Abstract
Microinjection of DNA is now the most widespread method for generating transgenic animals, but transgenesis rates achieved this way in higher mammals are extremely low. To address this longstanding problem, we used lentiviral vectors carrying a ubiquitously active promoter (phosphoglycerate kinase, LV-PGK) to deliver transgenes to porcine embryos. Of the 46 piglets born, 32 (70%) carried the transgene DNA and 30 (94%) of these pigs expressed the transgene (green fluorescent protein, GFP). Direct fluorescence imaging and immunohistochemistry showed that GFP was expressed in all tissues of LV-PGK transgenic pigs, including germ cells. Importantly, the transgene was transmitted through the germ-line. Tissue-specific transgene expression was achieved by infecting porcine embryos with lentiviral vectors containing the human keratin K14 promoter (LV-K14). LV-K14 transgenic animals expressed GFP specifically in basal keratinocytes of the skin. Finally, infection of bovine oocytes after and before in vitro fertilization with LV-PGK resulted in transgene expression in 45% and 92% of the infected embryos, respectively.
Introduction
The introduction of foreign genes into early embryos is a powerful tool for developmental studies and for the production of transgenic animals. Pronuclear injection of DNA into zygotes (Gordon et al., 1980) is now the most widespread technique used for generating transgenic mice. However, this technique has had only limited success in higher mammals because of inherent technical problems and low efficacy. Therefore, the costs for the production of transgenic livestock by pronuclear DNA microinjection are immense.
An alternative method is viral transgenesis—the use of recombinant viruses to deliver genes into the embryo. Retroviral vectors based on Moloney murine leukaemia virus transfer genes efficiently into murine, porcine and bovine embryos (Cabot et al., 2001; Chan et al., 1998; Jaenisch, 1976); however, retroviruses are subject to epigenetic modification, and retroviral expression is shut off during embryogenesis (Jaenisch, 1976) or shortly after birth (Chan et al., 1998).
Vectors derived from lentiviruses (for a review, see Pfeifer & Verma, 2001), which belong to the family of complex retroviruses, have been shown to transduce human embryonic stem cells and pre-implantation embryos of mice and rats (Lois et al., 2002; Pfeifer et al., 2002). However, attempts to generate transgenic monkeys by lentiviral infection of pre-implantation embryos were not successful and resulted in gene transfer only into extraembryonic tissues (Wolfgang et al., 2001), raising the question whether lentiviral vectors can be used for transgenesis in higher mammals.
We used lentiviral vectors to achieve reproducibly high transgenesis rates in swine and efficient gene transfer into bovine embryos and oocytes.
Results
Pig embryos were collected from gonadotropin-stimulated donor animals after artificial insemination. Single-cell embryos were infected with high titre (109–1010 infectious units per millilitre) recombinant lentiviral vector pseudotyped with vesicular stomatitis virus envelope glycoprotein G (Naldini et al., 1996) by injection into the perivitelline space. The lentiviral vector carries the green fluorescent protein (GFP) reporter transgene, a central polypurine tract (cPPT) and the post-transcriptional regulatory element of woodchuck hepatitis virus to increase transduction efficiency (Follenzi et al., 2000; Zufferey et al., 1999). The lentiviral vector construct LV-PGK contains the phosphoglycerate kinase promoter to achieve ubiquitous expression of the reporter transgene (GFP; Fig. 1A).
Figure 1.
