Abstract
The vertebrate eye forms by specification of the retina anlage and subsequent morphogenesis of the optic vesicles, from which the neural retina differentiates. chokh (chk) mutant zebrafish lack eyes from the earliest stages in development. Marker gene analysis indicates that retinal fate is specified normally, but optic vesicle evagination and neuronal differentiation are blocked. We show that the chk gene encodes the homeodomain-containing transcription factor, Rx3. Loss of Rx3 function in another teleost, medaka, has also been shown to result in an eyeless phenotype. The medaka rx3 locus can fully rescue the zebrafish mutant phenotype. We provide evidence that the regulation of rx3 is evolutionarily conserved, whereas the downstream cascade contains significant differences in gene regulation. Thus, these mutations in orthologous genes allow us to study the evolution of vertebrate eye development at the molecular level.
Introduction
The retina and lens of the vertebrate eye originate from an anterior territory, which comprises the neuroectodermal retina anlage and the abutting ectodermal lens-competence regions. During neurulation, the cells of the retina anlage converge at the midline in the prosencephalon, from where they then evaginate laterally to form the optic vesicles. These, in turn, induce lens formation in the adjacent competence regions.
Several genes required for the establishment of the retina anlage and its subsequent transition to optic vesicles have been identified. The homeodomain-containing transcription factors Six3 and Pax6 are essential for the determination of retinal fate in the neuroectoderm (Carl et al., 2002; Gehring, 2002; Loosli et al., 1999) and also function in lens formation (Ashery-Padan & Gruss, 2001). The retinal homeobox transcription factor Rx has a crucial function in the morphogenesis of the optic primordia to form the optic vesicles (Loosli et al., 2001; Mathers et al., 1997). Both Six3 and Rx have been shown to control proliferation in the optic primordium (Andreazzoli et al., 1999; Carl et al., 2002; Loosli et al., 2001; Mathers et al., 1997). For proper morphology of the optic vesicle, the homeobox factor Vsx2/Chx10 is required (Barabino et al., 1997).
Forward genetic screens in fish have been used to isolate an increasing number of mutations that cause defects in the developing eye (Malicki et al., 1996; Loosli et al., 2000; for a review, see Easter & Malicki, 2002). In zebrafish, two mutants have been described that affect early Wnt (Wingless/int-related)-signalling-dependent anterior–posterior patterning of the brain and, as a secondary consequence, result in eyeless or headless embryos (Kim et al., 2000; Heisenberg et al., 2001). These mutations are not specific to eye formation and also lead to severe patterning defects in other regions of the brain. So far, in the large-scale mutagenesis screens of zebrafish, surprisingly few mutations were identified that specifically affect the earliest stage of eye development.
Here, we describe the identification and analysis of a novel zebrafish mutant that lacks eyes from the earliest stages. The corresponding gene, named chokh (chk) after the Bangla word for 'eye', encodes Rx3. In view of the existing rx mutations in other vertebrate species, the isolation of a zebrafish rx3 mutation closes an important gap in our understanding of conserved regulatory networks that underlie optic vesicle morphogenesis and differentiation. This now provides the opportunity to study the evolution of vertebrate eye development by cross-species comparison at the molecular level.
Results
Zebrafish chokh mutants lack eyes
Mutants that are homozygous for the ethylnitrosourea-induced allele s399 of the chk locus lack eyes at all stages of development (Fig. 1). This phenotype is similar to medaka eyeless (el) mutants (Winkler et al., 2000). Both retinal pigmented epithelium and neuroretina are missing. A lens forms, but it is markedly reduced in size. The overall morphology of the head and trunk is not affected by the mutation. In addition, all the main subdivisions of the brain (that is, the forebrain, midbrain and hindbrain), as well as the inner ear, seem normal. The chk mutation is recessive and fully penetrant. Mutants do not show visual responses, but respond to touch and vibration; they hatch and show apparently normal swimming, but die at ∼3–4 weeks.
Figure 1.
