Abstract
Here we show, using the green fluorescent protein (GFP) fusion system, that an Arabidopsis thaliana zinc-metalloprotease (AtZn-MP) is targeted to both mitochondria and chloroplasts. A deletion mutant lacking the amino-terminal 28 residues, with translation initiation at the second methionine residue, was imported into chloroplasts only. However, a mutated form of the full-length targeting peptide, in which the second methionine residue is changed to leucine, was imported to both organelles. No GFP fluorescence was detected when a frame-shift mutation was introduced between the first and second ATG codons of the Zn-MP–GFP construct, suggesting no alternative translational initiation. Our results show that the dual targeting of the Zn-MP is due to an ambiguous targeting peptide. Furthermore, we show that the recombinant AtZn-MP degrades mitochondrial and chloroplastic targeting peptides, indicating its function as a signal peptide degrading protease in both mitochondria and chloroplasts.
Introduction
More than 98% of mitochondrial and chloroplastic proteins are encoded in the nucleus, synthesized on cytosolic polyribosomes as precursors carrying a cleavable amino-terminal targeting peptide and then imported post-translationally into the organelle. After import, targeting peptides are cleaved off by the mitochondrial processing peptidase (MPP) in mitochondria and the stromal processing peptidase (SPP) in chloroplasts. The accumulation of targeting peptides has been reported to have severe effects on the integrity and functions of mitochondria and chloroplasts (Nicolay et al., 1994; Wieprecht et al., 2000). The free targeting peptides are therefore rapidly removed by proteolytic degradation. We have isolated and identified a novel zinc-metalloprotease (Zn-MP) involved in the degradation of mitochondrial presequences (Ståhl et al., 2002).
Mitochondria and chloroplasts share a set of common metabolic activities; many enzymes that catalyse similar reactions are therefore localized in both organelles. These enzymes are usually isoenzymes encoded by different genes, expressed individually and imported into their respective organelles as distinct precursors. However, a subset of these proteins have been shown to be dual-targeted proteins that, although encoded by a single nuclear gene, are translated in the cytosol and targeted post-translationally to both mitochondria and chloroplasts (Peeters & Small, 2001). There are two possible mechanisms by which dual targeting can be achieved: through either a twin targeting sequence or an ambiguous targeting sequence. Twin targeting signals might be the result of alternative transcription or translation initiation, alternative splicing, or some post-translational modification that results in the formation of two proteins with distinct targeting peptides. The precursor proteins carrying an ambiguous targeting signal exist as a single polypeptide form but can be recognized and transported by the import machinery of more than a single organelle (Danpure, 1995; Small et al., 1998).
Here, we investigated the dual targeting and catalytic function of a recently identified signal peptide degrading Zn-MP. Our results show that Arabidopsis thaliana Zn-MP (AtZn-MP) is dual-targeted to mitochondria and chloroplasts in vivo, and that Zn-MP can proteolytically degrade both mitochondrial and chloroplastic targeting peptides.
Results and discussion
In vivo dual targeting of the Arabidopsis thaliana Zn-MP
The full-length protein of the Arabidopsis thaliana Zn-MP (AtZn-MP; AAL90904) is reported in the NCBI database to contain 1,080 amino-acid residues. The targeting peptide was predicted to be 85 amino acids long (Fig. 1A) and the mature part corresponds to 995 amino acids as determined by both MitoProt and ChloroP. The intracellular prediction program TargetP indicated that the AtZn-MP could be localized both to mitochondria and chloroplasts, whereas Predotar predicted only the mitochondrial localization.
Figure 1.