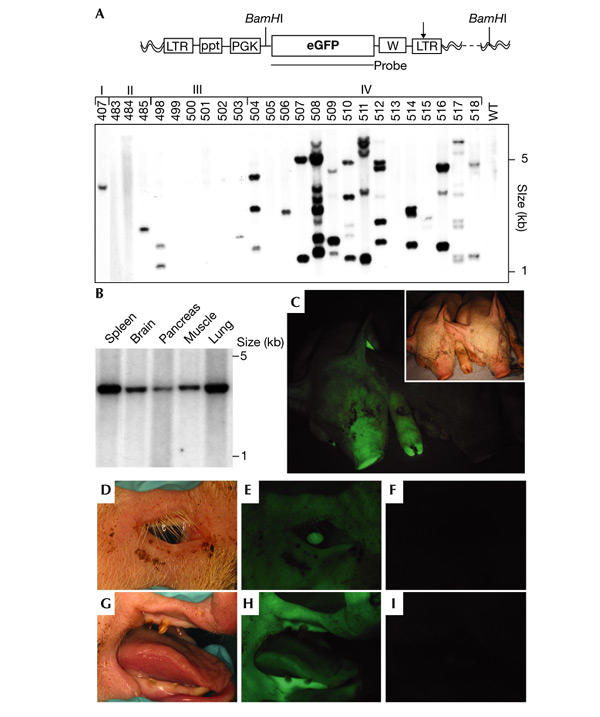
Southern blot analyses and in vivo imaging. (A) The lentiviral vector carrying the phosphoglycerate kinase promoter (LV-PGK, top). Arrow, self-inactivating mutation; eGFP, enhanced green fluorescent protein; LTR, long terminal repeat; ppt, polypurine tract; W, woodchuck hepatitis responsive element; wavy lines, pig genome. Southern blot of BamHI-digested genomic DNA (bottom) isolated from skin samples of piglets generated by subzonal injection of LV-PGK (pregnancies I–IV) and one age-matched control animal (WT). (B) Southern blot analysis of DNA extracted from animal #407. Spleen, brain, pancreas, muscle and lung carry one viral integrant. (C–I) In vivo fluorescence imaging. GFP expression was observed by direct epifluorescence in the skin and claws of #511 (C, left), but not in the age-matched control animal (C, right). Inset shows the bright-field image. Green fluorescence was also observed in the eye (D, E), gingival tissue and tongue (G, H) of the transgenic animal, but not in the eye (F) and snout (I) of the control.
After injection of LV-PGK, embryos were transferred endoscopically into hormonally synchronized recipient females. Six pregnancies resulted in the birth of 46 piglets (Table 1). No significant differences were observed between the number of pregnancies derived from transfers of virus-injected and control (buffer-injected) embryos (not shown).
Table 1.
Transgenesis rate and green fluorescent protein expression in the 46 piglets derived from subzonal injection of LV-PGK (six pregnancies) and 16 piglets from subzonal injection of LV-K14 (two pregnancies)
| LV-PGK | LV-K14 | |||||||
|---|---|---|---|---|---|---|---|---|
| Pregnancies | I | II | III | IV | V | VI | Ik14 | IIk14 |
| Embryos transferred | 58 | 27 | 46 | 43 | 32 | 38 | 52 | 34 |
| Animals born | 1 * | 3 | 6 | 15 | 10 | 11 | 8 | 8 |
| Transgenic animals | 1 | 1 | 2 | 13 | 5 | 10 | 2 | 0 |
| GFP+ animals | 1 | 0 | 2 | 13 | 4 | 10 | 2 | 0 |
*Stillborn pig. The ratio of viral titres of LV-PGK (phosphoglycerate kinase) to LV-K14 is 4:1. GFP, green fluorescent protein.
To analyse lentiviral integration and the number of proviral integrants, Southern blot analysis (Fig. 1A) was performed on genomic DNA digested with BamHI, which cuts only once in the lentiviral vector. Thirty-two (70%) of the animals that developed from subzonal injection of LV-PGK carried the provirus (Fig. 1A, and data not shown). In these animals, the number of proviruses present in the genome ranged from 1 to 20 copies, and the mean copy number was 4.6 ± 0.9. Integrated lentivirus was detected in organs derived from all three primary germ layers (mesoderm, ectoderm and endoderm) and extraembryonic tissues (Fig. 1B, and not shown). The number of transgene copies were identical in all tissues analysed (Fig. 1B).
Transgene expression was assayed first in whole animals by in vivo fluorescence imaging (Pfeifer et al., 2001). Of the 46 animals born, 26 pigs expressed GFP at levels detectable by direct fluorescence, whereas the age-matched control animals did not exhibit green fluorescence (Fig. 1C). In the GFP-positive animals, all tissues accessible to this non-invasive technique—skin, claws, eye, tongue and oral mucosa (Fig. 1C–I)—exhibited green fluorescence. Analysis of internal organs from animal #511 by direct fluorescence imaging showed that GFP was expressed in all organs (Fig. 2A–D). Skin (ectoderm), cerebellum (neuro-ectoderm), kidney (mesoderm) and pancreas (endoderm) showed strong green fluorescence, whereas organs of an age-matched control did not fluoresce (Fig. 2A–D).
