2 locus in mice lacking Gli2. Conversely, the defects seen in mice lacking Gli3 suggest that the transcriptional inhibitory activity of Gli2 alone is insufficient to generate the normal domains of gene expression seen in intermediate regions. This model predicts that it should be possible to recapitulate the graded activity of Shh by regulating the level of Gli transcriptional activity in neural cells.
Shh-independent signals in the ventral neural tube
Strikingly, several aspects of the severe ventral patterning defects seen in mice lacking Shh or its receptor, smoothened (Smo), can be rescued by the loss of Gli3. In mice lacking either Shh or Smo, MNs and most ventral neurons fail to develop, but the combined loss of Shh and Gli3 (Litingtung & Chiang, 2000; Persson et al., 2002) or Smo and Gli3 (Wijgerde et al., 2002) partially restores the patterning of ventral progenitor domains and the differentiation of MNs and some ventral neurons. Apart from providing evidence for the antagonistic relationship between Shh signalling and Gli3, these data suggest that there may be some Shh-independent positional information in the neural tube that is sufficient to direct the generation of cell types.
What might the nature of such Shh-independent signals be? Retinoids derived from somitic and presomitic mesoderm are known to be important for the differentiation of two subpopulations of interneurons in the intermediate spinal cord (Pierani et al., 1999), and a broader role for these factors in the neural tube cannot be discounted. Other extrinsic factors that influence patterning in this tissue include the Wingless/Int-related (Wnt) and bone morphogenetic protein (Bmp) signals that emanate from the dorsal neural tube (Muroyama et al., 2002; Lee & Jessell, 1999). Indeed, several lines of evidence suggest that Bmps may influence dorsoventral patterning in ventral as well as dorsal regions of the neural tube. In the presence of Bmps, neural progenitor cells that are exposed to a fixed concentration of Shh in vitro adopt a more dorsal identity and post-mitotic fate (Liem et al., 2000). Moreover, notochord-derived Bmp antagonists regulate the availability of Bmps in the ventral neural tube, and these antagonists modulate dorsoventral patterning of the neural tube in vitro and in vivo (McMahon et al., 1998; Liem et al., 2000; Patten & Placzek, 2002). Finally, the phenotypes of Bmp-mutant zebrafish have shown that a reduction in Bmp signalling leads to the expansion of ventral neural fates (Barth et al., 1999). Thus, Bmps and Shh have opposing activities in the specification of ventral neural identity, and Bmp signalling may be sufficient to provide positional information throughout the neural tube in embryos lacking Gli3 and Shh.
Several observations suggest that Gli proteins themselves are mediators of more than just the Shh signalling pathway. Mullor and colleagues (2001) proposed that Gli proteins are involved in Wnt signalling, and glycogen synthase kinase 3 (Gsk3), a component of the Wnt signalling pathway, is thought to influence Ci activity in Drosophila (Jia et al., 2002; Price & Kalderon, 2002). In addition, there is evidence to suggest that the Bmp signal-transduction pathway may converge on Gli proteins. Smad (similar to mothers against decapentaplegic) proteins, the transcriptional effectors of Bmp signalling, physically associate with processed Gli proteins (Liu et al., 1998), suggesting that Bmp-regulated post-translational control of Gli activity may operate in parallel with the Shh pathway to ensure that antagonistic Shh and Bmp signals are precisely coordinated during the development of the spinal cord. Thus, the modulation of Gli activity within progenitor cells by diverse extracellular patterning signals may provide a robust mechanism for the acquisition of distinct positional values along the dorsal–ventral axis of the spinal cord.
Conclusion
Recent studies have begun to clarify the mechanisms underlying spinal-cord patterning, and suggest a model to account for the generation of neuronal subtypes in the ventral spinal cord. The data also raise new questions: what signals in addition to Shh confer positional information on the neural tube? How do cells integrate the many patterning signals to which they are exposed in a manner that leads to the selection of distinct identities? And what roles do Gli proteins have in these processes? Answers to these questions are likely to emerge in the coming years.
Acknowledgments
We thank J. Ericson, D. Stamataki, F. Ulloa and D. Wilkinson for insightful discussions and comments on this manuscript. J.B. is the recipient of an EMBO Young Investigator Award and is supported by the Medical Research Council (UK).
