Abstract
Thyroid hormone receptors (TRs) have several regulatory functions in vertebrates. In the absence of thyroid hormone (T3; triiodothyronine), apo-TRs associate with co-repressors to repress transcription, whereas in the presence of T3, holo-TRs engage transcriptional coactivators. Although many studies have addressed the molecular mechanisms of T3 action, it is not known how specific physiological responses arise. We used T3-dependent amphibian metamorphosis to analyse how TRs interact with particular co-regulators to differentially regulate gene expression during development. Using chromatin immunoprecipitation to study tissue from pre-metamorphic tad-poles, we found that TRs are physically associated with T3-responsive promoters, whether or not T3 is present. Addition of T3 results in histone H4 acetylation specifically on T3-response genes. Most importantly, we show that individual T3-response genes have distinct co-regulator requirements, the T3-dependent co-repressor-to-coactivator switch being gene-specific for both co-regulator categories.
Introduction
The nuclear receptor superfamily, which includes the thyroid hormone receptors (TRs), which are encoded by the TRa (NR1A1; Nuclear Receptors Nomenclature Committee, 1999) and TRb (NR1A2) loci, affects vertebrate development, cell homeostasis and physiology. TRs bind as heterodimers with 9-cis-retinoic-acid receptor (RXR) to target genes through cis-acting DNA sequences known as T3 (thyroid hormone; triiodothyronine)-response elements (T3REs). In the absence of T3, apo-TRs repress basal transcription; in the presence of T3, holo-TRs relieve repression and activate transcription. The repression-to-activation switch involves changes in co-repressor to coactivator complexes, which are partnered by apo-TR and holo-TR, respectively (Glass & Rosenfeld, 2000). Given the diversity among co-regulators, their tissue-specific distribution and their variable expression levels, it is possible that many modes of gene regulation by TRs could involve different combinations of co-regulators on particular gene subsets or in specific physiological contexts.
We used amphibian metamorphosis to investigate TR action in a physiological context. Metamorphosis is controlled by T3 (Tata, 1993). As in mammals, both TR-α and TR-β exist in amphibians such as Xenopus laevis (Yaoita et al., 1990). The TRb gene is expressed at low levels before metamorphosis, and is upregulated by T3 during metamorphosis as a T3 direct-response gene (Ranjan et al., 1994). By contrast, TR-α is activated after completion of embryogenesis, but well before the production of endogenous T3. A working model of T3-dependent gene regulation during pre-metamorphosis is that apo-TRs repress the T3 direct-response genes and, later, the combination of T3 and TRs permits the activation of T3 direct-response genes, thus inducing metamorphosis (Sachs & Shi, 2000).
We used chromatin immunoprecipitation (ChIP) to analyse histone H4 acetylation and recruitment of TR and co-regulators on T3-response-gene promoters. We focused on four well-characterized co-regulators that are required for TR action (Glass & Rosenfeld, 2000): the nuclear co-repressor NCoR, the histone deacetylase (HDAC) Rpd3, steroid receptor coactivator 3 (SRC3), and the coactivator p300. Four important results were obtained: first, we demonstrated constitutive TR binding to T3 direct-response gene promoters in vivo in pre-metamorphic tadpoles; second, we showed that there is induction by T3 treatment of histone H4 acetylation on T3-response gene promoters; third, we showed that there is gene-specific recruitment of co-regulators at T3-response gene promoters; and finally, we showed that there is a gene-specific switch from co-repressor to coactivator recruitment after treatment with T3.
Results
Constitutive TR binding on T3-response-gene promoters
We first analysed the expression patterns of several control and T3-response genes in tadpole tails after T3 treatment (for 48 h). We chose tail tissue because it is the best-characterized organ that undergoes extensive remodelling during metamorphosis, disappearing completely due to apoptosis and showing strong upregulation of T3-response genes. The tail is composed of connective tissue, blood vessels, spinal cord, notochord and muscles. As skeletal muscle predominates, it provides a relatively homogeneous tissue for studying specific changes in gene regulation and chromatin remodelling. Treatment with T3 for 48 h was carried out to mimic the physiological conditions of metamorphosis as closely as possible and to obtain maximum activation of T3 direct-response genes (Furlow & Brown, 1999). Total RNA was extracted from tail tissue and used for RT–PCR (PCR after reverse transcription) analysis. TRa was selected as the only TR gene that is expressed at all tadpole stages; TRb and TH/bZIP (a basic leucine-zipper TH-response gene) are the only two Xenopus T3 direct-response genes with sequenced and characterized promoters. MyoD was chosen because it is a muscle-cell-specific gene. Intestinal fatty acid binding protein (IFABP) is an intestinal epithelial-cell-specific gene. IFABP provided a good negative control for these experiments, as it is not expressed in the tail. Finally, elongation factor 1a (EF1a) and ribosomal protein L8 (Rpl8) were used as controls, as they are housekeeping genes. T3 treatment significantly increased the messenger RNA levels of TRb, TH/bZIP and MyoD (Fig. 1A), but did not affect the levels of TRa, EF1a or Rpl8. As expected, IFABP was not expressed (Fig. 1A). These expression patterns are consistent with earlier data that were based on northern blots (Wang & Brown, 1993) and RNase protection assays (Kawahara et al., 1991). Our results provide the first evidence that MyoD is a T3-response gene in Xenopus tadpoles.