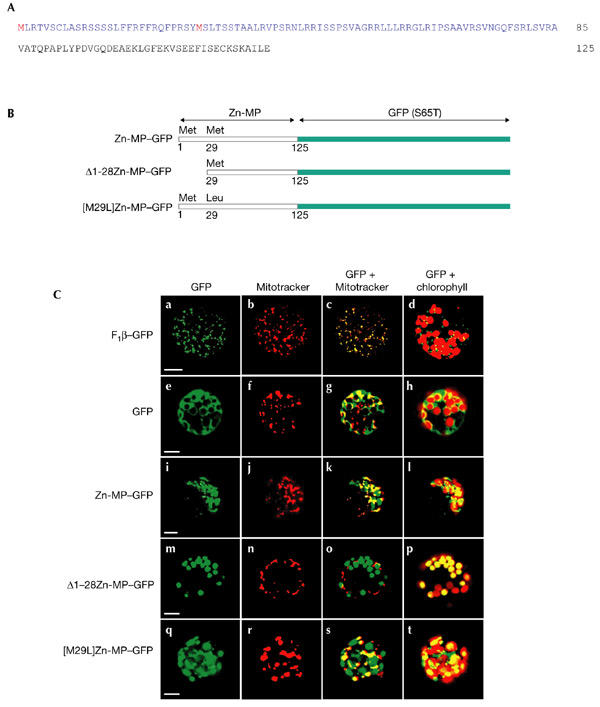
Targeting of the green fluorescent protein fusions to tobacco protoplasts. (A) The amino-terminal 125 amino-acid residues of the Arabidopsis thaliana zinc-metalloprotease (AtZn-MP) precursor protein. The predicted targeting sequence is shown in blue and two potential initiation codons are in red. (B) Schematic presentation of the three green fluorescent protein (GFP) fusion constructs used in the transient expression experiments. (C) Transient expression of the GFP fusion constructs in Nicotiana tabacum protoplasts: F1β–GFP (Ca–Cd), soluble GFP (Ce–Ch), Zn-MP–GFP (Ci–Cl), Δ1–28Zn-MP–GFP (Cm–Cp) and [M29L]Zn-MP–GFP (Cq–Ct), as described in Methods. The GFP column shows the signal detected in the green channel; the Mitotracker column shows the signal detected in the red channel; the GFP + Mitotracker column corresponds to the merging of the two previous columns, in which yellow represents the superposition of green and red; and the GFP + chlorophyll column corresponds to the merging of the green channel and the chlorophyll signal detected in the far-red channel. Scale bars, 10 μm.
To investigate targeting of the AtZn-MP, the targeting peptide and 40 amino-acid residues from the mature part of the protein were fused to green fluorescent protein (GFP; Fig. 1B) under the plant strong transcription promoter EN50PMA4 (Zhao et al., 1999). The mitochondrial presequence of the ATP synthase F1β-subunit from Nicotiana plumbaginifolia fused to GFP (F1β–GFP) was used to assay mitochondrial targeting (Duby et al., 2001). Transient expression of the GFP constructs was performed in tobacco protoplasts. Confocal microscopy analysis shows that, in protoplasts transformed with the F1β–GFP construct, GFP fluorescence is localized to small, punctuated structures (Fig. 1Ca), which were also labelled by the fluorescence of Mitotracker (Molecular Probes; Fig. 1Cb). Colocalization is shown in yellow in the merged image in Fig. 1Cc. GFP alone showed the characteristic fluorescence in the cytosol (Fig. 1Ce–h). Protoplasts transformed with the Zn-MP–GFP construct showed fluorescence in two different locations, the punctuated structures and large, round structures (Fig. 1Ci). Fluorescence in the punctuated structure colocalized with Mitotracker (Fig. 1Ck). In contrast, GFP fluorescence in the large, round structures colocalized with the chloroplasts as shown by the superposition of GFP and chlorophyll autofluorescence (Fig. 1Cl).
The targeting peptide of the AtZn-MP contains a second methionine residue at position 29 that might function as a second translational initiation site. We therefore used a deletion construct starting from the second methionine (Δ1–28Zn-MP–GFP) and a mutant with the second methionine changed to leucine ([M29L]Zn-MP–GFP) (Fig. 1B). The truncated targeting peptide in the Δ1–28Zn-MP–GFP construct targeted GFP to chloroplasts only, as shown by the colocalization of green fluorescence with the red autofluorescence of the chlorophyll (Fig. 1Cp). A few chloroplasts seem not to be labelled by GFP, as has been shown for other proteins in a protoplast transient expression system (Akashi et al., 1998; Hedtke et al., 2000). The mutant construct [M29L]Zn-MP–GFP targeted GFP to both mitochondria and chloroplasts, as revealed by the colocalization of GFP with Mitotracker and chlorophyll fluorescence (Fig. 1Cq–t). We can therefore conclude that the second part of the targeting peptide is sufficient for chloroplast targeting but not for mitochondrial targeting.