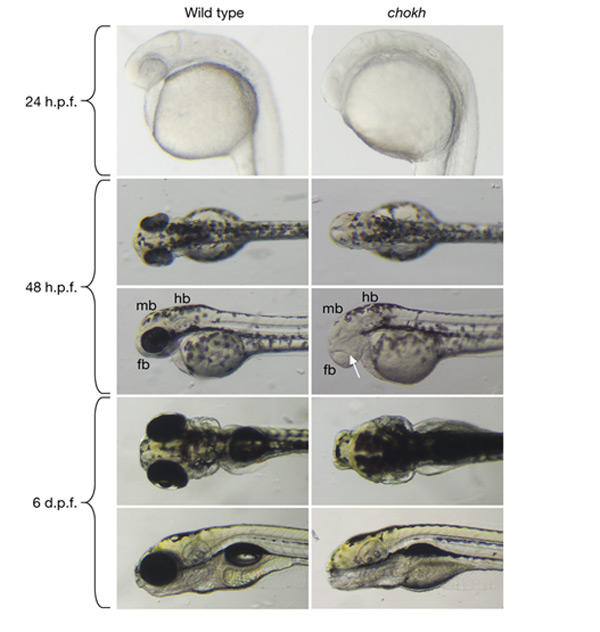
Morphological analysis of the chokh phenotype. Wild-type and mutant embryos are shown at 24 h post-fertilization (h.p.f.), 48 h.p.f. and 6 days post-fertilization (d.p.f.). Eyes are absent at all stages of development in chokh (chk) mutants. The overall morphology of the head and trunk is normal in mutants. At 48 h.p.f., a small lens is visible in chk mutants (arrow). fb, forebrain; hb, hindbrain; mb, midbrain.
The chokh mutation disrupts rx3
The chk mutation was mapped close to the polymorphic CA-repeat marker z10432 on chromosome 21 using 41 mutant embryos from a TL/WIK hybrid cross (see Methods). The zebrafish rx3 gene had previously been mapped to the same position using the T51 radiation-hybrid panel (Geisler et al., 1999). The similar phenotypes of chk and medaka el strongly suggested that rx3 might be a candidate gene. We therefore sequenced the coding region of rx3 in our mutant and discovered a nonsense mutation in the homeodomain of the gene (Fig. 2A). This mutation is expected to convert the highly conserved tyrosine codon at position 479 to a stop codon (Fig. 2B). Therefore, two-thirds of the homeodomain (including the third helix, which is indispensable for sequence-specific DNA binding) and the entire carboxy-terminal portion are deleted, resulting in a null allele (Fig. 2C).
Figure 2.
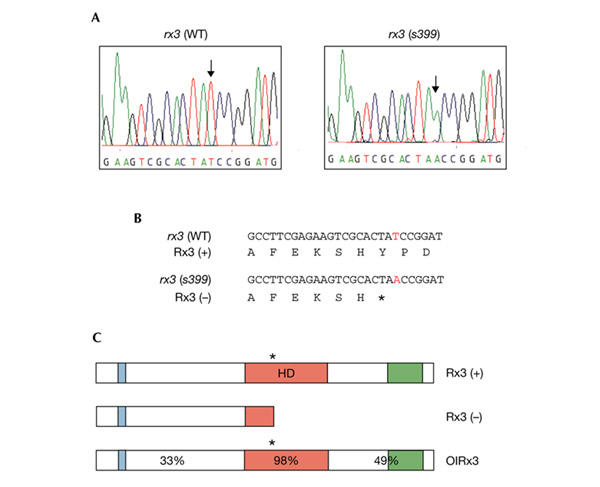
The chokh gene encodes Rx3. (A) Comparison of the sequencing trace data from wild-type and chk mutant rx3 complementary DNA. Sequencing reveals a T to A point mutation (arrows). (B) The predicted translation of the wild-type and mutant rx3 open reading frames. The mutation results in a premature stop codon (indicated by an asterisk) in the homeobox (red). (C) Comparison of zebrafish (Rx3) and medaka (OlRx3) proteins. The octapeptide (blue), homeodomain (HD; red) and the otp–aristaless–rx (OAR) domain (green) are indicated. Sequence identity at the amino-acid level is indicated for the amino- and carboxy-terminal regions and the homeodomain. The position of the nonsense mutation in the homeobox of chk and the frameshift mutation of the control plasmid (pOlRx3-fs) are indicated (asterisks). The mutation is predicted to cause a truncation of the protein.
