Abstract
Dorsoventral patterning in animal development is regulated by a morphogenetic gradient of Bone morphogenetic protein signalling, which is established by a set of proteins that are conserved from Drosophila to vertebrates. These include Chordin (Chd)/Short gastrulation, Xolloid/Tolloid and Twisted gastrulation. Here, we report the identification of a cell-surface component of this morphogenetic pathway. Prompted by the observation that Chd protein bound to the surface of certain cell lines with subnanomolar affinity, we identified two cell-surface proteins that bind to Chd, one of which corresponds to Integrin-α3. Integrin-α3 and Chd are co-expressed in the Xenopus embryo. Transfection of Integrin-α3 increased the binding of Chd to the cell surface, which was competed by an excess of soluble Integrin-α3. After binding to the cell surface, Chd was translocated into intracellular endocytic compartments in a temperature-dependent manner. We propose that Integrin-α3 may regulate the concentration of Chd protein in the extracellular space by endocytosis.
Introduction
Embryonic patterning arises through the generation of morphogen gradients during early development. Dorsoventral patterning of fly and vertebrate embryos has been well studied, and is one of the paradigms for understanding morphogen function (Freeman & Gurdon, 2002). The establishment of an extracellular gradient of Bone morphogenetic protein (BMP) determines cell-fate decisions along the dorsoventral axis. A gradient of this morphogen is generated in the extracellular space by the action of a set of evolutionarily conserved secreted proteins, including Chordin (Chd)/Short gastrulation (Sog), Xolloid (Xld)/Tolloid (Tld) and Twisted gastrulation (Tsg; De Robertis et al., 2000). The Drosophila Chd homologue, Sog, is a key component in the generation of embryonic BMP/Decapentaplegic (Dpp) gradients (Eldar et al., 2002). Sog can diffuse along the dorsoventral axis, and its diffusion and extracellular concentration levels are enhanced by a dynamin mutation that blocks endocytosis (Srinivasan et al., 2002). Chd and Sog encode secreted proteins of ∼120 kDa that contain four cysteine-rich repeats (CRs). The CR modules bind directly to BMP, preventing its binding to the BMP receptor (Piccolo et al., 1996; Larraín et al., 2000). The anti-BMP activity of Chd is controlled by two cofactors, Tld and Tsg. Tld is a zinc metalloproteinase that inactivates Chd by proteolytic cleavage at sites located just after CR1 and CR3 (Piccolo et al., 1997; Scott et al., 1999). Tsg is an evolutionarily conserved secreted protein that forms ternary complexes with BMP4 and Chordin that are unable to signal in the absence of Tld. Tsg binding also makes Chd a better substrate for cleavage by Tld, and competes the anti-BMP activity of Chd proteolytic fragments. Thus, Tsg promotes BMP activity in the presence of Tld (Oelgeschläger et al., 2000; Ross et al., 2001; Larraín et al., 2001).
This work was initiated in an attempt to identify other components of the Chd/BMP/Tsg/Tld regulatory pathway. It was found that Chd binds to the cell surface of various tissue-culture cell lines. To identify the molecular basis of this interaction, we used affinity-chromatography Chd columns and found that two cell membrane proteins bound to Chd. One of them was identified as the transmembrane receptor Integrin-α3. An excess of soluble purified Integrin-α3 prevented the binding of Chd to the cell surface. The cell-surface interaction between Chd and Integrin-α3 was followed by endocytosis at 37 °C. Chd and Integrin-α3 are coordinately expressed in the dorsal mesoderm of Xenopus embryos. We propose that the levels of Chd in the extracellular space may be regulated by endocytosis through Integrin-α3.
Results and Discussion
During screening for novel Chd-interacting proteins using a secretion-trap expression cloning method (Davis et al., 1996), we noticed that non-transfected COS-7 cells bound detectable amounts of a Chd–alkaline-phosphatase fusion protein (Chd–AP). When binding was quantified using a biochemical fluorescent AP assay, Chd–AP bound to COS-7 cells in amounts significantly higher than background levels (Fig. 1A). As negative controls, we used a secreted version of AP (s-AP) and a Tsg–AP fusion protein, neither of which bound to COS-7 cells in detectable amounts (Fig. 1A; and data not shown). We were able to saturate the binding of Chd–AP to COS-7 cells at 4 °C using increasing concentrations of Chd–AP protein, with an estimated dissociation constant (Kd) of 0.24 nM (Fig. 1B). This binding was specific, as it was competed by excess purified mouse Chd protein (Fig. 1C).