The same constructs that were used for protoplast transformation were cloned into a binary pBI101 (Clontech) vector, and transient expression into tobacco leaves was performed. The leaf area infiltrated with the Agrobacterium suspension carrying the control F1β–GFP construct showed green fluorescence in the characteristic mitochondrial structures of small punctuate morphology (Fig. 2A). The green fluorescence in the punctuated structures was colocalized with the immunostaining of a mitochondrial-specific marker, lipoamide dehydrogenase (data not shown). With the GFP construct alone, fluorescence was detected in both the nucleus and the cytosol surrounding the large central vacuole (Fig. 2C). The presence of GFP in the nucleus has been also observed previously (Grebenok et al., 1997). The leaf area infiltrated with Agrobacterium carrying the Zn-MP–GFP construct again showed green fluorescence in mitochondria and chloroplasts (Fig. 2E,F). Note that the mitochondrial labelling follows the border of the puzzle-piece-like epidermal cell. Note also that not all the cells are transformed in this transient expression system. This results in the identification of chloroplasts of non-transformed cells (for example, in the upper left part of Fig. 2F) that are not labelled by GFP. These results further confirmed the dual targeting properties of the Zn-MP targeting peptide.
Figure 2.
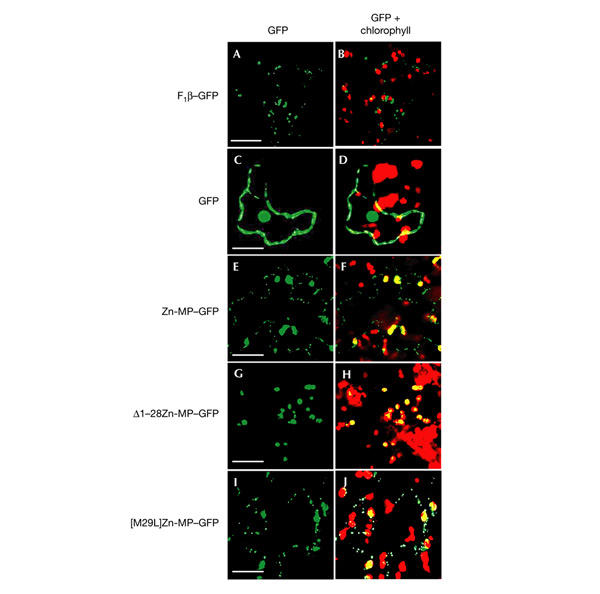
Targeting of the green fluorescent protein fusions to tobacco-leaf epidermal cells. Agrobacterium-mediated transient expression of the green fluorescent protein (GFP) fusion constructs into the Nicotiana tabaccum leaves: F1β–GFP (A,B), soluble GFP (C,D), Zn-MP–GFP (E,F), Δ1–28Zn-MP–GFP (G,H) and [M29L]Zn-MP–GFP (I,J) as described in Methods. Scale bars, 10 μm.
When the leaves were infiltrated with Agrobacterium carrying the truncated construct Δ1–28Zn-MP–GFP (Fig. 2G,H), GFP was targeted to the chloroplasts only. However, GFP in the [M29L]Zn-MP–GFP construct was targeted to both mitochondria and chloroplasts (Fig. 2I,J).
Furthermore, no GFP fluorescence was detected when a Zn-MP–GFP construct that contained a frameshift mutation between the first and second ATG codon of the Zn-MP–GFP construct was introduced into tobacco leaves (data not shown). These results exclude the dual targeting of the Zn-MP as a result of alternative translational initiation in vivo. In conclusion, the AtZn-MP harbours an ambiguous targeting signal, which is recognized and transported by both mitochondrial and chloroplastic import machinery.