Figure 1.
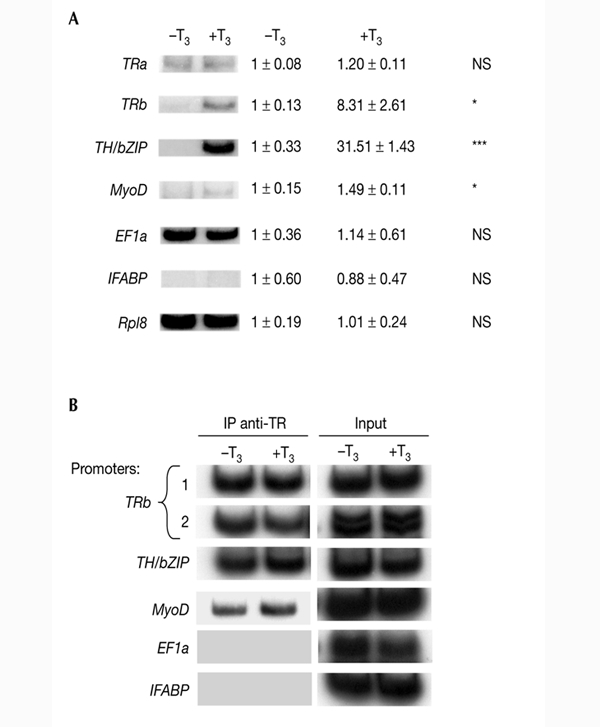
Effects of T3 on transcription and DNA binding by thyroid hormone receptor at T3-response genes in pre-metamorphic stage NF55 tadpole tail. (A) T3 induces transcription of T3-response genes. Tadpoles were treated for 48 h with 10 nM T3. Total RNA was extracted from tail tissue and used for RT–PCR (PCR after reverse transcription) analysis of thyroid hormone receptor b (TRb), TH/bZIP (a basic leucine-zipper TH-response gene), MyoD, intestinal fatty acid binding protein (IFABP), elongation factor 1a (EF1a) and ribosomal protein L8 (Rpl8) expression. The internal control was Rpl8. The results were also quantified by phosphoimager scanning. The average values ± s.e.m. of three independent experiments are expressed as multiples of induction, where 1 is equal to expression in the absence of T3 (control level). For each sample, densitometry readings were normalized against the value for Rpl8 RNA (except for the Rpl8 data, which were not normalized). Statistical significance as compared with untreated animals is indicated as NS (not significant), * (p < 0.05) or *** (p < 0.001). (B) T3 does not affect TR binding to T3 response elements. Chromatin isolated from tails of T3-treated tadpoles (10 nM T3 for 48 h) was immunoprecipitated (IP) with antibodies against TR and analysed by PCR. Aliquots of the chromatin taken before immunoprecipitation were used directly for PCR as a control (input). For TRb promoters, we distinguished two sequences containing T3REs (sequence 1 at position +266 and sequence 2 at positions −800 to −500). All experiments were carried out at least three times. T3, thyroid hormone (triiodothyronine).