Figure 1.
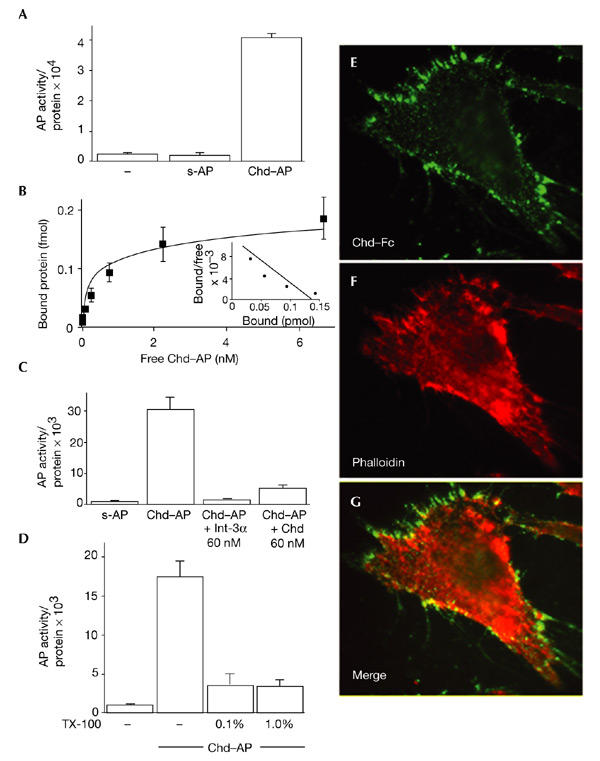
Chordin binds to the cell surface. (A) Chordin–alkaline-phosphatase (Chd–AP) binds to COS-7 cells. COS-7 cells were incubated with Chd–AP or secreted AP (s-AP) for 2 h at 4 °C, and the amount of activity of bound AP was quantified using a fluorescent AP substrate. Equal loading was confirmed by protein measurements. (B) Binding curve and Scatchard analysis of Chd–AP binding to COS-7 cells. A dissociation constant (Kd) of 0.24 nM was estimated. (C) COS-7 cells were incubated with Chd–AP in the presence of 60 nM recombinant Integrin-α3 (Int-α3; Chemicon) or mouse Chd (R&D Systems), and the amount of bound Chd–AP was quantified using a fluorescent AP substrate. Other cysteine-rich-repeat-containing proteins were not tested because they are not available in purified form. (D) Chd binding requires an intact cell membrane. COS-7 cells were incubated with 0.1% or 1.0% Triton X-100 (TX-100), and Chd–AP was bound and quantified. (E) Chd binding colocalizes with actin filaments. 10T1/2 fibroblasts were incubated with pure Chd–Fc at 4 °C, fixed and permeabilized, and binding was visualized with an anti-human IgG antibody conjugated to Alexa Fluor 486. (F) The same cell as in (E), stained with phalloidin-Alexa558 to visualize actin. (G) Merge of (E) and (F). Note the overlap between Chd–Fc and actin (yellow).
Chd–AP binding was lost from COS-7 cells that were pretreated with 0.1% or 1% Triton X-100 (Fig. 1D); these amounts of detergent are known to solubilize integral plasma membrane proteins, but not extracellular matrix (ECM) components (Mehta et al., 1985). This suggested that Chd binds to a cell membrane protein. To visualize binding to the cell surface, 10T1/2 fibroblasts were incubated at 4 °C with a protein consisting of Chd fused to the Fc fragment of human IgG (Chd–Fc), fixed, and stained with a fluorescent anti-human IgG. Chd–Fc binding was detected in membrane ruffles, filopodia and structures reminiscent of focal-adhesion plaques, particularly at the leading edge of the cell (Fig. 1E). Chd–Fc co-localized with actin filaments stained by fluorescent phalloidin, indicating that some Chd binding sites overlap with focal-adhesion plaques (Fig. 1F,G, note yellow staining where the Chd and actin signals overlap). These results show that Chd can bind with high affinity (Kd in the subnanomolar range) to the cell surface in cultured cell lines.