In vitro import of the Arabidopsis thaliana Zn-MP
To investigate whether the targeting fidelity of the AtZn-MP is influenced by its mature part and to verify the dual targeting of the Zn-MP targeting peptide, in vitro import assays of the full-length Zn-MP, Δ1–28Zn-MP and [M29L]Zn-MP were performed with isolated mitochondria and chloroplasts (Fig. 3). Incubation of the Zn-MP precursor with isolated mitochondria resulted in import and processing of the precursor protein (Fig. 3A, lanes 2 and 3), detected as protection of the mature Zn-MP inside mitochondria after treatment with proteinase K (PK). The truncated presequence (Δ1–28Zn-MP) could not target the Zn-MP into mitochondria (Fig. 3A, lanes 7 and 8). However, incubation of the mutant precursor ([M29L]Zn-MP) with isolated mitochondria resulted in import and partial processing of the precursor (Fig. 3A, lanes 12 and 13). Import of the precursors into mitochondria was dependent on membrane potential, because no imported precursor or mature form was protected from degradation by PK in the presence of valinomycin (Fig. 3A, lanes 5 and 15).
Figure 3.
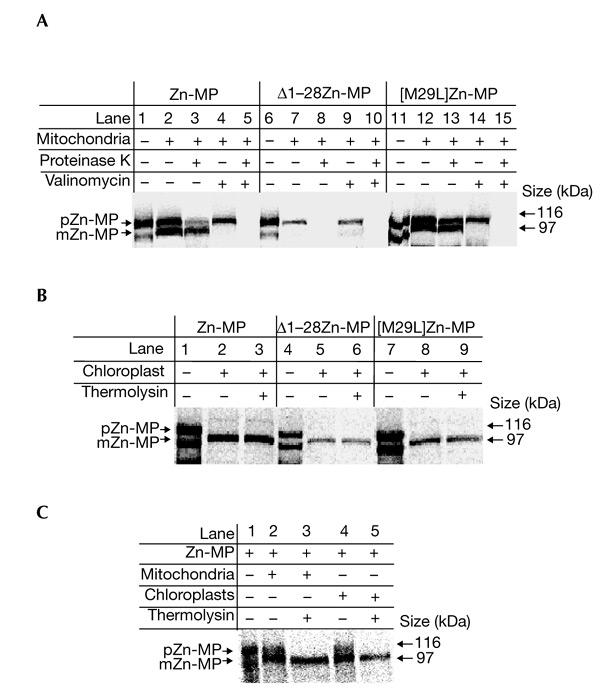
In vitro import of the Arabidopsis thaliana zinc-metalloprotease into mitochondria and chloroplasts. (A) The indicated precursors were incubated with mitochondria as described in Methods. Proteinase K (15 μg ml−1) was added after import or 1 μM valinomycin was added before import, as indicated. (B) The indicated precursors were incubated with chloroplasts. Thermolysin (5 μg ml−1) was added after import where indicated. (C) In vitro import of the Zn-MP precursor into the dual import system. The Zn-MP precursor was incubated with mitochondria and chloroplasts together. Mitochondria and chloroplasts were re-isolated on a 4% Percoll gradient after import, as described in Methods. Zn-MP, zinc-metalloprotease; Δ1-28Zn-MP, truncated Zn-MP presequence; [M29L]Zn-MP, mutant Zn-MP precursor. m, mature form; p, precursor.
Incubation of the Zn-MP precursor with isolated chloroplasts resulted in import and processing of the precursor protein to a mature-sized protein identical to that in the mitochondrial import experiments (Fig. 3B, lane 2). The mature protein was resistant to treatment with thermolysin (Fig. 3B, lane 3). Both the truncated (Δ1–28Zn-MP) and mutant ([M29L]Zn-MP) precursor proteins were also imported and processed to a mature-sized form after incubation with chloroplasts (Fig. 3B, lanes 6 and 9).