To obtain direct information about whether these T3-induced changes in gene expression correlate with the binding of TR to chromatin, we analysed TR binding in vivo to the promoters of TRb, TH/bZIP, MyoD, IFABP and EF1a using ChIP assays. Antibodies that recognize both TR-α and TR-β were used to immunoprecipitate formaldehyde-crosslinked, fragmented chromatin from nuclei that were isolated from tadpole tails and either treated with T3 or left untreated. The TR-bound DNA fragments were then analysed by semi-quantitative PCR (Fig. 1B). Primers flanking the T3REs were used for the TRb and TH/bZIP promoters. For the TRb promoter, we distinguished two T3RE-containing sequences ('promoter region 1', which has one T3RE at position +266, and 'promoter region 2', which has three putative T3REs between positions −800 and −500; Urnov & Wolffe, 2001). Primers corresponding to the 300 bp immediately upstream of the transcription start site were used for the MyoDa promoter (Leibham et al., 1994). This promoter has never been studied for regulation by T3. However, sequence analysis revealed two imperfect putative T3REs (data not shown). Finally, for the EF1a promoter (Johnson & Krieg, 1995) and the IFABP promoter (Gao et al., 1998), which are not regulated by T3, we chose primers in the 500-bp region upstream of the transcription start site. As shown in Fig. 1B, TR was constitutively present on the TRb, TH/bZIP and MyoD promoters, but was absent from T3-insensitive promoters (EF1a and IFABP). Furthermore, T3 does not have any effect on TR binding (Fig. 1B). These results indicate that, first, as previously described (Sachs & Shi, 2000), apo-TR and holo-TR bind T3REs in chromatin in vivo, and second, that TR binds in vivo to both of the T3RE-containing sequences in the TRb promoter. Moreover, our results highlight the fact that the levels of TR occupancy correlate with the levels of gene expression. Finally, the presence of TR on the MyoD promoter suggests that MyoD might be a T3 direct-response gene. However, more data will be necessary to confirm this.
Histone acetylation correlates with gene regulation
We next investigated histone acetylation of promoters. Co-repressor complexes have HDAC activity, and many coactivators have intrinsic histone acetyl transferase (HAT) activity, which suggests that TRs might regulate transcription by modification of local histone acetylation levels (Wolffe, 1997). As shown in Fig. 2, using a ChIP assay with an antibody specific to acetylated histone H4 (AcH4), we showed that histone H4 acetylation increased on TRb promoter regions 1 and 2 and on the TH/bZIP and MyoD promoters, but not on the EF1a and IFABP promoters. Comparison of Fig. 1A with Fig. 2 shows that the levels of histone H4 acetylation correlate with the levels of gene expression. As a control for the specificity of local histone acetylation, we analysed the acetylation levels of the MyoD, EF1a and IFABP promoters in intestine. As expected, as MyoD is not expressed in intestine, its promoter chromatin did not contain AcH4 (Fig. 2). However, given that IFABP and EF1a are highly expressed in this tissue, it was not surprising to find that chromatin from their promoters contained AcH4 (Fig. 2). Finally, for TRb, which is strongly repressed in the absence of T3, AcH4 was detected at region 2 of the TRb promoter in the absence of T3, whereas there was no detectable AcH4 at region 1 (Fig. 2, compare TRb lanes 1 and 2). However, both regions contained AcH4 when T3 was present.
Figure 2.
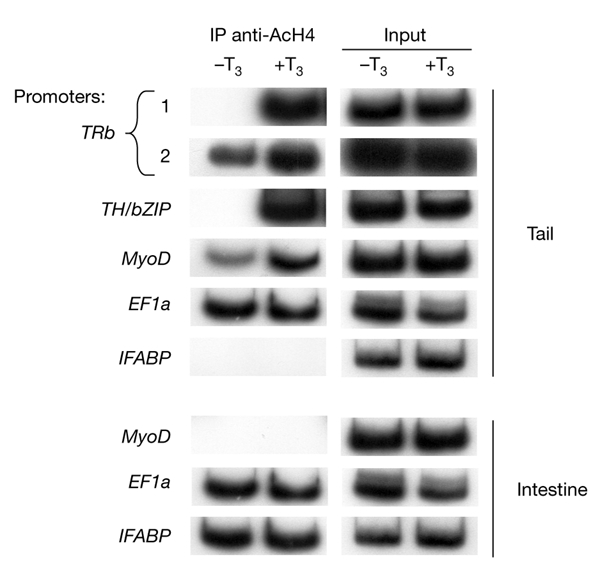
T3 treatment increases histone H4 acetylation specifically at the T3-response elements of T3-response genes in pre-metamorphic tadpoles. Chromatin isolated from tail or intestine of T3-treated stage NF55 tadpoles (treated with 10 nM T3 for 48 h) was immunoprecipitated (IP) with antibodies against acetylated histone H4 (AcH4) and analysed by PCR, as described for Fig. 1. Each experiment was carried out at least twice. EF1a, elongation factor 1a; IFABP, intestinal fatty acid binding protein; T3, thyroid hormone (triiodothyronine); TH/bZIP, a basic leucine-zipper Th-response gene; TRb, thyroid hormone receptor b.