Expression of pax6 and six3 are unaffected by loss of rx3
The homeobox transcription factors Six3 and Pax6 are key regulators of vertebrate retina specification and have been shown to function in a cross-regulatory interaction (Ashery-Padan & Gruss, 2001; Carl et al., 2002). Similar to other vertebrate species, in the zebrafish embryo Pax6 and Six3 are expressed in the eye field starting at late gastrula stages (Kobayashi et al., 1998; Püschel et al., 1992; Seo et al., 1998). We therefore used the expression of six3a (six3.1) and pax6a (pax6.1) as markers to examine the effects of loss of rx3 function on early retina development in chk mutant embryos.
At 10.5 h post-fertilization (h.p.f.), just before optic vesicle evagination, six3a expression in the anterior neural plate comprises the retina anlage. Whole-mount in situ hybridization showed that six3a expression is unaltered in chk mutant embryos (data not shown). At 11.5 h.p.f., the optic vesicles start to evaginate in wild-type embryos. This morphogenetic process does not occur in chk mutant embryos. At this stage, pax6a is expressed in the prosencephalon, which is adjacent to the head ectoderm, and more posteriorly in the hindbrain and spinal cord. In chk mutant embryos, pax6a is expressed normally in all of these domains (data not shown). Hence, the normal early expression of six3a and pax6a indicate that forebrain patterning, and particularly retinal specification, occur normally in the absence of rx3 function.
At 19 h.p.f., pax6a expression is prominent in the diencephalon, the evaginated optic vesicles and the abutting lens placodes (Fig. 3A). In chk mutant embryos, the diencephalic expression domain extends further anteriorly, which suggests that retinal progenitor cells remain in the forebrain instead of evaginating. The expression in the lens placodes is unaltered in mutant embryos, which indicates that rx3 is not required for lens induction (Fig. 3B). Consistent with this, small lenses are visible at 48 h.p.f. (see also Fig. 1). More posterior pax6a expression in the hindbrain and spinal cord is unaffected at this stage, too, which is a further indication that central nervous system (CNS) regionalization is not affected by loss of rx3.
Figure 3.
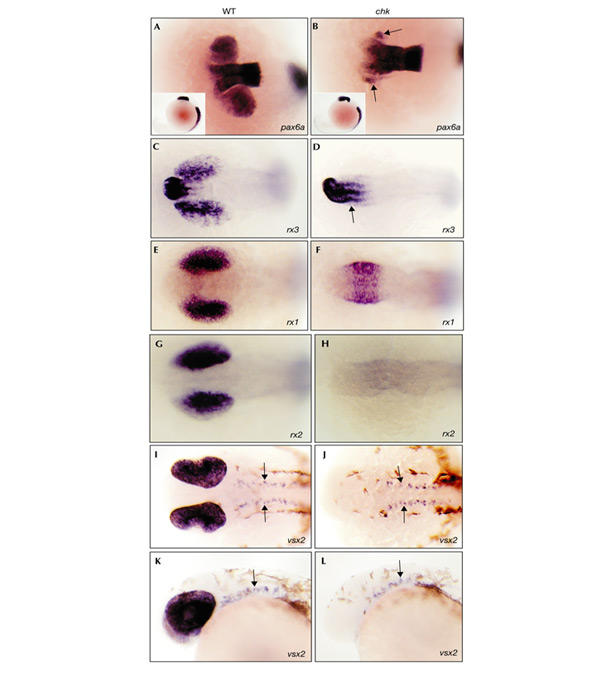
Whole-mount in situ hybridization analysis of marker genes in wild-type embryos and chokh mutants. (A–J) Dorsal views. Lateral views are shown in (K) and (L), and in the insets in (A) and (B). Anterior is to the left in all panels. (A,B) Wild-type (WT) (A) and mutant (B) pax6a expression in the forebrain and optic vesicles at 19 h.p.f. Note the anteriorly extended pax6a expression in the mutant. Arrows indicate pax6a expression in the lens placodes. pax6a expression along the anterior–posterior axis is identical in wild-type and mutant embryos (insets in (A) and (B), respectively). (C,D) rx3 expression in the ventral forebrain and optic vesicles of wild-type (C) and mutant (D) embryos at 14 h.p.f. Note the ectopic rx3 expression in the forebrain of mutants (D) (arrow). (E,F) rx1 is expressed in the optic vesicles