To identify the cell-surface proteins that bind to Chd, we prepared a Chd affinity-chromatography column. Chd–Fc and secreted Fc (s-Fc) fusion proteins were produced in S2 cells, purified with protein A, and covalently crosslinked to protein-A–agarose beads using dimethylpimelimidate (DMP). Cell-surface proteins from COS-7 cells were biotinylated (using EZ-link-Biotin; Pierce), solubilized with 0.1% Triton X-100 (as in Fig. 1D), and loaded on Chd–Fc or s-Fc affinity columns. Bound proteins were eluted with SDS and visualized by performing a streptavidin–horseradish-peroxidase (SA–HRP) immunoblot. As shown in Fig. 2A, lane 3, the Chd–Fc column bound two proteins that migrated in non-reducing SDS gels with apparent molecular weights of 170 and 200 kDa. Neither protein bound to the s-Fc control column (Fig. 2A, lane 2). The molecular weight of these proteins and the localization of the Chd–Fc staining on focal-adhesion plaques (Fig. 1E–G) suggested integrins as candidate cell-surface receptors for Chd.
Figure 2.
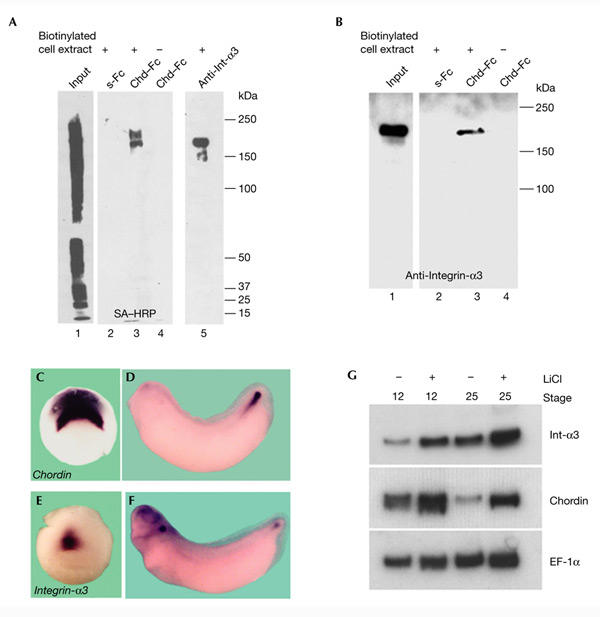
Chordin affinity columns bind biotinylated cell-surface Integrin-α3. (A) A Chordin (Chd)–Fc affinity matrix binds two distinct cell-surface proteins in COS-7 cells. Cell extracts containing biotinylated surface proteins from COS-7 cells were bound to secreted Fc (s-Fc; lane 2) or Chd–Fc (lane 3) columns, or were immunoprecipitated using an anti-Integrin-α3 (Int-α3) antibody (lane 5). Proteins bound to the columns were analysed by immunoblotting with streptavidin–horseradish-peroxidase (SA–HRP; Pierce). Lane 1 shows loading of 1% of the total biotinylated cell lysate. (B) A Chordin affinity matrix binds Integrin-α3. COS-7 cell extracts were bound to s-Fc (lane 2) or Chd–Fc affinity columns (lane 3), eluted, and analysed by immunoblotting with anti-Integrin-α3. (C–G) Chordin and Integrin-α3 are co-expressed during embryonic development. In situ hybridization analysis of chordin (C,D) and integrin-a3 (E,F) expression at stages 10.5 and 32. (G) RT–PCR (PCR after reverse transcription) analysis of chordin and integrin-a3 messenger RNA levels in control and LiCl-treated embryos. Elongation factor-1α (EF-1α) was used as a loading control.