Figure 2.

Analysis of green fluorescent protein expression by fluorescence imaging and immunohistochemistry. (A–D) Fluorescence imaging of skin (A), cerebellum (B), kidney (C) and pancreas (D). All organs of transgenic animal #511 fluoresced (A and C, left; B and D, bottom), whereas no significant fluorescence was detectable in the control organs (A and C, right; B and D, top). Insets, bright-field photographs. (E–L) Histological analysis of green fluorescent protein (GFP) expression by direct fluorescence and immunohistochemistry. (E, I) Expression of GFP in the skin. Epidermal and dermal cells are GFP positive. The inset shows a representative example of an epidermal ridge with epidermal (white asterisk) and dermal cells being GFP positive. (F, J) Analysis of GFP expression in the cerebellum. Red arrowheads indicate Purkinje cells; white asterisk, granule cell layer; inset, border between molecular and granule cell layer. (G, K) Cortical sections of transgenic kidney express GFP in glomeruli (red arrowheads), proximal tubules (white arrows) and distal tubules (yellow arrow). The inset shows a higher magnification of the lower left glomerulus. (H, L) Detection of GFP- and insulin-expressing cells in the pancreas by anti-GFP (brown) and anti-insulin staining (blue). Red arrowheads, double-stained cells; inset, islet of Langerhans. Shown are fluorescence images of organs (top), epifluorescence (middle) and immunohistochemistry against GFP (bottom) of consecutive sections. Nuclear stain (blue staining in E–H), DAPI; scale bars, 50 μm.
At a cellular level, GFP expression was analysed by direct fluorescence imaging of sections and by immunohistochemistry. We focused on skin, cerebellum, kidney and pancreas. In skin sections the transgene was expressed in all layers of the epidermis and dermis (Fig. 2E,I). In the cerebellum, GFP expression was strongest in the granular layer, where numerous granule cells were GFP positive (Fig. 2F,J; asterisk). Expression of GFP was also observed in Purkinje cells (Fig. 2J, arrowheads) and in the molecular layer (Fig. 2F,J). Cortical sections of the kidney contained GFP-positive cells in glomeruli and distal tubules (Fig. 2G,K, arrowheads and yellow arrow). The highest level of GFP expression was observed in the proximal tubules (Fig. 2K, white arrows). Sections of the pancreas showed bright fluorescence (Fig. 2H). Immunofluorescence staining with polyclonal antibodies (Abs) against insulin performed simultaneously with GFP staining using monoclonal anti-GFP Abs showed expression of the lentivirally delivered transgene in insulin-expressing pancreatic islets (Fig. 2L, arrowheads).
To study whether recombinant lentiviruses can be used to express transgenes in specific porcine tissues, we constructed a lentiviral vector (LV-K14) in which transgene expression is driven by a skin-specific promoter. We chose the human keratin K14 gene promoter because it has been shown to be strongly active in the dividing cells of the epidermal basal layer (basal keratinocytes), hair follicles and oral epithelia. Most importantly, the K14 promoter has been used to establish skin-specific expression of various transgenes in mice (Munz et al., 1999; Vassar et al., 1989). As described above, LV-K14 was injected into the perivitelline space of single-cell porcine embryos. Two animals were born alive that carried the LV-K14 provirus (#519 and #526). Fluorescence imaging showed that GFP was expressed in the skin and snout of these piglets (Fig. 3A), whereas all other tissues were GFP negative—including cerebellum, pancreas and kidney (Fig. 3B–D). Epifluorescent examination and immunostaining of frozen skin sections showed that GFP expression was limited to the basal layer of the epidermis and hair follicles (Fig. 3E,I). Transgene expression was not detectable in suprabasal epidermal layers and in the dermis. Sections of the cerebellum (Fig. 3F,J), kidney (Fig. 3G,K) and pancreas (Fig. 3H,L) were GFP negative. However, Southern blot analyses of animal #526 showed the presence of an identical number of LV-K14 proviruses in all organs including pancreas, kidney and skin (Fig. 4A). Western blotting confirmed the restriction of transgene expression to the skin in LV-K14 transgenic pigs (Fig. 4B).
Figure 3.