of wild-type embryos (E) and in the forebrain of mutant embryos (F) at 14 h.p.f. Expression in the forebrain of the mutant (F) coincides with the position of the evaginated optic vesicles in wild-type embryos (E). (G,H) rx2 is expressed in wild-type optic vesicles (G) but not in mutants (H) at 14 h.p.f. (I–L) vsx2 expression in the optic cup and ventral midbrain and hindbrain (arrows) of wild-type (I,K) and mutant (J,L) embryos at 26 h.p.f. Midbrain and hindbrain expression is unaffected by the mutation.
Rx3 is required for normal expression of Rx1 and Rx2
The enlarged pax6a expression domain in the forebrain of mutant embryos suggested that retinal progenitor cells, once specified, remain in the forebrain. To test this further, we used other marker genes that are expressed in retinal progenitor cells. rx3 is initially expressed in the anteriormost neural plate, in a region which gives rise to the ventral forebrain and the optic primordia (Chuang et al., 1999). At 14 h.p.f., its expression is detectable in the presumptive anterior hypothalamus and the proximal optic vesicles (Fig. 3C). In chk mutant embryos, the rx3 expression domain in the ventral forebrain extends posteriorly into the region from which the optic vesicles should evaginate, consistent with the abnormal location of retinal progenitor cells (Fig. 3D). Furthermore, the finding that rx3 is expressed normally both in the presumptive hypothalamus and in retinal progenitor cells shows that rx3 does not function in an autoregulatory feedback loop, similar to the situation in medaka (Loosli et al., 2001).
We used the retina-specific expression of the homeobox genes rx1 and rx2 to examine retina development in chk mutants in more detail. From 14 h.p.f. onwards, rx1 and rx2 are specifically expressed in the retinal progenitor cells of the optic vesicle (Fig. 3E,G). At that stage, rx1 is expressed, although at reduced levels, in the ventral forebrain of mutant embryos at the position from which the optic vesicles normally evaginate, whereas at 26 h.p.f. rx1 expression is absent (Fig. 3F; and data not shown). rx2 expression, however, is completely abolished at all stages in mutant embryos (Fig. 3H). Thus, the retinal progenitor-specific expression of rx1 in the forebrain of chk mutant embryos provides further independent evidence for abnormally located retinal progenitor cells due to failure of optic vesicle evagination.
Retinal progenitors do not differentiate in chk mutants
We examined whether retinal progenitor cells in the ventral forebrain of chk mutant embryos undergo differentiation. At 26 h.p.f., the homeobox gene vsx2 is expressed in mitotically active retinal progenitor cells before terminal differentiation. In the midbrain and hindbrain, vsx2 is expressed in a ventrally located, paired row of cells (Barabino et al., 1997). Retinal expression of vsx2 is not detectable at 26 h.p.f. in chk mutant embryos, whereas the expression in the double row of the midbrain and hindbrain is not affected (Fig. 3J,L). Thus, retinal progenitor cells do not reach this early stage of development in mutant embryos.