Integrins constitute a large transmembrane receptor family of at least 24 distinct heterodimers, and were originally described as one of the main components of focal-adhesion plaques in fibroblasts (Hynes, 2002). Among them, Integrin-α3 stood out as an attractive candidate because it is co-expressed with Chd in the dorsal blastopore lip and its derivatives (the notochord and chordoneural hinge) and is coordinately upregulated with Chd in embryos that are dorsalized by lithium chloride treatment (Fig. 2C–G; Sasai et al., 1994; Whittaker & DeSimone, 1991; Meng et al., 1997). As Chd and Integrin-α3 are coordinately regulated in the dorsal mesoderm, it seemed possible that they might function in the same biochemical pathway. Furthermore, Integrin-α3 migrates with an apparent molecular weight of 170 kDa in non-reducing SDS gels (Shang et al., 2001). For these reasons, we tested whether Integrin-α3 is one of the Chd cell-surface receptors that is expressed in COS-7 cells. An anti-Integrin-α3 antibody immunoprecipitated a biotinylated protein with a molecular weight similar to the smaller band bound by the Chd–Fc column (Fig. 2A, compare lanes 3 and 5). Furthermore, the 170-kDa protein purified by the Chd–Fc affinity matrix was specifically recognized by the anti-Integrin-α3 antibody in immunoblots (Fig. 2B, lane 3). Thus, one of the cellsurface proteins that interact with Chd corresponds to Integrin-α3. The nature of the 200-kDa protein remains unknown; one possibility is that it corresponds to Integrin-β1, the heterodimerization partner of Integrin-α3, but this seems unlikely as the expected molecular weight of Integrin-β1 in non-reducing gels is 110 kDa (Shang et al., 2001).
We next tested whether the binding of Chd to COS-7 cells could be mediated by Integrin-α3. First, an excess of recombinant purified Integrin-α3 blocked the binding of Chd–AP to COS-7 cells (Fig. 1C). Second, we found that the amount of Chd–AP bound to the different cell lines correlated with the endogenous levels of Integrin-α3 expression (Fig. 3A). P19 cells, which express low levels of Integrin-α3 bound approximately six times less Chd–AP than did COS-7 cells, which express higher levels of Integrin-α3 protein (Fig. 3A, inset). 293T cells, which do not express detectable levels of Integrin-α3, did not bind Chd–AP (Fig. 3B). Third, to test whether Integrin-α3 was sufficient for binding of Chd to the cell surface, 293T cells were transfected with a full-length human integrin-α3 complementary DNA and stained with Chd–AP. We found that 293T cells transfected with integrin-α3 bound Chd–AP to the cell surface (Fig. 3C). We conclude from these experiments that Integrin-α3 mediates the binding of Chd to the cell surface.
Figure 3.
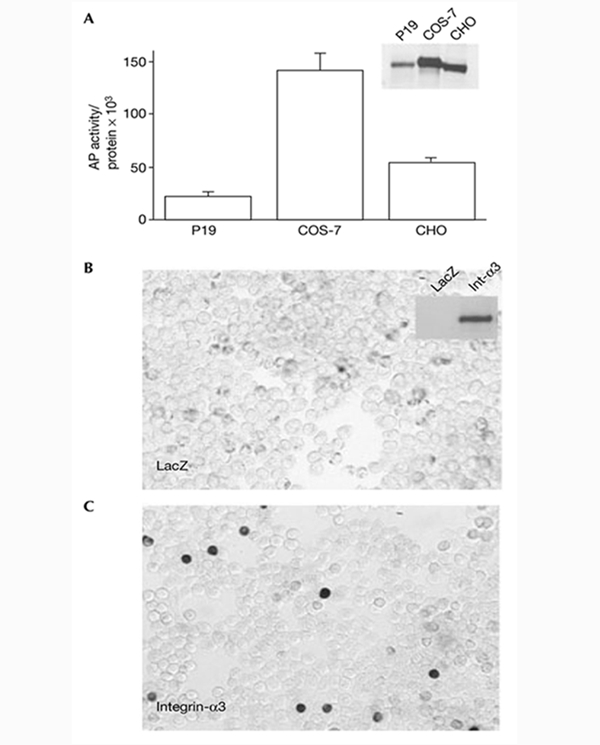
Chordin binds to the cell surface through Integrin-α3. (A) Binding of Chordin–alkaline-phosphatase (Chd–AP) to P19, COS-7 and CHO cell lines. The inset shows an anti-Integrin-α3 immunoblot of cell extracts from the three cell lines. The amount of Chd–AP binding correlates with endogenous levels of Integrin-α3. (B,C) Chd–AP binds to 293T cells transfected with integrin-a3. 293T cells were transfected with LacZ or human integrin-a3 full-length complementary DNAs, incubated with recombinant Chd–AP and stained for AP activity. The inset shows an anti-Integrin-α3 (Int-α3) immunoblot of the corresponding cell extracts.