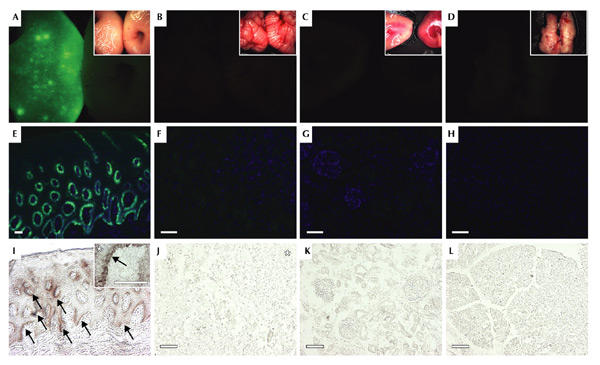
Tissue-specific transgene expression in LV-K14 transgenic pig. (A–D) Fluorescence imaging of isolated tissues. The skin (A, left) of pig #526 showed green fluorescence, whereas cerebellum (B, left), kidney (C, left) and pancreas (D, left) did not fluoresce. No fluorescence was detectable in the organs of the age-matched control (A–D, right). Insets, bright-field photographs of the corresponding fluorescence images. (E–L) Histological analysis of green fluorescent protein (GFP) expression in animal #526. (E, I) Analysis of transgene expression in skin sections. GFP expression was only detected in the basal layer of the epidermis and hair follicles (I, arrows). The inset shows a higher magnification of a typical junction of epidermis (white asterisk) and dermis; the inset arrow indicates basal keratinocytes. In the cerebellum (F, J), (asterisk, granule cell layer), kidney (G, K) and the pancreas (H, L), neither green fluorescence nor GFP-specific immunostaining were detectable. Shown are fluorescence images of organs (top), epifluorescence images (middle) and immunohistochemistry against GFP (bottom) of consecutive sections. Nuclear stain (blue in E–H), DAPI; scale bars, 50 μm.
Figure 4.
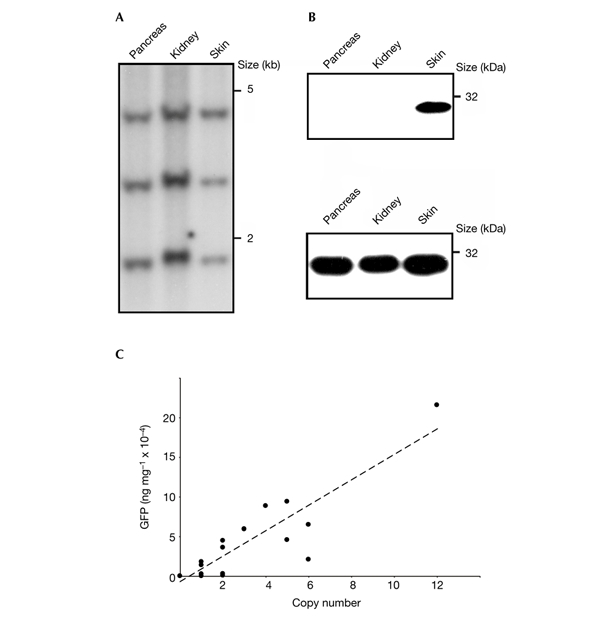
Analyses of transgene expression. (A) Southern blot analysis of vector integration in pancreas, kidney and skin of animal #526 (see also Fig. 3). (B) Western blot analyses of transgene expression using anti-green flourescent protein (GFP) antibodies on tissue samples isolated from a LV-K14 (#526, top) and a LV-PGK transgenic pig (#511, bottom). (C) Correlation between proviral copy number and GFP expression levels quantified by immunoblotting.
To determine the effect of copy number on transgene expression levels, we performed western blot analyses on tissue samples isolated from pigs carrying LV-PGK (pregnancies I–IV, Fig. 4C). Interestingly, the relationship between copy number and GFP concentration was almost identical in low (for example, two integrants) and high (for example, 12 integrants) copy animals. Transgene expression was detected by immunoblotting in 30 (94%) out of 32 transgenic animals and increased over a broad range almost linearly with increasing lentiviral vector number (Fig. 4C, and data not shown). All animals that carried more than two proviral copies expressed GFP (Fig. 4C and Table 1).