Incubation of the Zn-MP precursor simultaneously with isolated mitochondria and chloroplasts in a dual import system, followed by re-isolation of the organelles (Rudhe et al., 2002), also resulted in import and processing of the precursor protein to a mature-sized protein in both organelles (Fig. 3C, lanes 2 and 4). The mature-sized protein was further resistant to treatment with thermolysin, indicating import of the precursor into mitochondria (Fig. 3C, lane 3) and chloroplasts (Fig. 3C, lane 5). These results further verified dual targeting of the Zn-MP.
Dual organellar localization of Zn-MP
Isolated spinach mitochondria and chloroplasts were probed with an antibody against AtZn-MP (Fig. 4). A protein band with an apparent molecular mass of 100 kDa was detected in both mitochondria and chloroplasts. These results showed dual localization of Zn-MP in spinach. Purity of the mitochondria and chloroplasts was tested by using antibodies raised against organelle-specific proteins; there was less than 0.3% cross-contamination (data not shown).
Figure 4.
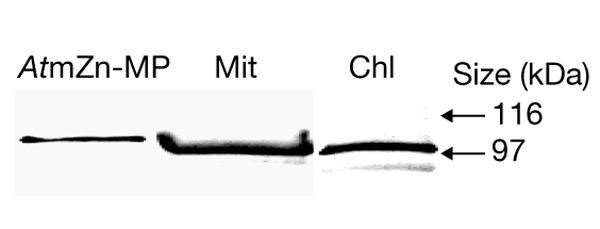
Dual localization of the zinc-metalloprotease in spinach. Recombinant Arabidopsis thaliana zinc-metalloprotease (AtmZn-MP), spinach mitochondria (Mit) and chloroplasts (Chl) were immunodecorated with antibodies raised against the carboxy-terminal part of the AtmZn-MP, as described in Methods. m, mature form.
Dual function of the Arabidopsis thaliana Zn-MP
Previous results from our laboratory showed that the mitochondrial presequences are degraded by a metalloprotease and that the protease was identified as the AtZn-MP (Ståhl et al., 2002). Chloroplastic transit peptides were shown to be degraded by an ATP-dependent metalloprotease activity; however, the protease responsible for the degradation remains unknown (Richter & Lamppa, 2002). Incubation of a Nicotiana mitochondrial presequence from the ATP synthase F1β-subunit (N5.7pF1β(2–54)-hsl; Ståhl et al., 2002) and a Pisum sativum chloroplast transit peptide of the small subunit of ribulose bisphosphate carboxylase/oxygen-ase (SStpPs) with the recombinant mature part of the AtZn-MP (Fig. 5, lanes 2 and 5) resulted in the complete degradation of both targeting peptides. No proteolytic activity was seen in the presence of a metal chelator, o-phenanthroline (Fig. 5, lanes 3 and 6). We therefore conclude that the Zn-MP degrades targeting peptides in both organelles.
Figure 5.
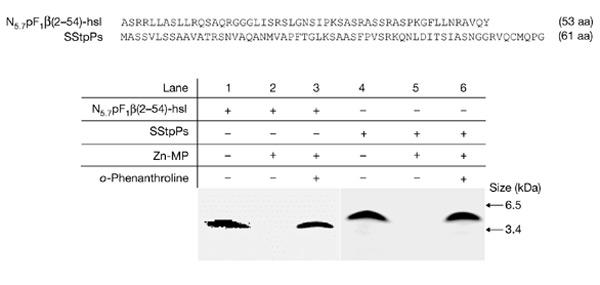
Dual proteolytic activity of the recombinant Arabidopsis thaliana zinc-metalloprotease. Lanes 1 and 4, the mitochondrial presequence peptide, N5.7pF1β(2–54)-hsl and the chloroplastic transit peptide, SStpPs alone. N5.7pF1β(2–54)-hsl and SStpPs peptides were either incubated (lanes 2, 3, 5 and 6) or not (lanes 1 and 4) with recombinant Arabidopsis thaliana zinc-metalloprotease (AtZn-MP) in the absence (lanes 2 and 5) or presence (lanes 3 and 6) of o-phenanthroline, as described in Methods. The sequence of both peptides is depicted at the top.