Recent studies have highlighted the importance of receptor-mediated endocytosis in the formation of morphogen gradients in the embryo (Lander et al., 2002; Seto et al., 2002; Vincent & Dubois, 2002). For example, BMP/Dpp diffusion can be regulated by endocytosis after binding to its signalling receptor in imaginal discs (Entchev et al., 2000), and the secreted protein Dickkopf regulates Wingless/Int-related (Wnt) signalling by inducing the rapid removal of the Wnt co-receptor lipoprotein-receptor-related protein 6 (LRP6) by endocytosis, together with the transmembrane protein Kremen (Mao et al., 2002). Integrins are transmembrane glycoproteins that link the ECM to intracellular cytoskeletal and signalling networks, which, in concert, regulate cell adhesion, migration, cell–cell communication, proliferation and survival (Kreidberg, 2000). One of the functions described for integrins is their ability to mediate the internalization of ECM components, adenoviruses and apoptotic cells through endocytosis (Memmo & McKeown-Longo, 1998; Li et al., 2001; Hanayama et al., 2002).
To determine whether Integrin-α3 regulates extracellular levels of Chd through endocytosis, we analysed the cellular localization of Chd–Fc in COS-7 cells using a fluorescent probe that marks endocytic vesicles (Entchev et al., 2000; Texas Red Dextran; molecular weight 3,000 Da). When incubated at 4 °C, the Chd–Fc signal was detected on the cell surface (Fig. 4A–D). To allow internalization, cells were shifted to 37 °C for 30 min. After this treatment, Chd–Fc colocalized with Texas Red Dextran in punctate intracellular vesicles (Fig. 4E–H; note yellow spots in the inset in Fig. 4G). When COS-7 cells were transfected with full-length integrin-a3 cDNA and stained with Chd–AP, the amount of intracellular Chd–AP was greatly increased compared with untransfected COS-7 cells (data not shown). These results indicate that binding of Chd to Integrin-α3 on the surface of COS-7 cells results in its endocytosis and removal from the extracellular space in a temperature-dependent manner.
Figure 4.
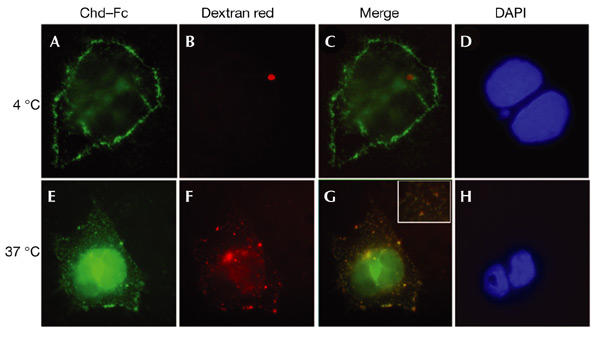
Chordin–Fc translocates into intracellular endocytic compartments at 37 °C. Cells were incubated on ice with Chordin (Chd)–Fc for 2 h, washed, and then incubated with Texas-Red–dextran for 30 min on ice (A–D) or at 37 °C (E–H). (A,E) Localization of Chd–Fc (green) bound to COS-7 cells. (B,F) Localization of Texas-Red–dextran (red) in punctate endocytic compartments after shifting to 37 °C. (C,G) Merged images. (D,H) 4,6-diamidino-2-phenylindole (DAPI) staining of nuclear DNA. Note that at 4 °C, most of the Chd–Fc is detected at the cell surface, but after 30 min at 37 °C it colocalizes with endocytic vesicles in the cytoplasm that contain Texas-Red–dextran (G). The red spot in (B) is artefactual, but shows that red fluorescence does not bleed into the green fluorescence channel.