Co-repressor recruitment to T3-response-gene promoters
Apo-TR is known to interact with co-repressor complexes containing HDAC, whereas holo-TR is known to interact with coactivator complexes containing HATs. We examined the recruitment and T3-dependent release of two co-repressors, NCoR (Sachs et al., 2002) and Rpd3 (Wong et al., 1998). After confirming that NCoR and Rpd3 are expressed in tail (Fig. 3A), a ChIP assay was performed using polyclonal antibodies to Rpd3, the only characterized Xenopus HDAC (Wong et al., 1998), and to Xenopus NCoR, a co-repressor that seems to function through mechanisms involving HDACs. As shown in Fig. 3B, Rpd3 is recruited to the TRb promoter (regions 1 and 2) in a T3-independent manner. However, Rpd3 is recruited to the TH/bZIP promoter only in the absence of T3 (Fig. 3B), and is never recruited to the MyoD, EF1a and IFABP promoters (Fig. 3B). We found that NCoR recruitment to the TRb (regions 1 and 2), TH/bZIP and MyoD promoters decreased after T3 treatment (Fig. 3B). Noticeably, NCoR is never present on the EF1a and IFABP promoters (Fig. 3B). Thus, all the T3-response genes that were studied recruit NCoR only in the absence of T3. By contrast, the recruitment of Rpd3 is gene-specific, and is not always affected by T3 treatment.
Figure 3.
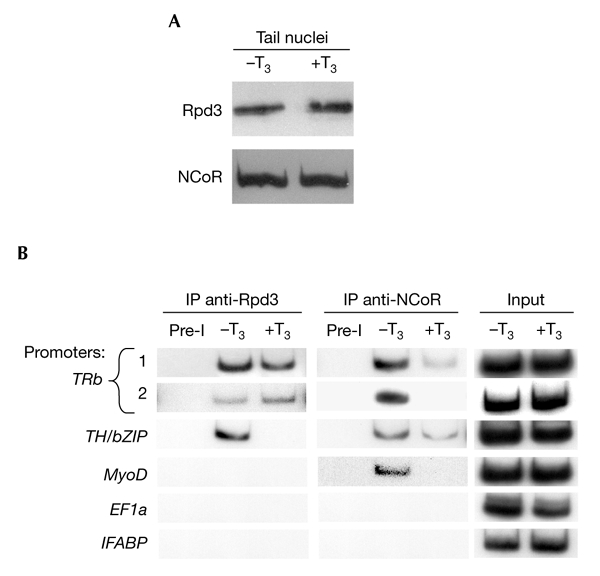
Effects of T3 on Rpd3 and NCoR co-repressor expression and recruitment on T3-response elements of T3-response genes in pre–metamorphic tadpoles. (A) Rpd3 and NCoR protein levels in tail nuclei are not affected by T3 treatment. Western blot analysis of protein extracts from the tail nuclei of tadpoles treated with 10 nM T3 for 48 h. (B) Chromatin isolated from tails of T3-treated tadpoles (treated with 10 nM T3 for 48 h) was immunoprecipitated (IP) with antibodies against Rpd3 or NCoR and analysed by PCR, as described for Fig. 1. Pre-immune serum (Pre-I) was used as a control for antibody specificity. The data represent one of several independent experiments with identical results. EF1a, elongation factor 1a; IFABP, intestinal fatty acid binding protein; NCoR, nuclear co-repressor; T3, thyroid hormone (triiodothyronine); TH/bZIP, a basic leucine-zipper Th-response gene; TRb, thyroid hormone receptor b.
Coactivator recruitment to T3-response-gene promoters
Finally, we examined, in tadpole tail, the effects of T3 on the recruitment of the TR receptor coactivators SRC3 (Kim et al., 1998) and p300 (Fujii et al., 1998), which have HAT activity. After verifying that these coactivator proteins are expressed in the tail at significant levels (Fig. 4A), we analysed whether they were present on the promoters of the genes studied. Three different situations were found: SRC3 and p300 are continually present on the promoter of TRb (regions 1 and 2); these coactivators are only present on TH/bZIP and MyoD promoters if T3 is present. In the case of the EF1a and IFABP promoters, these coactivators are never present (Fig. 4B).
Figure 4.

Effects of T3 on steroid receptor coactivator 3 and p300 coactivator expression and recruitment on T3-response elements of T3-response genes in pre-metamorphic tadpoles. (A) Steroid receptor coactivator 3 (SRC3) and p300 protein levels in tail nuclei are not affected by T3 treatment. Western blot analysis of protein extracts fron tail nuclei of tadpoles treated with 10 nM T3 for 48 h. (B) Chromatin isolated from tails of T3-treated tadpoles (treated with 10 nM T3 for 48 h) was immunoprecipitated (IP) with antibodies against SRC3 or p300 and analysed by PCR, as described for Fig. 1. Pre-immune serum (Pre-I) was used as a control for antibody specificity. All experiments were carried out at least three times. EF1a, elongation factor 1a; IFABP, intestinal fatty acid binding protein; T3, thyroid hormone (triiodothyronine); TH/bZIP, a basic leucine-zipper Th-response gene; TRb, thyroid hormone receptor b.