An important question is whether lentiviral gene transfer into pre-implantation embryos results in germ-line transgenic animals. Direct fluorescence imaging of sections of the testis of newborn pigs derived from subzonal injection of LV-PGK revealed GFP-positive cells in the seminiferous tubules (Fig. 5A,B). Staining with the Dolichos biflorus agglutinin (DBA), which specifically binds spermatogonial cells during the first weeks after birth (Ertl et al., 1992), identified the GFP-positive cells as spermatogonia (Fig. 5C–F). To address whether the lentivirally delivered transgene can be transmitted through the germ line, we obtained sperm from pig #507 (see also Fig. 1A). The presence of LV-PGK in spermatozoa was detected by fluorescence in situ hybridization (FISH) analysis (Fig. 5G, inset). Flow cytometer analysis showed that GFP was expressed in sperm of animal #507 (Fig. 5G). In addition, the transgenic sperm was used to inseminate one wild-type female pig. Subsequently, embryos at the 2–4-cell stage were isolated and analysed by nested PCR. We detected the transgene in 4 out of 11 embryos, which clearly shows that LV-PGK is transmitted through the germ line (Fig. 5H).
Figure 5.
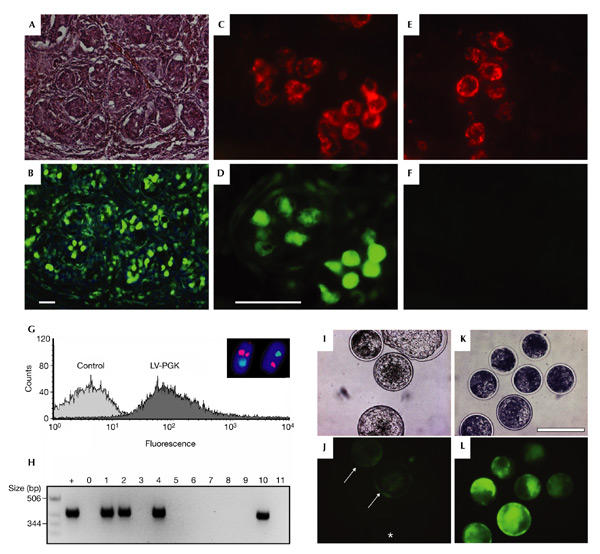
Analyses of transgene expression, transduction of germ cells and lentiviral transduction of bovine blastocysts. (A, B) Histological analysis of testicular sections of a LV-PGK transgenic piglet. Green fluorescent protein (GFP)-positive cells can be seen in the seminiferous tubules. Shown are the haematoxylin and eosin staining (A) and epifluorescent image (B). Scale bar, 50 μm. (C–F) Detection of transgene expression in spermatogonia of a newborn LV-PGK piglet (C, D), as detected by Dolchios biflorus agglutinin (DBA) staining (red). Control spermatogonia are GFP-negative (E, F). Scale bar, 50 μm. (G) Flow cytometer analysis of GFP expression in spermatozoa of a control animal and animal #507 (LV-PGK). Inset, photomicrograph showing representative fluorescence in situ hybridization results. Sperm nuclei of #507 bearing red signals for LV-PGK and blue signals for the Y chromosome. (H) Detection of the transgene by nested PCR in 2–4-cell stage embryos derived from a mating of animal #507 with a wild-type female. Four of the 11 embryos carry the transgene; +, positive control (skin sample of #407); 0, empty lane; 1–11, embryos analysed. (I–L) Analysis of GFP expression in bovine blastocysts derived from subzonal injection of zygotes (I, J) and oocytes (K, L) with LV-PGK. Arrowheads and asterisk, GFP-positive and -negative zygote-derived blastocysts, respectively. Shown are the bright fields (I, K) and the fluorescence images (J, L). Nuclear stain (blue in B and inset G), DAPI. Scale bar, 200 μm.