Recently, a gradient of the Sog protein has been directly visualized in the Drosophila embryo and modified using the temperature-sensitive shibire (shits) endocytosis mutant (Srinivasan et al., 2002). Interestingly, at the nonpermissive temperature, blockage of endocytosis increases the extracellular levels of Sog and enhances its diffusion towards the dorsal pole of the fly embryo (Srinivasan et al., 2002). Thus, the Sog gradient is regulated and maintained by endocytosis. Chd and Integrin-α3 are co-expressed during Xenopus development. Integrin-α3 may limit Chd diffusion by endocytosis and removal from the extracellular space or, alternatively, may facilitate transcytosis, regulating the shape of a hypothetical Chd gradient. The experiments presented here show that Chd can bind to the cell surface through Integrin-α3 in cell culture, and that this binding can result in the removal of Chd from the extracellular space. Previous work has shown that endocytosis constitutes an important step in the formation of morphogenetic gradients (Entchev et al., 2000; Lander et al., 2002; Seto et al., 2002; Vincent & Dubois, 2002). A possible functional interaction between Chd and Integrin-α3 during Xenopus embryonic development is independently supported by recent observations in Drosophila (Araujo et al., 2003). Genetic interactions have been detected between sog and several integrin-coding genes (myospheroid, multiple edematous wing and scab) during the formation of wing veins in pupal development. In addition, in co-immunoprecipitation experiments, it was found that α-PS1 integrin, which is encoded by multiple edematous wing, binds to the Sog protein (Araujo et al., 2003). Interestingly, Drosophila α-PS1 integrin is most similar to vertebrate integrin-α3. We propose that the interaction between Integrin-α3 and Chordin provides another evolutionarily conserved mechanism for modulating BMP/Dpp signalling in the extracellular space.
Methods
Affinity chromatography and biotinylation.
Chd–Fc and s-Fc fusion proteins were produced in stable Drosophila S2 cell lines. For purification of Fc fusion proteins, 1 l of Drosophila Serum-free Medium (Invitrogen) containing the secreted proteins was bound to 1 ml of protein-A–sepharose, and the column was washed with 50 ml of 0.01 M sodium phosphate, pH 8.0, 150 mM NaCl. Proteins bound to protein A were eluted with five column-volumes of 0.1 M glycine, pH 3.0, and fractions of 1 ml were collected and neutralized in tubes containing 50 μl of 1 M Tris-HCl, pH 9.0. The proteins purified in this way were bound to protein-A–agarose beads (∼0.5 mg protein per ml of wet beads) and crosslinked using DMP (Pierce; Harlow & Lane, 1988). For biotinylation, COS-7 cells were grown to confluency, washed twice with ice-cold PBS, and incubated on ice for 30 min with 1.0 mg ml−1 EZ-link sulpho-NHS-biotin (Pierce) in PBS. The reaction was stopped by washing cells twice with ice-cold PBS and incubating them for 30 min with 0.1 M glycine in PBS. Two 100-mm plates of cells were lysed with lysis buffer (10 mM Tris pH 7.5, 0.15 M NaCl, 10% glycerol, 0.1% Triton X-100; with complete protease inhibitor cocktail, EDTA-free (Roche Biochemicals)). Chd–Fc and s-Fc beads were incubated for 16 h at 4 °C with cell lysates in lysis buffer supplemented with 2 mM of each of the following divalent cations: Mg2+, Ca2+ and Mn2+. Beads were washed four times with ice-cold lysis buffer, and bound proteins were eluted with SDS loading buffer and analysed by immunoblotting using SA–HRP (Pierce) at a dilution of 1 in 4,000.
Cell-surface binding.
Cells were grown to 80% confluence in 24-well plates, washed twice with 1 ml of ice-cold HBSS containing 0.5 mg ml−1 BSA and 20 mM Hepes, pH 7.0, and incubated for 2 h on ice with 250 μl of AP fusion protein (Flanagan & Cheng, 2000). Cells were then washed on ice six times with 1 ml of ice-cold HBSS and extracted with 100 μl of 10 mM Tris-HCl, pH 7.5, containing 1% Triton X-100. Endogenous AP activity was inactivated by incubation of the plate at 65 °C for 1 h. AP activity resulting from fusion proteins bound to the cells was detected by a fluorescent assay in 96-well FluoroNunc microplates. Cell extracts (25 μl) were mixed with 25 μl of 2× assay buffer containing 0.2 mM 4-methylumbelliferyl-phosphate (Sigma) in 0.1 M diethanolamine–HCl, pH 9.8, 1 mM MgCl2. After 1 h of incubation at 37 °C, the reaction was stopped with 200 μl of 0.5 M glycine–NaOH. Fluorescence was read at 465 nm (emission) on an HTS 7000 Plus Bio Assay plate-reader (Perkin Elmer). To equalize loading, protein concentration was measured using a Lowry-modified colorimetric assay compatible with detergents (DC Protein Assay; BioRad).