Discussion
We exploited the absolute dependence of amphibian metamorphosis on T3 and TRs to analyse successive stages of TR/co-regulator association during a physiologically defined sequence of events.
Recruitment of NCoR with and without Rpd3
The first model of transcriptional repression by apo-TR, proposed by Wolffe (1997), described the recruitment of a multiprotein complex that included NCoR and Rpd3. Recently, on the basis of results from the TR regulation of the Xenopus TRb promoter, this model was refined to conclude that apo-TR specifically recruits an NCoR–HDAC3 complex, and not an NCoR–Rpd3 complex (Li et al., 2002a). Nevertheless, Rpd3 is constitutively associated with chromatin and contributes to chromatin deacetylation in a non-targeted manner (Li et al., 2002a). Here, by examining three T3-response-gene promoters, we show that NCoR is present at all promoters in the absence of T3, but that NCoR is absent from the same promoters after T3 treatment. However, Rpd3 recruitment is gene-specific. Rpd3 is associated with the TRb promoter whether T3 is present or not, thus confirming the finding of Li and collaborators (2002a). However, Rpd3 is recruited to the TH/bZIP promoter only in the absence of T3, and is never recruited to the MyoD promoter. However, we did observe the presence of NCoR and Rpd3 on the TH/bZIP promoter, showing that such complexes can be recruited by apo-TR. Thus, it is still possible that NCoR–Rpd3 or NCoR–HDAC3 might function through targeting to different T3 direct-response gene promoters.
Ligand-dependent co-repressor to coactivator switches
It is thought at present that TRs repress transcription by recruiting co-repressors and activate transcription by recruiting coactivators. Our results from the TH/bZIP and MyoD promoters are consistent with this model. By contrast, at the TRb promoter, Rpd3, SCR3 and p300 are present simultaneously both in the presence and absence of T3, emphasizing that NCoR release is a key event in the activation of TRb transcription. This finding supports an earlier hypothesis that TRb expression results from the relief of repression, rather than from activation by holo-TR (Collingwood et al., 1999).
The direct interaction of coactivators and co-repressors in a single regulatory unit has been described before. Examples of these are NCoR and SRC3 (Li et al., 2002b), and NCoR and CBP/p300 (Saleh et al., 2000). Direct interactions such as these provide an integral control mechanism that affects the timing of repression and activation. Indeed, the induction of TRb expression precedes TH/bZIP expression (Furlow & Brown, 1999). These more rapid kinetics could be due to the simultaneous association of co-repressors and coactivators on the TRb promoter. In such a complex, the presence of co-repressors inhibits transcription, and their release rapidly activates transcription. In this context, it would be interesting to compare the occupancy of the various promoters at various times after T3 treatment. However, when using an in vivo approach, one has to take into account the fact that all the cells are not simultaneously T3 responsive.
A growing body of evidence suggests that co-regulators are themselves highly regulated by covalent modification. Such modifications not only alter protein function, but can also confer specificity to ubiquitous factors. The acetylation of SRC3 by p300 has been shown to induce its release from the holo-ER form of the oestrogen receptor to attenuate transcriptional activation by oestradiol (Chen et al., 1999). In addition, covalent modification of CBP/p300 induces changes in HAT activity, substrate specificity, protein–protein interactions and stability (Gamble & Freedman, 2002). For example, on binding of NCoR and CBP to the homeobox heterodimer pbx–hox, protein kinase A stimulation of CBP has been found to facilitate the switch from transcriptional repression to activation in this system (Saleh et al., 2000).
Transcriptional activation might also involve chromatin structure. Binding by apo-TR to the TRb promoter T3RE is potentiated by assembly of the DNA into a mature array of transitionally positioned nucleosomes, and holo-TR disrupts this array, creating a lower-affinity template for itself (Urnov & Wolffe, 2001). Another possibility is the recruitment of other types of coactivator complexes by T3 treatment. Indeed, TR can recruit SRC–p300 and Mediator complexes in at least two sequential steps. SRC and p300 are recruited first and rapidly induce histone acetylation, followed by the recruitment of the Mediator complex (Sharma & Fondell, 2002). However, sequential models are usually cyclic, with windows of time in the range of minutes (Shang et al. 2000; Sharma & Fondell, 2002), and these complexes are all required for correct gene transcription (Huang et al., 2003).