The basic helix–loop–helix gene ath5 (lak, atoh7) is the earliest known marker for differentiating retinal ganglion cells and is essential for their development during the first differentiation wave in the retina (Kay et al., 2001; Masai et al., 2000). In wild-type embryos at 36 h.p.f., ath5 is expressed in the proximal retina in presumptive ganglion cells (Fig. 4D). In mutant embryos, ath5 expression is not detectable, indicating that the first step of retinal differentiation is already disrupted in chk mutant embryos (Fig. 4E).
Figure 4.
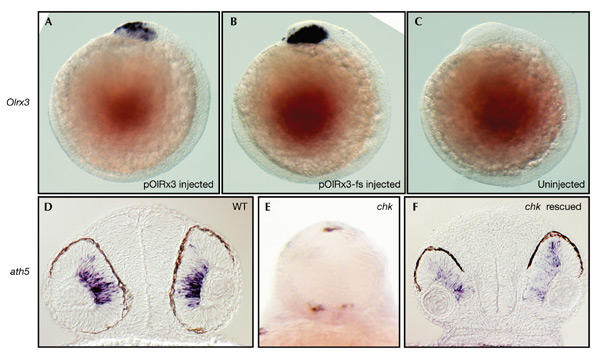
Rescue of chokh mutants by the medaka rx3 locus. (A–C) Lateral views, with the anterior to the left. (D) and (F) show transverse sections at the level of the eye. (E) is a frontal view. (A,B) Medaka rx3 (Olrx3) expression at 12 h post-fertilization in wild-type (WT) embryos injected with the rescue plasmid (pOlRx3; A) and control plasmid (pOlRx3-fs; B). Note the specific expression in the ventral forebrain and optic vesicles of injected embryos. (C) The same Olrx3 probe does not cross-hybridize with uninjected control embryos. (D–F) ath5 is expressed in ganglion cells of wild-type retinae (D) at 36 h.p.f., but not in the mutant (E) (whole-mount, to show the absence of staining). The dark spots in (E) are due to pigmentation. Injection of pOlRx3 restores morphology and wild-type ath5 expression (F, compare with D).
The chk mutant is rescued by medaka rx3
We analysed whether injection at the one-cell stage of a plasmid containing medaka rx3 and its regulatory regions (Loosli et al., 2001) is able to rescue the chk phenotype. As a control, we injected a frameshift derivative of the plasmid (Loosli et al., 2001). Because a conserved BspEI restriction site was used to introduce the frameshift mutation, the same portion of the medaka rx3 protein was deleted in the control plasmid and by the chk mutation (Fig. 2C). Wild-type embryos injected with the control plasmid were analysed for medaka rx3 (Olrx3) expression at 12 h.p.f. by whole-mount in situ hybridization. For both plasmids, specific and strong Olrx3 expression was detectable in the ventral forebrain and evaginating optic vesicles at this stage (Fig. 4A–C). Thus, both plasmids contain the essential regulatory elements for stage- and region-specific rx3 expression in zebrafish embryos.
In the rescue experiments, we genotyped the injected embryos by PCR, followed by digestion with BspEI, which cuts the wild-type DNA, but not the chk mutant DNA. In a representative experiment, on injecting the pOlRx3 plasmid, we obtained 15 eyeless (non-rescued) mutants among 68 injected embryos at 48 h.p.f. We genotyped the 53 embryos that had eyes and found that 7 of them had homozygous mutant genotypes. This corresponds to a rescue rate of 32% (7 out of 22 injected mutants), which is similar to the result obtained with the same plasmid in medaka el mutants (37%; Loosli et al., 2001). Injection of the control plasmid, pOlRx3-fs (which contains a frameshift mutation in rx3), did not rescue any of the mutants. For this plasmid, all 34 genotyped embryos (with eyes) were either heterozygous or were homozygous wild-type.
Interestingly, most rescued mutants (six out of seven in the experiment described above) seemed to be morphologically normal. The size and morphology of their eyes were indistinguishable from wild-type embryos. This result was reproducible and was obtained by double-blind scoring of rescued, control-injected and uninjected embryos.