To determine whether lentiviral transgenesis could also work in other relevant livestock species, we infected bovine zygotes or oocytes with the lentiviral vectors. Two hundred and twenty-seven zygotes were injected subzonally with LV-PGK and 45% ± 22% (n = 4 experiments) of the in vitro developing blastocysts expressed GFP (Fig. 5I,J). Lentiviral infection of zygotes resulted in GFP-positive blastocysts at more than double the efficiency of conventional pronuclear injection (17%; Chan et al., 1998). The data shown so far resulted from injection of virus after fertilization of oocytes. Next, we injected lentivirus before in vitro fertilization, to allow for viral integration into the bovine genome before a nuclear membrane encloses the chromatin. Subzonal injection of LV-PGK into bovine oocytes resulted in the expression of GFP in 92% ± 8% (n = 3 experiments) of the developing blastocysts (Fig. 5K,L). Analysis of GFP expression in blastocysts derived from lentivirus-injected oocytes and zygotes by confocal microscopy clearly showed that GFP was expressed in the trophectoderm as well as the inner cell mass (see supplementary information online). The percentage of embryos developing to blastocysts after virus injection (40% ± 17%, n = 227) was not significantly different when compared with control treatment (47% ± 16%, n = 83). So, subzonal injection of lentiviruses does not affect embryonic development.
Discussion
The GFP transgene was not found in neonates developed from rhesus monkey embryos infected with lentiviral vectors (Wolfgang et al., 2001). Further analyses revealed that the transgene was present only in extraembryonic tissues such as the placenta (Wolfgang et al., 2001). By contrast, we observed efficient transduction of the embryo proper and extraembryonic cells after infection of bovine embryos and oocytes. In transgenic pigs, the derivatives of the three primary germ layers, germ cells and extraembryonic tissues contained the provirus and expressed GFP. In addition, the lentiviral provirus was transmitted through the germ line. The different efficacies of lentiviral transgenesis in monkey versus livestock might be due to basic biological differences as well as technical aspects such as the time-point of virus injection (single-cell embryos versus blastocysts; Wolfgang et al., 2001) and virus titre.
An important feature of lentiviral transgenesis in livestock is the linear correlation of transgene expression and number of integrants. This shows that lentiviral transgene expression is controlled predominantly by the number of proviral integrants. In contrast to previous studies that used retroviral vectors for generating transgenic livestock (Chan et al., 1998), silencing does not seem to have an important role in lentiviral transgenesis. In addition, ubiquitous as well as tissue-specific expression can be easily achieved by incorporating different internal promoters in the lentiviral vector construct.
Owing to the fact that transgenesis rates are the most important determinant of production costs, the inefficiencies of pronuclear DNA microinjection (only ∼1% to 10% of the injected embryos are transgenic) mean that the production costs of transgenic pigs and cattle are very high (Moffat, 1998; Wells et al., 1999). Lentiviral transfer of genes into porcine and bovine genomes resulted in a reproducibly high efficiency of transgenesis. On the basis of previous estimations of the impact of transgenesis rates on production costs, lentiviral transgenesis could reduce costs of transgenic livestock to about one-tenth of the present costs (Wells et al., 1999). Low production costs are essential for the widespread use of transgenic swine as disease models and donor animals for xenotransplantation, as well as for the genetic improvement of agricultural populations.
Methods
Virus production.
The vector LV-PGK (Follenzi et al., 2000) was recently described. LV-K14 was constructed by replacing the PGK promoter of LV-PGK with the promoter of the human K14 gene (Munz et al., 1999). Recombinant lentivirus was produced as previously described (Pfeifer et al., 2002).
Pig embryo collection and virus injection.
Embryos were collected from 6-month-old gilts after slaughter. These donors were superovulated with 1200 IU gonadotropin (pregnant mare serum gonadotropin, PMSG; Intergonan, Intervet, Unterschleissheim, Germany), and ovulation was stimulated with 750 IE human chorionic gonadotropin (HCG; Ovogest, Intervet) 3 days later. During the following 24–36 h donor animals were artificially inseminated twice and slaughtered 36 h after the first insemination. Their oviducts were flushed with 38 °C flush-media (PBS supplemented with 20% lamb serum, Invitrogen, Karlsruhe, Germany) and 50 mg gentamicin sulphate (Sigma, Germany). Embryos were collected in flush-media and used directly for subzonal virus injection with glass capillaries containing concentrated virus.
Pig embryo transfer.
Six-month-old prepuberal gilts were used as recipients and synchronized by oral administration of altrenogest (Regumate, Serumwerk, Bernburg, Germany) over a 15-day period, followed by administration of 750 IE PMSG 1 day after the last gestagen feeding. Ovulation was induced 3 days later with 750 IE HCG. Transfers were performed by laparoscopy under general anaesthesia with a combination of 1.2 ml per 10 kg ketamine hydrochloride (Ursotamin, Serumwerk) and 0.5 ml per 10 kg xylazine (Xylazin 2%, WDT, Germany) injected intravenously. To each recipient, 30–40 injected or control embryos were transferred in one oviduct.