Our results suggest that during metamorphosis, combinatorial associations of TR, co-repressor and/or coactivator molecules that are influenced by cell history and promoter context provide the specificity of the response of genes to T3. This study underlines the importance of tissue specificity with regard to promoter occupancy. We are now analysing this problem in tissues such as intestine and brain that show different metamorphic organizational responses to those of tail tissue. Finally, promoter specificity for co-regulator requirements is a particularly interesting phenomenon, as the different gene regulatory mechanisms revealed in this study might be correlated with the multiple T3-induced cellular responses that underlie tissue remodelling during metamorphosis.
Methods
Animals.
Xenopus laevis tadpoles were staged in accordance with the method of Nieuwkopp & Faber (NF staging; 1956). For T3 treatment, stage NF55 tadpoles were kept for 48 h in 5 l of dechlorinated tap water with 10 nM 3,5,3′ triiodothyronine (T3; Sigma). Tadpoles were sacrificed by decapitation after anaesthesia. Animal care was carried out in accordance with institutional guidelines.
RT–PCR.
Tissues were stored at 4 °C in RNAlater (Ambion). RNA extractions and RT–PCR were carried out as described in Sachs et al. (2002). The primers used are shown in Table 1. To define for each gene the optimal number of PCR cycles for quantitative analysis, 10–24 cycles were carried out (data not shown). The numbers of cycles chosen were as follows: EF1a, 14 cycles; TH/bZIP, 18 cycles; TRa and MyoD, 20 cycles; TRb and IFABP, 22 cycles. PCR products were loaded onto acrylamide gels (6%) in 1 × TBE buffer and visualized by autoradiography. Phosphoimager scanning (Molecular Dynamics) was used to quantify each of the PCR products. A Student's t-test was used to assess statistical differences between means.
Table 1.
Primers used in this study
| Gene | Reverse primer | Forward primer | Reference |
|---|---|---|---|
| RT–PCR | |||
| TRa | 5′-CTCTATCTTGTCCGTGCAGAT-3′ | 5′-ATGGCTTCCATGCCGGATGGG-3′ | Yaoita et al. (1990) |
| TRb | 5′-CTTTTCTATTCTCTCCACGCTAGC-3′ | 5′-TAGTTAATGCGCCCGAGGGTGGA-3′ | Yaoita et al. (1990) |
| TH/bZIP | 5′-CTTAAACCTCAGCTTATGTGGAAG-3′ | 5′-ACTGGGAAAAGAGGCGCAAGAAC-3′ | GenBank U37375 |
| MyoD | 5′-GACAGTTGAGTGCAGGTA-3′ | 5′-GGACTCAGATGCCTCAAG-3′ | GenBank X16106 |
| EF1a | 5′-TGAGGAAGAGAGCGAACC-3′ | 5′-TGCACAGTTGGCGCAGTG-3′ | Johnson & Krieg, 1995 |
| IFABP | 5′-GCCTCTCTTGAAAATCCTTTTTG-3′ | 5′-GGAACTTGGAAGGTTGACAGA-3′ | GenBank L19946 |
| Rpl8 | 5′-GACGACCAGTACGACGA-3′ | 5′-AAAGAGAAACTGCTGGC-3′ | GenBank U00920 |
| Promoter | |||
| TRb 1 | 5′-GACAGTCAGAGGAACTG-3′ | 5′-GTAAGCTGCCTGTGTCTATA-3′ | Ranjan et al. (1994) |
| TRb 2 | 5′-GACTATGGCATGTTACAGC-3′ | 5′-TAGATGCTGCACCTGCTC-3′ | Ranjan et al. (1994) |
| TH/bZIP | 5′-CTCCCAACCCTACAGAGTTCA-3′ | 5′-TCTCCCTGTTGTGTATAATGG-3′ | Furlow & Brown (1999) |
| MyoD | 5′-CCTCTGCCCTCACTCTAT-3′ | 5′-AGTTCCTTGGATCGTTCG-3′ | Leibham et al. (1994) |
| EF1a | 5′-TGAGGAAGAGAGCGAACC-3′ | 5′-TGCACAGTTGGCGCAGTG-3′ | Johnson & Krieg (1995) |
| IFABP | 5′-GGCCACAAGATCTACTCG-3′ | 5′-ATAGCAGCAGGTGGTTGCG-3′ | Gao et al. (1998) |
EF1a, elongation factor 1a; IFABP, intestinal fatty acid binding protein; Rpl8, ribosomal protein L8; T3, thyroid hormone (triiodothyronine); TH/bZIP, a basic leucine-zipper TH-response gene; TR, thyroid hormone receptor.