References
- Aza-Blanc P., Ramirez-Weber F.A., Laget M.P., Schwartz C. & Kornberg T.B. ( 1997) Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell, 89, 1043–1053. [DOI] [PubMed] [Google Scholar]
- Aza-Blanc P., Lin H.Y., Ruiz i Altaba A. & Kornberg T.B. ( 2000) Expression of the vertebrate Gli proteins in Drosophila reveals a distribution of activator and repressor activities. Development, 127, 4293–4301. [DOI] [PubMed] [Google Scholar]
- Bai C.B. & Joyner A.L. ( 2001) Gli1 can rescue the in vivo function of Gli2. Development, 128, 5161–5172. [DOI] [PubMed] [Google Scholar]
- Bai C.B., Auerbach W., Lee J.S., Stephen D. & Joyner A.L. ( 2002) Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development, 129, 4753–4761. [DOI] [PubMed] [Google Scholar]
- Barth K.A., Kishimoto Y., Rohr K.B., Seydler C., Schulte-Merker S. & Wilson S.W. ( 1999) Bmp activity establishes a gradient of positional information throughout the entire neural plate. Development, 126, 4977–4987. [DOI] [PubMed] [Google Scholar]
- Böse J., Grotewold L. & Rüther U. ( 2002) Pallister–Hall syndrome phenotype in mice mutant for Gli3. Hum. Mol. Genet., 11, 1129–1135. [DOI] [PubMed] [Google Scholar]
- Chen C.H., von Kessler D.P., Park W., Wang B., Ma Y. & Beachy P.A. ( 1999) Nuclear trafficking of Cubitus interruptus in the transcriptional regulation of Hedgehog target gene expression. Cell, 98, 305–316. [DOI] [PubMed] [Google Scholar]
- Cheng S.Y. & Bishop J.M. ( 2002) Suppressor of Fused represses Gli-mediated transcription by recruiting the SAP18–mSin3 corepressor complex. Proc. Natl Acad. Sci. USA, 99, 5442–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai P., Akimaru H., Tanaka Y., Maekawa T., Nakafuku M. & Ishii S. ( 1999) Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J. Biol. Chem., 274, 8143–8152. [DOI] [PubMed] [Google Scholar]
- Ding Q., Motoyama J., Gasca S., Mo R., Sasaki H., Rossant J. & Hui C.C. ( 1998) Diminished Sonic hedgehog signaling and lack of floor plate differentiation in Gli2 mutant mice. Development, 125, 2533–2543. [DOI] [PubMed] [Google Scholar]
- Hui C.C., Slusarski D., Platt K.A., Holmgren R. & Joyner A.L. ( 1994) Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev. Biol., 162, 402–413. [DOI] [PubMed] [Google Scholar]
- Hynes M., Stone D.M., Dowd M., Pitts-Meek S., Goddard A., Gurney A. & Rosenthal A. ( 1997) Control of cell pattern in the neural tube by the zinc finger transcription factor and oncogene Gli-1. Neuron, 19, 15–26. [DOI] [PubMed] [Google Scholar]
- Ingham P.W. & McMahon A.P. ( 2001) Hedgehog signaling in animal development: paradigms and principles. Genes Dev., 15, 3059–3087. [DOI] [PubMed] [Google Scholar]
- Jessell T.M. ( 2000) Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nature Rev. Genet., 1, 20–29. [DOI] [PubMed] [Google Scholar]
- Jia J., Amanai K., Wang G., Tang J., Wang B. & Jiang J. ( 2002) Shaggy/GSK3 antagonizes Hedgehog signaling by regulating Cubitus interruptus. Nature, 416, 548–552. [DOI] [PubMed] [Google Scholar]
- Jiang J. ( 2002) Degrading Ci: who is Cul-pable? Genes Dev., 16, 2315–2321. [DOI] [PubMed] [Google Scholar]
- Karlstrom R.O., Tyurina O.V., Kawakami A., Nishioka N., Talbot W.S., Sasaki H. & Schier A.F. ( 2003) Genetic analysis of zebrafish gli1 and gli2 reveals divergent requirements for gli genes in vertebrate development. Development, 130, 1549–1564. [DOI] [PubMed] [Google Scholar]
- Lee J., Platt K.A., Censullo P. & Ruiz i Altaba A. ( 1997) Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development, 124, 2537–2552. [DOI] [PubMed] [Google Scholar]
- Lee K.J. & Jessell T.M. ( 1999) The specification of dorsal cell fates in the vertebrate central nervous system. Annu. Rev. Neurosci., 22, 261–294. [DOI] [PubMed] [Google Scholar]
- Liem K.F., Jessell T.M. & Briscoe J. ( 2000) Regulation of the neural patterning activity of sonic hedgehog by secreted BMP inhibitors expressed by notochord and somites. Development, 127, 4855–4866. [DOI] [PubMed] [Google Scholar]
- Litingtung Y. & Chiang C. ( 2000) Specification of ventral neuron types is mediated by an antagonistic interaction between Shh and Gli3. Nature Neurosci., 3, 979–985. [DOI] [PubMed] [Google Scholar]
- Litingtung Y., Dahn R.D., Li Y., Fallon J.F. & Chiang C. ( 2002) Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature, 418, 979–983. [DOI] [PubMed] [Google Scholar]
- Liu F., Massague J. & Ruiz i Altaba A. ( 1998) Carboxy-terminally truncated Gli3 proteins associate with Smads. Nature Genet., 20, 325–326. [DOI] [PubMed] [Google Scholar]