Several techniques are available for studying organellar protein targeting, not all of which are ideal for studying dual targeting. There are a few reports showing mistargeting of chloroplastic proteins to mitochondria with the use of in vitro import methods. However, GFP as a marker in an in vivo GFP fusion system has been frequently used to study the subcellular localization of dual-targeted proteins with rare examples of mistargeting. The only published example in which GFP has given results that were in conflict with another technique was for Arabidopsis mercaptopyruvate sulphurtransferase (reviewed by Peeters & Small, 2001). We have confirmed our results of dual targeting with a combination of complementary techniques, both in vivo and in vitro. In addition to the targeting and immunodetection studies, the proteolytic activity of the recombinant Zn-MP against both the mitochondrial and chloroplastic targeting peptides strongly suggests that the Zn-MP functions as a targeting-peptide-degrading protease in both organelles.
Methods
Green fluorescent protein fusion constructs.
The full-length cDNA of AtZn-MP (AtZn-MP) was originally obtained from the Kazusa DNA Research Institute in Japan (http://www.kazusa.or.jp/en/plant). To investigate the targeting properties of the AtZn-MP, three different constructs named Zn-MP–GFP, Δ1–28Zn-MP–GFP and [M29L]Zn-MP–GFP were made that contained 125 N-terminal amino acids of the Zn-MP precursor fused to GFP mGFP4 S65T. In the construct Zn-MP–GFP, the Zn-MP sequence was introduced by using the primers 5′zmpgfp (5′-agatctCAGTAGCAGTCTCCGCCG-3′) and 3′zmpgfp (5′-ggtaccGAAGAGTATCGCCTTTGATTTAC-3′); in the construct Δ1–28Zn-MP–GFP it was introduced by using the primers 5′-Δ28zmpgfp (5′-agatcttacatgtctctcacatcgtcga-3′) and 3′zmpgfp. The PCR products were directly cloned into a TOPO zero-blunt vector (Invitrogen) and digested with BglII and KpnI. The digested fragments were gel-extracted and cloned into pTZ19U-derived vector between the EN50PMA4 promoter and GFP (Duby et al., 2001). By site-directed mutagenesis (Stratagene), the methionine residue at position 29 was changed to leucine in [M29L]Zn-MP–GFP by using the primers 5′mutzmp (5′-CAGTTCCCTCGCTCTTACTTGTCTCTCTCACATC-3′; mutation underlined) and 3′mutzmp (5′-GATGTGAGAGACAAGTAAGAGCGAGGGAACTG-3′). To perform Agrobacterium-mediated transient expression, the expression cassettes used for protoplast transformation were transferred into a pBI101-derived vector (Clontech). All constructs were verified by sequencing.
In vitro targeting constructs.
The first 28 N-terminal amino acids of the AtZn-MP were deleted by using the primers 5′zmpp (5′-cccgggATGTCTCTCACATCGTCGACGGC-3′) and 3′zmp (5′-ctcgagGAGAGCTTTCTTCACCTCGAAAAAGTTG-3′). The methionine residue at position 29 in [M29]LZn-MP was changed to leucine as described above.
Transient expression into tobacco protoplasts and leaves.
Protoplasts were prepared from leaves of Nicotiana tabacum cv. SR1 (Maliga et al., 1973) and transformed as described by Lukaszewicz et al. (1998). Transient expression into tobacco leaves, mediated by Agrobacterium tumefaciens, was performed as described by Batoko et al. (2000). The Agrobacterium suspension with an absorbance of 0.3 at 600 nm was infiltrated into tobacco leaves.
Staining of mitochondria and analysis by confocal microscopy.
Protoplasts were incubated in culture medium with 400-nM Mitotracker Red CM-H2Xros (Molecular Probes) for 40 min, and washed three times before confocal analysis.