Antibodies.
Rabbit polyclonal antibodies against Xenopus Rpd3 and Xenopus NCoR have been described previously (Vermaak et al., 1999; Sachs et al., 2002). Rabbit polyclonal antibodies against Xenopus SRC3 were generated against two synthetic peptides (amino acids 1–20, MSGLGENSLDPLASETRKRK, and amino acids 62–77, DNFNVKPDKCAILKETVR) and those against Xenopus p300 were generated against two synthetic peptides (amino acids 1033–1049, KSEPVELEEKKEEVKTE, and amino acids 1487–1506, KPRLQEWYKKMLDKSVSER).
ChIP assays.
ChIP assays were carried out as described by Sachs & Shi (2000). 5 μl of anti-AcH4 antiserum (Upstate Biotechnology) or 8 μl of antibodies against the Xenopus proteins TR, Rpd3, NCoR, p300 and SRC3 were used for immunoprecipitation. Pre-immune serum was used as a control for antibody specificity. Immunoprecipitated DNA was analysed by semi-quantitative PCR, as described in Sachs & Shi (2000). The primers used are shown in Table 1. 10 μl of PCR product was resolved on a 6% acrylamide–TBE gel, and bands were visualized by autoradiography.
Protein isolation and western blotting.
After the isolation of tail nuclei (Sachs & Shi, 2000), protein extraction and western blotting were carried out as described in Sachs et al. (2001). One modification to this method was the time of transfer to nitrocellulose membranes (Bio-Rad), which was 1 h for Rpd3 and SRC3 and overnight for NCoR and p300.
Acknowledgments
We thank G. Benisti, J.-P. Chaumeil and E. LeGoff for animal care. The Association Pour la Recherche Contre le Cancer, the CNRS and the Muséum National d'Histoire Naturelle supported this work.
References
- Chen H., Lin R.J., Schiltz R.L., Chakravarti D., Nash A., Nagy L., Privalsky M.L., Nakatani Y. & Evans R.M. ( 1999) Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell, 90, 569–580. [DOI] [PubMed] [Google Scholar]
- Collingwood T.N., Urnov F.D. & Wolffe A.P. ( 1999) Nuclear receptors: coactivators, corepressors and chromatin remodeling in the control of transcription. J. Mol. Endocrinol., 23, 255–275. [DOI] [PubMed] [Google Scholar]
- Fujii G., Tsuchiya R., Itoh Y., Tashiro K. & Hirohashi S. ( 1998) Molecular cloning and expression of Xenopus p300/CBP. Biochim. Biophys. Acta, 1443, 41–54. [DOI] [PubMed] [Google Scholar]
- Furlow J.D. & Brown D.D. ( 1999) In vitro and in vivo analysis of the regulation of a transcription factor gene by thyroid hormone during Xenopus laevis metamorphosis. Mol. Endocrinol., 13, 2076–2089. [DOI] [PubMed] [Google Scholar]
- Gamble M.J. & Freedman L.P. ( 2002) A coactivator code for transcription. Trends Biochem. Sci., 27, 165–167. [DOI] [PubMed] [Google Scholar]
- Gao X., Sedgwick T., Shi Y.-B. & Evans T. ( 1998) Distinct functions are implicated for the GATA-4, -5, and -6 transcription factors in the regulation of intestine epithelial cell differentiation. Mol. Cell. Biol., 18, 2901–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C.K. & Rosenfeld M.G. ( 2000) The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev., 14, 121–141. [PubMed] [Google Scholar]
- Huang Z.-Q., Li J., Sachs L.M., Cole P.A. & Wong J. ( 2003) A role for cofactor–cofactor and cofactor–histone interactions in targeting p300, SWI:SNF and Mediator for transcription. EMBO J., 22, 2146–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.D. & Krieg P.A. ( 1995) A Xenopus laevis gene encoding EF-1αS, the somatic form of elongation factor 1α: sequence, structure, and identification of regulatory elements required for embryonic transcription. Dev. Genet., 17, 280–290. [DOI] [PubMed] [Google Scholar]
- Kawahara A., Baker B.S. & Tata J.R. ( 1991) Developmental and regional expression of thyroid hormone receptor genes during Xenopus metamorphosis. Development, 112, 933–943. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Lee S.K., Na S.Y., Choi H.S. & Lee J.W. ( 1998) Molecular cloning of xSRC-3, a novel transcription coactivator from Xenopus, that is related to AIB1, p/CIP, and TIF2. Mol. Endocrinol., 12, 1038–1047. [DOI] [PubMed] [Google Scholar]