We analysed ath5 expression in chk mutant embryos that were injected with the pOlRx3 plasmid and found that ath5 expression is restored in the optic cups of rescued embryos at 36 h.p.f. (Fig. 4F). Thus, molecular analysis confirms the result of our morphological inspections, showing that morphogenesis and differentiation of retinal progenitor cells in chk mutants is fully restored by the medaka rx3 locus.
Discussion
We have identified a novel point mutation in zebrafish that results specifically in a lack of eyes. In summary, map position, sequence analysis and rescue by complementation show that the chk gene encodes the zebrafish orthologue of the homeodomain transcription factor Rx3. The nonsense mutation that we identified is predicted to lead to a complete loss of function, as it deletes most of the DNA-binding homeodomain and the entire C-terminal portion. We showed that retinal progenitor cells fail to evaginate as optic vesicles, and that subsequent differentiation of the optic primordia is blocked. Other regions of the brain are not affected. Thus, in contrast to other zebrafish genes that affect anterior brain development, chk/rx3 is specific for the developing optic primordia.
Our analysis of marker gene expression shows that rx3 requirement is limited to the portion of the neural plate that will give rise to the optic primordia. Retinal specification and the formation of the lens placode are normal, consistent with neural-plate-specific expression of rx3.
Recently, a loss-of-function mutation of rx3 has been described in the teleost medaka that also results in the complete absence of retinae (Loosli et al., 2001). In crossspecies rescue experiments, we show that injection of the medaka rx3 locus results in stage- and region-specific expression of this gene in the zebrafish embryo that resembles the endogenous pattern. This indicates that, in addition to the open reading frame of rx3, the respective regulatory elements are also highly conserved. This is further supported by the high frequency of complete rescues that were obtained. Therefore, the regulatory region of rx3 can be expected to share conserved motifs, an issue that will be addressed in the future.
In zebrafish, all three rx paralogues are expressed in the evaginating optic vesicles (Chuang et al., 1999), whereas in medaka only rx3 is expressed during optic vesicle evagination. Medaka rx1 and rx2 are first expressed in the fully evaginated optic vesicle just before the transition to optic cups (Loosli et al., 2001; F.L. and J.W., unpublished data). Thus, there is considerably more temporal overlap of rx gene expression during the critical period in zebrafish than in medaka. However, the phenotypic consequences of loss of rx3 are equally dramatic in both species. This suggests that the weak and transient rx1 expression in chk mutants is not sufficient for optic vesicle evagination because either the expression level is too low or, alternatively, rx1 and rx3 have non-redundant functions. Future experiments will address this issue.
The closely related genes rx1 and rx2 are specifically expressed in retinal progenitor cells of both species. Interestingly, rx1 and rx2 are differentially affected by the respective rx3 mutations. Whereas in the medaka el mutation both rx1 and rx2 are still expressed, albeit at reduced levels (Loosli et al., 2001; F.L. and J.W., unpublished data), only rx1 is initially expressed in the zebrafish chk mutant, whereas rx2 expression is completely abolished. Hence, only in zebrafish are rx1 and rx2 completely dependent on rx3 function. This finding shows a different transcriptional control of orthologues in these two vertebrates, which predicts an interesting divergence of the respective regulatory elements.
In contrast to rx1 and rx2, retinal vsx2 is lost in both el and chk mutants, suggesting that residual rx1 and rx2 expression cannot rescue vsx2 expression in the medaka embryo. This indicates that, probably indirectly, vsx2 depends mainly on rx3 function.
Although they are highly conserved in both structure and expression pattern, several genes of the downstream cascade are differentially affected by the medaka and zebrafish rx3 mutations. The availability of rx3 mutations in both species offers a unique paradigm to study the divergence of gene function by changes in gene regulation. The ongoing whole-genome sequencing projects of medaka and zebrafish will provide the tools that are needed to compare the regulatory elements of these genes and address the evolution of regulatory interactions at the molecular level.
Methods
Fish stocks and mapping.