Confocal microscopy was performed with a Bio-Rad MRC-1024 laser-scanning confocal imaging system. For the detection of GFP, excitation was at 488 nm and detection was between 506 and 538 nm. Mitotracker staining was detected between 589 and 621 nm with excitation at 568 nm; chloroplast autofluorescence was detected between 664 and 696 nm with excitation at 488 nm.
In vitro import.
All the precursors and translated products were synthesized by using the in vitro transcription/translation coupled reticulocyte lysate TNT system (SDS-Promega) in the presence of [35S]methionine (Amersham).
Potato tuber mitochondria were isolated and the import experiments were performed as described by von Stedingk et al. (1997). Spinach mitochondria and chloroplasts were isolated from spinach leaves, and import experiments were performed as described by Eriksson et al. (1994) and Bruce et al. (1994). Dual import was performed with isolated spinach mitochondria and chloroplasts as described by Rudhe et al. (2002).
Western blotting.
Samples (100 μg) of mitochondria and chloroplasts (20 μg chlorophyll) from spinach were solubilized in Laemmli sample buffer and subjected to 12% SDS–polyacrylamide gel electrophoresis (SDS–PAGE) in the presence of 4 M urea (Laemmli, 1970). Immunological cross-reactivity was analysed by western blotting with antibodies raised against the C-terminal part of Zn-MP (residues 904–922) followed by detection with horseradish peroxidase (HRP)-conjugated secondary antibodies.
Proteolytic activity of the Arabidopsis thaliana Zn-MP.
Proteolytic activity was determined by degradation of the mitochondrial presequence N5.7pF1β(2–54) and the chloroplastic ztransit peptide SStpPs. Overexpression of the mature part of the AtZn-MP was performed in Escherichia coli using the glutathione S-transferase fusion system (P. Moberg, A. Ståhl, S. Bhushan, S. Wright, A.C. Eriksson, B. Bruce & E. Glaser, unpublished data). The proteolytic reaction contained 1 μg of the recombinant Zn-MP and 10 μM of either N5.7pF1β(2–54)-hsl or SStpPs in the degradation buffer containing 20 mM HEPES-KOH (pH 8.0) and 10 mM MnCl2. Degradation was performed for 30 min at 30 °C and the reaction was stopped by the addition of Laemmli sample buffer. For the detection of N5.7pF1β(2–54), samples were analysed on 10–20% Tris-Tricine gels (Bio-Rad) and stained with Coomassie Brilliant Blue. For the detection of SStpPs, samples were subjected to 12–20% SDS–PAGE in the presence of 4 M urea and immunological cross-reactivity was analysed by western blotting with an antibody raised against SStpPs followed by detection with horseradish peroxidase-conjugated secondary antibodies. For the inhibition studies, 20 mM o-phenanthroline was added and pre-incubated with the reaction mixture for 10 min.
Acknowledgments
This work was supported by grants from The Swedish Research Council and The Swedish Foundation for International Cooperation in Research and Higher Education to E.G., and from the Interuniversity Poles of Attraction Program–Belgian State, the Prime Minister's Office for Scientific, Technical and Cultural Affairs and the Belgian Fund for Scientific Research to M.B. and from The National Science Foundation, USA, to B.D.B.