- Leibham D., Wong M.-W., Cheng T.-C., Schroeder S., Weil P.A., Olson E.N. & Perry M. ( 1994) Binding of TFIID and MEF2 to the TATA element activates transcription of the Xenopus MyoDa promoter. Mol. Cell. Biol., 14, 686–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Lin Q., Wang W., Wade P. & Wong J. ( 2002a) Specific targeting and constitutive association of histone deacetylase complexes during transcriptional repression. Genes Dev., 16, 687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Kimbrel E.A., Kenan D.J. & McDonnel D.P. ( 2002b) Direct interactions between corepressors and coactivators permit the integration of nuclear receptor-mediated repression and activation. Mol. Endocrinol., 16, 1482–1891. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P. & Faber J. ( 1956) Normal table of Xenopus laevis. North Holland, Amsterdam, The Netherlands. [Google Scholar]
- Nuclear Receptors Nomenclature Committee ( 1999) A unified nomenclature system for the nuclear receptor superfamily. Cell, 97, 161–163. [DOI] [PubMed] [Google Scholar]
- Ranjan M., Wong J. & Shi Y.-B. ( 1994) Transcriptional repression of Xenopus TRβ gene is mediated by a thyroid hormone response element located near the start site. J. Biol. Chem., 269, 24699–24705. [PubMed] [Google Scholar]
- Sachs L.M. & Shi Y.-B. ( 2000) Targeted chromatin binding and histone acetylation in vivo by thyroid hormone receptor during amphibian development. Proc. Natl Acad. Sci. USA, 97, 13138–13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs L.M., Amano T. & Shi Y.-B. ( 2001) An essential role of histone deacetylases in postembryonic organ transformations in Xenopus laevis. Int. J. Mol. Med., 8, 595–601. [DOI] [PubMed] [Google Scholar]
- Sachs L.M., Jones P.L., Havis E., Rouse N., Demeneix B.A. & Shi Y.-B. ( 2002) Nuclear receptor corepressor recruitment by unliganded thyroid hormone receptor in gene repression during Xenopus laevis development. Mol. Cell. Biol., 22, 8527–8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M., Rambaldi I., Yang X.-J. & Featherstone M. ( 2000) Cell signaling switches HOX–PBX complexes from repressors to activators of transcription mediated by histone deacetylases and histone acetyltransferases. Mol. Cell. Biol., 20, 8623–8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y., Hu X., DiRenzo J., Lazar M.A. & Brown M. ( 2000) Cofactor dynamics and sufficiency in oestrogen receptor-regulated transcription. Cell, 103, 843–852. [DOI] [PubMed] [Google Scholar]
- Sharma D. & Fondell J.D. ( 2002) Ordered recruitment of histone acetyltransferases and TRAP/mediator complex to thyroid hormone-responsive promoters in vivo. Proc. Natl Acad. Sci. USA, 99, 7934–7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J.R. ( 1993) Gene expression during metamorphosis: an ideal model for post-embryonic development. Bioessays, 15, 239–248. [DOI] [PubMed] [Google Scholar]
- Urnov F.D. & Wolffe A.P. ( 2001) An array of positioned nucleosomes potentiates thyroid hormone receptor action in vivo. J. Biol. Chem., 276, 19753–19761. [DOI] [PubMed] [Google Scholar]
- Vermaak D., Wade P.A., Jones P.L., Shi Y.-B. & Wolffe A.P. ( 1999) Functional analysis of the Sin3-histone deacetylase RPD3-RbAp48-histone H4 connection in the Xenopus oocyte. Mol. Cell. Biol., 19, 5847–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. & Brown D.D. ( 1993) Thyroid hormone-induced gene expression program for amphibian tail resorption. J. Biol. Chem., 268, 16270–16278. [PubMed] [Google Scholar]
- Wolffe A.P. ( 1997) Sinful repression. Nature, 387, 16–17. [DOI] [PubMed] [Google Scholar]
- Wong J., Patterton D., Imhof A., Guschin D., Shi Y.-B. & Wolffe A.P. ( 1998) Distinct requirements for chromatin assembly in transcriptional repression by thyroid hormone receptor and histone deacetylase. EMBO J., 17, 520–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaoita Y., Shi Y.-B. & Brown D.D. ( 1990) Xenopus laevis α and β thyroid hormone receptors. Proc. Natl Acad. Sci. USA, 87, 7090–7094. [DOI] [PMC free article] [PubMed] [Google Scholar]