- Matise M.P., Epstein D.J., Park H.L., Platt K.A. & Joyner A.L. ( 1998) Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development, 125, 2759–2770. [DOI] [PubMed] [Google Scholar]
- McMahon J.A., Takada S., Zimmerman L.B., Fan C.M., Harland R.M. & McMahon A.P. ( 1998) Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev., 12, 1438–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methot N. & Basler K. ( 1999) Hedgehog controls limb development by regulating the activities of distinct transcriptional activator and repressor forms of Cubitus interruptus. Cell, 96, 819–831. [DOI] [PubMed] [Google Scholar]
- Methot N. & Basler K. ( 2001) An absolute requirement for Cubitus interruptus in Hedgehog signaling. Development, 128, 733–742. [DOI] [PubMed] [Google Scholar]
- Mullor J.L., Dahmane N., Sun T. & Ruiz i Altaba A. ( 2001) Wnt signals are targets and mediators of Gli function. Curr. Biol., 11, 769–773. [DOI] [PubMed] [Google Scholar]
- Muroyama Y., Fujihara M., Ikeya M., Kondoh H. & Takada S. ( 2002) Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev., 16, 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlmeyer J.T. & Kalderon D. ( 1998) Hedgehog stimulates maturation of Cubitus interruptus into a labile transcriptional activator. Nature, 396, 749–753. [DOI] [PubMed] [Google Scholar]
- Park H.L., Bai C., Platt K.A., Matise M.P., Beeghly A., Hui C.C., Nakashima M. & Joyner A.L. ( 2000) Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development, 127, 1593–1605. [DOI] [PubMed] [Google Scholar]
- Patten I. & Placzek M. ( 2002) Opponent activities of Shh and BMP signaling during floor plate induction in vivo. Curr. Biol., 12, 47–52. [DOI] [PubMed] [Google Scholar]
- Persson M., Stamataki D., te Welscher P., Andersson E., Bose J., Ruther U., Ericson J. & Briscoe J. ( 2002) Dorsal–ventral patterning of the spinal cord requires Gli3 transcriptional repressor activity. Genes Dev., 16, 2865–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierani A., Brenner-Morton S., Chiang C. & Jessell T.M. ( 1999) A sonic hedgehog-independent, retinoid-activated pathway of neurogenesis in the ventral spinal cord. Cell, 97, 903–915. [DOI] [PubMed] [Google Scholar]
- Price M.A. & Kalderon D. ( 2002) Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by Glycogen Synthase Kinase 3 and Casein Kinase 1. Cell, 108, 823–835. [DOI] [PubMed] [Google Scholar]
- Robbins D.J., Nybakken K.E., Kobayashi R., Sisson J.C., Bishop J.M. & Therond P.P. ( 1997) Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell, 90, 225–234. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A. ( 1997) Catching a Gli-mpse of Hedgehog. Cell, 90, 193–196. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A. ( 1998) Combinatorial Gli gene function in floor plate and neuronal inductions by Sonic Hedgehog. Development, 125, 2203–2212. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A. ( 1999) Gli proteins encode context-dependent positive and negative functions: implications for development and disease. Development, 126, 3205–3216. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Hui C., Nakafuku M. & Kondoh H. ( 1997) A binding site for Gli proteins is essential for HNF-3β floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development, 124, 1313–1322. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Nishizaki Y., Hui C., Nakafuku M. & Kondoh H. ( 1999) Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development, 126, 3915–3924. [DOI] [PubMed] [Google Scholar]
- Wang B., Fallon J.F. & Beachy P.A. ( 2000) Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell, 100, 423–434. [DOI] [PubMed] [Google Scholar]
- Wang Q.T. & Holmgren R.A. ( 2000) Nuclear import of cubitus interruptus is regulated by hedgehog via a mechanism distinct from Ci stabilization and Ci activation. Development, 127, 3131–3139. [DOI] [PubMed] [Google Scholar]
- Wijgerde M., McMahon J.A., Rule M. & McMahon A.P. ( 2002) A direct requirement for Hedgehog signaling for normal specification of all ventral progenitor domains in the presumptive mammalian spinal cord. Genes Dev., 16, 2849–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J.W., Kita Y., Frank D.J., Majewski R.R., Konicek B.A., Nobrega M.A., Jacob H., Walterhouse D. & Iannaccone P. ( 2002) Gene expression profiling leads to identification of GLI1-binding elements in target genes and a role for multiple downstream pathways in GLI1-induced cell transformation. J. Biol. Chem., 277, 5548–5555. [DOI] [PubMed] [Google Scholar]