References
- Akashi K., Grandjean O. & Small I. ( 1998) Potential dual targeting of an Arabidopsis archaebacterial-like histidyl-tRNA synthetase to mitochondria and chloroplasts. FEBS Lett., 431, 39–44. [DOI] [PubMed] [Google Scholar]
- Batoko H., Zheng H.Q., Hawes C. & Moore I. ( 2000) A Rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell, 12, 2201–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce B.D., Perry S., Froelich J. & Keegstra K. ( 1994) in Plant Molecular Biology Manual 2nd edn (eds Gelvin, S.B. & Schilferoot, R.A.), J1–J15. Kluwer Academic, Dordrecht, the Netherlands. [Google Scholar]
- Danpure C.J. ( 1995) How can the products of a single gene be localized in more than one intracellular compartment? Trends Cell Biol., 5, 230–238. [DOI] [PubMed] [Google Scholar]
- Duby G., Oufattole M. & Boutry M. ( 2001) Hydrophobic residues within the predicted N-terminal amphiphilic α-helix of a plant mitochondrial targeting presequence play a major role in in vivo import. Plant J., 27, 539–549. [DOI] [PubMed] [Google Scholar]
- Eriksson A.C., Sjöling S. & Glaser E. ( 1994) The ubiquinol cytochrome c oxidoreductase complex of spinach leaf mitochondria is involved in both respiration and protein processing. Biochim. Biophys. Acta, 1186, 221–231. [Google Scholar]
- Grebenok R.J., Pierson E., Lambert G.M., Gong F.C., Afonso C.L., Haldeman-Cahill R., Carrington J.C. & Galbraith D.W. ( 1997) Green-fluorescent protein fusions for efficient characterization of nuclear targeting. Plant J., 11, 573–586. [DOI] [PubMed] [Google Scholar]
- Hedtke B., Borner T. & Weihe A. ( 2000) One RNA polymerase serving two genomes. EMBO Rep., 1, 435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U.K. ( 1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz M., Jerouville B. & Boutry M. ( 1998) Signs of translational regulation within the transcript leader of a plant plasma membrane H+-ATPase gene. Plant J., 14, 413–423. [DOI] [PubMed] [Google Scholar]
- Maliga P., Sz-Breznovits A. & Marton L. ( 1973) Streptomycin-resistant plants from callus culture of haploid tobacco. Nature New Biol., 244, 29–30. [DOI] [PubMed] [Google Scholar]
- Nicolay K., Laterveer F. & Heerde W. ( 1994) Effects of amphipathic peptides, including presequences, on the functional integrity of rat liver mitochondrial membranes. J. Bioenerg. Biomembr., 26, 327–334. [DOI] [PubMed] [Google Scholar]
- Peeters N. & Small I. ( 2001) Dual targeting to mitochondria and chloroplasts. Biochim. Biophys. Acta, 12, 54–63. [DOI] [PubMed] [Google Scholar]
- Richter S. & Lamppa G.K. ( 2002) Determinants for removal and degradation of transit peptides of chloroplast precursor proteins. J. Biol. Chem., 277, 43888–43894. [DOI] [PubMed] [Google Scholar]
- Rudhe C., Chew O., Whelan J. & Glaser E. ( 2002) A novel in vitro system for simultaneous import of precursor proteins into mitochondria and chloroplasts. Plant J., 30, 213–220. [DOI] [PubMed] [Google Scholar]
- Small I., Wintz H., Akashi K. & Mireau H. ( 1998) Two birds with one stone: genes that encode products targeted to two or more compartments. Plant Mol. Biol., 38, 265–277. [PubMed] [Google Scholar]
- Ståhl A., Moberg P., Ytterberg J., Panfilov O., Brockenhuus Von Lowenhielm H., Nilsson F. & Glaser E. ( 2002) Isolation and identification of a novel mitochondrial metalloprotease (PreP) that degrades targeting presequences in plants. J. Biol. Chem., 277, 41931–41939. [DOI] [PubMed] [Google Scholar]
- Von Stedingk E.M., Pavlov P.F., Grinkevich V.A. & Glaser E. ( 1997) Mitochondrial protein import: modification of sulfhydryl groups of the inner mitochondrial membrane import machinery in Solanum tuberosum inhibits protein import. Plant Mol. Biol., 35, 809–820. [DOI] [PubMed] [Google Scholar]
- Wieprecht T., Apostolov O., Beyermann M. & Seelig J. ( 2000) Interaction of a mitochondrial presequence with lipid membranes: role of helix formation for membrane binding and perturbation. Biochemistry, 19, 15297–15305. [DOI] [PubMed] [Google Scholar]
- Zhao R.M., Moriau L. & Boutry M. ( 1999) Expression analysis of the plasma membrane H+-ATPase pma4 transcription promoter from Nicotiana plumbaginifolia activated by the CaMV 35S promoter enhancer. Plant Sci., 149, 157–165. [Google Scholar]