In vitro production of bovine embryos.
Bovine cumulus oocyte complexes (COCs) were collected by aspirating ovarian follicles obtained from slaughtered animals. The in vitro production of bovine embryos was performed as previously described (Stojkovic et al., 2001). Briefly, oocytes were matured in vitro for 22 h in modified tissue culture medium 199 (Invitrogen) at 39 °C in 5 % CO2.
After maturation, COCs were distributed among different treatment groups. In group I, COCs were co-cultured with frozen–thawed semen (106 spermatozoa per ml; capacitated in a swim-up procedure) for 18 h. Presumptive zygotes were then denuded by vortexing and injected subzonally with LV-PGK. In group II, oocytes were stripped free from cumulus cells and injected subzonally with LV-PGK; this was followed by in vitro fertilization as described above. Embryos were cultured in modified synthetic oviduct fluid supplemented with 10% (v/v) oestrous cow serum (ECS; Stojkovic et al., 2001) at 39 °C in a humidified atmosphere of 5% CO2, 5% O2 and 90% N2.
Southern blotting and nested PCR.
BamHI-digested DNA was separated by electrophoresis and transferred to Gene Screen Plus Hybridization Transfer Membranes (PerkinElmer, Boston, Massachusetts, USA). The blot was hybridized with a full-length 32P-labelled enhanced GFP (eGFP) cDNA probe. For nested PCR, porcine embryos at the 2–4-cell stage were isolated and incubated with Protease K (Roche). Thirty cycles were performed with GFP-specific primers (forward, 19–38 nucleotide (nt); reverse, 716–734 nt of the EGFP cDNA); thereafter, 35 cycles were done using nested primers (forward, 150–168 nt; reverse, 542–561 nt).
In vivo fluorescence imaging.
Excitation of green fluorescence was achieved using a Schott 2500 light source and a 485-nm filter (Zeiss, Jena, Germany). The emitted fluorescence was visualized using a long-pass filter (HQ 500, Zeiss).
Immunohistochemistry and FISH technology.
Frozen 10-μm sections were incubated with antibody against eGFP (Clontech, Palo Alto, California, USA), followed by incubation with the secondary biotinylated antibody (goat anti-mouse, Dianova, Hamburg, Germany) and staining with VECTASTAIN ABC Peroxidase Kit (Vector, Burlingame, CA, USA) and 3′,3-diaminobenzidine (270 μg ml−1; Sigma). Staining of pancreatic islets was performed using a polyclonal guinea pig anti-insulin antibody (BioTrend, Köln, Germany). Direct fluorescence imaging was performed on untreated sections after washing with PBS and mounting (Permafluor, Shandon, Lipshaw, Pittsburgh, USA). Nuclear staining was carried out with 1 μg ml−1 4′,6-diamidino-2-phenylindole (DAPI).
For fluorescence in situ hybridization technology, sperm samples were prepared as recently described (Pellestor et al., 1996). A Y-chromosome-specific probe (Rubes et al., 1999) and a GFPspecific probe (forward, 19–39 nt; reverse, 542–561 nt) were directly labelled with either fluorescein-12-dUTP or tetramethyl-rhodamine-dUTP (Roche) in a PCR reaction.
Western blotting.
Tissue samples were homogenized in lysis buffer (0.5% Triton X-100, 150 mM NaCl, 2 mM CaCl2 and protease inhibitors). After separation on SDS–polyacrylamide gel electrophoresis (PAGE) and transfer to polyvinylidene fluoride membranes (Immobilon-P, Millipore, Bedford, USA.), GFP was visualized using eGFP antibodies (Clontech) and enhanced chemoluminescent labelling (Amersham). Quantification of GFP expression was performed by immunoblotting. Protein samples were diluted according to the number of lentiviral integrants present in their genome, and limiting dilutions were performed to ensure that the signal was in the linear range. GFP concentration was calculated by comparing individual bands with the band intensity of a recombinant eGFP standard (Clontech) and given as ng μg−1 total protein.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/4-embor7400007-s1.pdf).