Cell staining and immunohistochemistry.
For histochemical staining, cells were grown to 80% confluence in six-well plates and incubated with the AP fusion proteins for 2 h at 25 °C. Cells were washed for 5 min on ice, four times with 2 ml of ice-cold HBSS and once with 2 ml of AP buffer (0.1 M Tris-HCl, pH 9.5, 0.1 M NaCl). To inactivate endogenous AP, 1 mM levamisole (Sigma) was included in the last two washes. To develop AP activity, cells were incubated at 25 °C with 2 ml of a NBT (nitroblue tetrazolium chloride)/BCIP (5-bromo-4-chloro-3-indolyl-phosphate) solution filtered using a 0.2-μm filter (Roche Biochemicals) until positive purple cells were seen (Oelgeschläger et al., 2000; Flanagan & Cheng, 2000).
For immunohistochemistry, COS-7 or 10T1/2 fibroblast cells were grown on coverslips coated with poly-L-lysine until 80% confluency was reached, incubated with Chd–Fc for 2 h on ice, and washed three times with ice-cold PBS. Some samples were incubated on ice and others at 37 °C for 30 min in PBS containing 0.5 mM Texas-Red-dextran (lysine fixable; molecular weight 3,000 Da; Molecular Probes) to mark endocytic vesicles (Entchev et al., 2000). Cells were fixed with 4% paraformaldehyde in PBS for 20 min and permeabilized with 0.1% Triton X-100 for 10 min. Fluorescence was visualized after incubation with an anti-human IgG antibody conjugated to Alexa Fluor® 488 (1:200 dilution; Molecular Probes). For actin staining, Alexa Fluor® 568 phalloidin (1:200 dilution; Molecular Probes) was used after cell permeabilization.
Acknowledgments
We thank E. Bier for communicating results before publication, S. Millard and K.-W. Zhao for help with cell staining and fluorescent AP assays, and O. Wessely, C. Coffinier, H. Lee and L. Fuentealba for reviewing the manuscript. J.L. was a Pew Latin American Fellow. C.B. is supported by a National Institutes of Health (NIH) Minority Supplement. This work was supported by NIH grant R37 HD21502-17 and the Howard Hughes Medical Institute, of which E.M.D.R. is an Investigator.
References
- Araujo H., Negreiros E. & Bier E. ( 2003) Integrins modulate Sog activity in the Drosophila wing. Development, in the press. [DOI] [PubMed] [Google Scholar]
- Davis S. et al. ( 1996) Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell, 87, 1161–1169. [DOI] [PubMed] [Google Scholar]
- De Robertis E.M., Larraín J., Oelgeschläger M. & Wessely O. ( 2000) The establishment of Spemann's Organizer and patterning of the vertebrate embryo. Nature Rev. Genet., 1, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar A., Dorfman R., Weiss D., Ashe H., Shilo B.Z. & Barkai N. ( 2002) Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature, 419, 304–308. [DOI] [PubMed] [Google Scholar]
- Entchev E.V., Schwabedissen A. & González-Gaitán M. ( 2000) Gradient formation of the TGF-β homolog Dpp. Cell, 103, 981–991. [DOI] [PubMed] [Google Scholar]
- Flanagan J.G. & Cheng H.J. ( 2000) Alkaline phosphatase fusion proteins for molecular characterization and cloning of receptors and their ligands. Meth. Enzymol., 327, 198–210. [DOI] [PubMed] [Google Scholar]
- Freeman M. & Gurdon J.B. ( 2002) Regulatory principles of developmental signaling. Annu. Rev. Cell Dev. Biol., 18, 515–539. [DOI] [PubMed] [Google Scholar]
- Hanayama R., Tanaka M., Miwa K., Shinohara A., Iwamatsu A. & Nagata S. ( 2002) Identification of a factor that links apoptotic cells to phagocytes. Nature, 417, 182–187. [DOI] [PubMed] [Google Scholar]
- Harlow E. & Lane D. ( 1988) Antibodies. A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, USA. [Google Scholar]
- Hynes R.O. ( 2002) Integrins: bidirectional, allosteric signaling machines. Cell, 110, 673–687. [DOI] [PubMed] [Google Scholar]
- Kreidberg J.A. ( 2000) Functions of α3β1 integrin. Curr. Opin. Cell Biol., 12, 548–553. [DOI] [PubMed] [Google Scholar]