The chks399 mutation was originally discovered in D. Stainier's laboratory in an unrelated screen for ethylnitrosourea-induced mutations that disrupt organ development (H.A. Field, E.O., H.V. and D. Stainier, unpublished data) and is now maintained in a TL background. The mutant name chokh (chk) was submitted to the Zebrafish Information Network database (http://www.zfin.org). For mapping, an s399 TL carrier was crossed to a WIK wild-type fish, and heterozygous F1 progeny were identified in the next generation. Bulk-segregant analysis was performed on 41 homozygous mutants and 48 siblings from one cross to determine the linkage group, using a panel of 192 simple-sequence repeat markers (CA repeats), which were chosen for their high rate of polymorphisms between TL and WIK strains (K.F.-B. and H.B., unpublished data). Single-embryo analysis was used to confirm bulk linkage.
Cloning of zebrafish rx3.
Total RNA was isolated from 2–3-day wild-type and chk mutant embryos using Trizol (Gibco BRL). The BD Biosciences SMART RACE complementary DNA amplification kit was used to generate 5′ cDNA. The coding region of rx3 was amplified by nested PCR (outer primers: NUP primer and 5′-AGTGGCCAGCTGCATTGT-3′; 35 cycles of 94 °C for 30 s, 52 °C for 30 s, 72 °C for 1.5 min; inner primers: 5′-CTTTTCTGCCGGGACAGTCT-3′ and 5′-AAAACGGATCCAGACCACTG-3′, 35 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 1.5 min). The PCR product was gel extracted and sequenced using internal primers of the nested PCR reaction.
DNA injections and genotyping of rescued embryos.
One-cell embryos from an incross of two heterozygous chk carriers were injected with 200 ng μl−1 of a plasmid that contains medaka rx3 and regulatory regions (pOlRx3) or a control plasmid that contains a frameshift mutation in rx3 (pOlRx3-fs). pOlRx3 carries the entire rx3 locus. This plasmid fully rescues the medaka el mutant, whereas the pOlRx3-fs plasmid has no rescue activity (Loosli et al., 2001). Embryos were scored for the presence or absence of eyes at 48 h.p.f., and DNA was isolated from individual embryos. A 426-bp fragment of rx3 was amplified by PCR with the following primers: 5′-GGATGATGAAAACCCGAAGA-3′ and 5′-TGGGCTGAATAAACAAACG-3′ (35 cycles of 94 °C for 30 s, 52 °C for 30 s, 72 °C for 1 min). PCR products were digested with BspE1 overnight and run on a 2% agarose gel.
In situ hybridizations and vibratome sections.
Whole-mount in situ hybridizations using digoxygenin-labelled antisense RNA probes and vibratome sections were carried out as described in Loosli et al. (2001).
Acknowledgments
We thank D. Stainier and H.A. Field for generously providing the chokh mutant, and P. Page-McCaw for assistance with primer design and sequence analysis. We thank S. Wilson, M. Jamrich and C. Neumann for probes, M. Carl and C. Neumann for critical comments on the manuscript, and A. Krone and E. Grzebisz for excellent technical assistance. This work was supported by grants from the National Insitutes of Health (EY13855, to H.B.), the Packard foundation (to H.B), the European Union (to J.W.) and the Human Frontier Science Program Organization (to J.W.).