Supplementary Material
Supplementary information
Acknowledgments
We thank H. Sebald and T. Holy for expert technical assistance. We also thank the Bayerische Forschungsstiftung (BFS 219/96, 492/02), Fonds der Chemischen Industrie and the Deutsche Forschungsgemeinschaft.
References
- Cabot R.A. et al. ( 2001) Transgenic pigs produced using in vitro matured oocytes infected with a retroviral vector. Anim. Biotechnol., 12, 205–214. [DOI] [PubMed] [Google Scholar]
- Chan A.W.S., Homan E.J., Ballou L.U., Burns J.C. & Bremel D.R. ( 1998) Transgenic cattle produced by reverse-transcribed gene transfer in oocytes. Proc. Natl Acad. Sci. USA, 95, 14028–14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl C. & Wrobel K.H. ( 1992) Distribution of sugar residues in the bovine testis during postnatal ontogenesis demonstrated with lectin–horseradish peroxidase conjugates. Histochemistry, 97, 161–171. [DOI] [PubMed] [Google Scholar]
- Follenzi A., Ailles L.E., Bakovic S., Geuna M. & Naldini L. ( 2000) Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nature Genet., 25, 217–222. [DOI] [PubMed] [Google Scholar]
- Gordon J.W., Scangos G.A., Plotkin D.J., Barbosa J.A. & Ruddle F.H. ( 1980) Genetic transformation of mouse embryos by microinjection of purified DNA. Proc. Natl Acad. Sci. USA, 77, 7380–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R. ( 1976) Germ line integration and Mendelian transmission of the exogenous Moloney leukemia virus. Proc. Natl Acad. Sci. USA, 73, 1260–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C., Hong E.J., Pease S., Brown E.J. & Baltimore D. ( 2002) Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science, 295, 868–872. [DOI] [PubMed] [Google Scholar]
- Moffat A.S. ( 1998) Improving gene transfer into livestock. Science, 282, 1619–1620. [DOI] [PubMed] [Google Scholar]
- Munz B. et al. ( 1999) Overexpression of activin A in the skin of transgenic mice reveals new activities of activin in epidermal morphogenesis, dermal fibrosis and wound repair. EMBO J., 18, 5205–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L. et al. ( 1996) In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science, 272, 263–267. [DOI] [PubMed] [Google Scholar]
- Pellestor F. et al. ( 1996) FISH and PRINS, a strategy for rapid chromosome screening: application to the assessment of aneuploidy in human sperm. Cytogenet. Cell Genet., 72, 34–36. [DOI] [PubMed] [Google Scholar]
- Pfeifer A. & Verma I.M. ( 2001) Gene therapy: promises and problems. Annu. Rev. Genomics Hum. Genet., 2, 177–211. [DOI] [PubMed] [Google Scholar]
- Pfeifer A. et al. ( 2001) Transduction of liver cells by lentiviral vectors: analysis in living animals by fluorescence imaging. Mol. Ther., 3, 319–322. [DOI] [PubMed] [Google Scholar]
- Pfeifer A., Ikawa M., Dayn Y. & Verma I.M. ( 2002) Transgenesis by lentiviral vectors: lack of gene silencing in mammalian embryonic stem cells and preimplantation embryos. Proc. Natl Acad. Sci. USA, 99, 2140–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubes J., Vozdova M. & Kubickova S. ( 1999) Aneuploidy in pig sperm: multicolor fluorescence in situ hybridization using probes for chromosomes 1, 10, and Y. Cytogenet. Cell Genet., 85, 200–204. [DOI] [PubMed] [Google Scholar]
- Stojkovic M. et al. ( 2001) Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol. Reprod., 64, 904–909. [DOI] [PubMed] [Google Scholar]
- Vassar R., Rosenberg M., Ross S., Tyner A. & Fuchs E. ( 1989) Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. Proc. Natl Acad. Sci. USA, 86, 1563–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells K., Moore K. & Wall R. ( 1999) Transgene vectors go retro. Nature Biotechnol., 17, 25–26. [DOI] [PubMed] [Google Scholar]
- Wolfgang M.J. et al. ( 2001) Rhesus monkey placental transgene expression after lentiviral gene transfer into preimplantation embryos. Proc. Natl Acad. Sci. USA, 98, 10728–10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R., Donello J.E., Trono D. & Hope T.J. ( 1999) Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J. Virol., 73, 2886–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