- Lander A.D., Nie Q. & Wan F.Y.M. ( 2002) Do morphogen gradients arise by diffusion? Dev. Cell, 2, 785–796. [DOI] [PubMed] [Google Scholar]
- Larraín J., Bachiller D., Lu B., Agius E., Piccolo S. & De Robertis E.M. ( 2000) BMP-binding modules in chordin: a model for signalling regulation in the extracellular space. Development, 127, 821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larraín J., Oelgeschläger M., Ketpura N.I., Reversade B., Zakin L. & De Robertis E.M. ( 2001) Proteolytic cleavage of Chordin as a switch for the dual activities of Twisted gastrulation on BMP. Development, 128, 4439–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E., Brown S.L., Stupack D.G., Puente X.S., Cheresh D.A. & Nemerow G.R. ( 2001) Integrin α(v)β1 is an adenovirus coreceptor. J. Virol., 75, 5405–5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B. et al. ( 2002) Kremen proteins are Dickkopf receptors that regulate Wnt/β-catenin signalling. Nature, 417, 664–667. [DOI] [PubMed] [Google Scholar]
- Mehta H., Orphe C., Todd M.S., Cornbrooks C.J. & Carey D.J. ( 1985) Synthesis by Schwann cells of basal lamina and membrane-associated heparan sulfate proteoglycans. J. Cell Biol., 101, 660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memmo L.M. & McKeown-Longo P. ( 1998) The αvβ5 integrin functions as an endocytic receptor for vitronectin. J. Cell Sci., 111, 425–433. [DOI] [PubMed] [Google Scholar]
- Meng F., Whittaker C.A., Ransom D.G. & DeSimone D.W. ( 1997) Cloning and characterization of cDNAs encoding the integrin α2 and α3 subunits from Xenopus laevis. Mech. Dev., 67, 141–155. [DOI] [PubMed] [Google Scholar]
- Oelgeschläger M., Larrain J., Geissert D. & De Robertis E.M. ( 2000) The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature, 405, 757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S., Sasai Y., Lu B. & De Robertis E.M. ( 1996) Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of Chordin to BMP-4. Cell, 86, 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S., Agius E., Lu B., Goodman S., Dale L. & De Robertis E.M. ( 1997) Cleavage of Chordin by the Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell, 91, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J.J. et al. ( 2001) Twisted gastrulation is a conserved extracellular BMP antagonist. Nature, 410, 479–483. [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., Geissert D., Gont L.K. & De Robertis E.M. ( 1994) Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell, 79, 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott I.C. et al. ( 1999) Mammalian BMP-1/Tolloid-related metalloproteinases, including novel family member mammalian Tolloid-like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis. Dev. Biol., 213, 283–300. [DOI] [PubMed] [Google Scholar]
- Seto E.S., Bellen H.J. & Lloyd T.E. ( 2002) When cell biology meets development: endocytic regulation of signaling pathways. Genes Dev., 16, 1314–1336. [DOI] [PubMed] [Google Scholar]
- Shang M., Koshikawa N., Schenk S. & Quaranta V. ( 2001) The LG3 module of laminin-5 harbors a binding site for integrin α3β1 that promotes cell adhesion, spreading, and migration. J. Biol. Chem., 276, 33045–33053. [DOI] [PubMed] [Google Scholar]
- Srinivasan S., Rashka K.E. & Bier E. ( 2002) Creation of a Sog morphogen gradient in the Drosophila embryo. Dev. Cell, 2, 91–101. [DOI] [PubMed] [Google Scholar]
- Vincent J.P. & Dubois L. ( 2002) Morphogen transport along epithelia, an integrated trafficking problem. Dev. Cell, 3, 615–623. [DOI] [PubMed] [Google Scholar]
- Whittaker C.A. & DeSimone D.W. ( 1991) Integrin α subunit mRNAs are differentially expressed in early Xenopus embryos. Development, 117, 1239–1249. [DOI] [PubMed] [Google Scholar]