References
- Andreazzoli M., Gestri G., Angeloni D., Menna E. & Barsacchi G. ( 1999) Role of Xrx1 in Xenopus eye and anterior brain development. Development, 126, 2451–2460. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R. & Gruss P. ( 2001) Pax6 lights-up the way for eye development. Curr. Opin. Cell Biol., 13, 706–714. [DOI] [PubMed] [Google Scholar]
- Barabino S.M., Spada F., Cotelli F. & Boncinelli E. ( 1997) Inactivation of the zebrafish homologue of Chx10 by antisense oligonucleotides causes eye malformations similar to the ocular retardation phenotype. Mech. Dev., 63, 133–143. [DOI] [PubMed] [Google Scholar]
- Carl M., Loosli F. & Wittbrodt J. ( 2002) Six3 inactivation reveals its essential role for the formation and patterning of the vertebrate eye. Development, 129, 4057–4063. [DOI] [PubMed] [Google Scholar]
- Chuang J.C., Mathers P.H. & Raymond P.A. ( 1999) Expression of three Rx homeobox genes in embryonic and adult zebrafish. Mech. Dev., 84, 195–198. [DOI] [PubMed] [Google Scholar]
- Easter S.S. & Malicki J.J. ( 2002) The zebrafish eye: developmental and genetic analysis. Results Probl. Cell Differ., 40, 346–370. [DOI] [PubMed] [Google Scholar]
- Gehring W.J. ( 2002) The genetic control of eye development and its implications for the evolution of the various eye-types. Int. J. Dev. Biol., 46, 65–73. [PubMed] [Google Scholar]
- Geisler R. et al. ( 1999) A radiation hybrid map of the zebrafish genome. Nature Genet., 23, 86–89. [DOI] [PubMed] [Google Scholar]
- Heisenberg C.P. et al. ( 2001) A mutation in the Gsk3-binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes Dev., 15, 1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay J.N., Finger-Baier K.C., Roeser T., Staub W. & Baier H. ( 2001) Retinal ganglion cell genesis requires lakritz, a zebrafish atonal homolog. Neuron, 30, 725–736. [DOI] [PubMed] [Google Scholar]
- Kim C.H., Oda T., Itoh M., Jiang D., Artinger K.B., Chandrasekharappa S.C., Driever W. & Chitnis A.B. ( 2000) Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature, 407, 913–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Toyama R., Takeda H., Dawid I.B. & Kawakami K. ( 1998) Overexpression of the forebrain-specific homeobox gene six3 induces rostral forebrain enlargement in zebrafish. Development, 125, 2973–2982. [DOI] [PubMed] [Google Scholar]
- Loosli F., Winkler S. & Wittbrodt J. ( 1999) Six3 overexpression initiates the formation of ectopic retina. Genes Dev., 13, 649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loosli F., Köster R.W., Carl M., Kuhnlein R., Henrich T., Mucke M., Krone A. & Wittbrodt J. ( 2000) A genetic screen for mutations affecting embryonic development in medaka fish (Oryzias latipes). Mech. Dev., 97, 133–139. [DOI] [PubMed] [Google Scholar]
- Loosli F. et al. ( 2001) Medaka eyeless is the key factor linking retinal dertermination and eye growth. Development, 128, 4035–4044. [DOI] [PubMed] [Google Scholar]
- Malicki J. et al. ( 1996) Mutations affecting development of the zebrafish retina. Development, 123, 263–273. [DOI] [PubMed] [Google Scholar]
- Masai I., Stemple D.L., Okamoto H. & Wilson S.W. ( 2000) Midline signals regulate retinal neurogenesis in zebrafish. Neuron, 27, 251–263. [DOI] [PubMed] [Google Scholar]
- Mathers P.H., Grinberg A., Mahon K.A. & Jamrich M. ( 1997) The Rx homeobox gene is essential for vertebrate eye development. Nature, 387, 603–607. [DOI] [PubMed] [Google Scholar]
- Püschel A.W., Gruss P. & Westerfield M. ( 1992) Sequence and expression pattern of pax-6 are highly conserved between zebrafish and mice. Development, 114, 643–651. [DOI] [PubMed] [Google Scholar]
- Seo H.C., Drivenes O., Ellingsen S. & Fjose A. ( 1998) Expression of two zebrafish homologues of the murine Six3 gene demarcates the initial eye primordia. Mech. Dev., 73, 45–57. [DOI] [PubMed] [Google Scholar]
- Winkler S., Loosli F., Henrich T., Wakamatsu Y. & Wittbrodt J. ( 2000) The conditional medaka mutation eyeless uncouples patterning and morphogenesis of the eye. Development, 127, 1911–1919. [DOI] [PubMed] [Google Scholar]


