Abstract
Human immunodeficiency virus 1 (HIV-1) expresses several accessory proteins that manipulate various host-cell processes to achieve optimum replicative efficiency. One of them, viral protein U (Vpu), has been shown to interfere with the cellular degradation machinery through interaction with SCFβ-TrCP complexes. To learn more about Vpu function in vivo, we used the genetically tractable fruit fly, Drosophila melanogaster. Our results show that the directed expression of Vpu, but not the non-phosphorylated form, Vpu2/6, in fat-body cells affects Drosophila antimicrobial responses. In flies, the Toll and Imd pathways regulate antimicrobial-peptide gene expression. We show that Vpu specifically affects Toll pathway activation by inhibiting Cactus degradation. Given the conservation of the Toll/nuclear factor-κB (NF-κB) signalling pathways between flies and mammals, our results suggest a function for Vpu in the inhibition of host NF-κB-mediated innate immune defences and provide a powerful genetic approach for studying Vpu inhibition of NF-κB signalling in vivo.
Introduction
Human immunodeficiency virus 1 (HIV-1) expresses several accessory proteins that manipulate various host-cell processes to achieve optimum replicative efficiency and viral pathogenesis. These factors include the HIV-1 proteins Nef, Vpr, viral protein U (Vpu) and Vif (Emerman & Malim, 1998). Vpu has been implicated in targeting CD4, the cellular receptor for HIV-1, for degradation by the proteasome. Cell-culture experiments suggest that Vpu mediates this targeted degradation through a phosphorylation-dependent interaction with the F-box/WD40 protein β-TrCP, the receptor component of the E3 ubiquitin ligase complex SCFβ-TrCP (Margottin et al., 1998). The Vpu/β-TrCP interaction requires the phosphorylation of Vpu at a DSGXXS motif, implicating Ser 52 and Ser 56 in this activation (Margottin et al., 1998). This motif is found in several cellular signalling proteins that are known to be degraded by the proteasome, such as I-κB-α, the inhibitor of the transcription factor nuclear factor-κB (NF-κB; Karin & Ben-Neriah, 2000). Recently, it was shown that I-κB-α is a cellular substrate of β-TrCP (Kroll et al., 1999; Yaron et al., 1998). Bour and colleagues (2001) showed that Vpu is resistant to β-TrCP-mediated degradation and that it competitively inhibits β-TrCP-dependent degradation of I-κB-α in response to tumour necrosis factor-α (TNF-α), resulting in the suppression of NF-κB activity in Vpu-expressing cells. In addition, Akari and co-workers (2001) showed that Vpu inhibits NF-κB-dependent expression of anti-apoptotic genes, with a consequent activation of the caspase pathway leading to induction of apoptosis. All these studies were performed using cell cultures, and the exact in vivo function of Vpu is not fully understood.
There are striking similarities between the NF-κB signalling cascades that regulate Drosophila antimicrobial defence responses and those that regulate vertebrate innate immune responses. In particular, the Toll and Imd cascades, which regulate the expression of antimicrobial-peptide-encoding genes by the fat body, the functional equivalent of the mammalian liver, are similar to the mammalian interleukin 1/TLR and TNF receptor pathways that regulate NF-κB in mammals (Hultmark, 2003). These parallels prompted us to study the effects of the HIV-1 accessory protein Vpu on Drosophila NF-κB signalling pathways in vivo. We have used the UAS–Gal4 system to express Vpu and the non-phosphorylated mutant Vpu2/6, which is incapable of binding β-TrCP, in flies. Our results show that the directed expression of Vpu, but not Vpu2/6, in fat-body cells strongly affects the Toll pathway, but not the Imd pathway, in response to microbial infection.
Results
Directed expression of Vpu in fat-body cells
We generated transgenic flies that carry either a UAS–Vpu or a UAS–Vpu2/6 (S52N/S56N) mutated construct. We crossed the UAS–Vpu and UAS–Vpu2/6 transgenic flies to flies carrying the yolk–GAL4 driver, which expresses the yeast transactivator Gal4 in the fat-body cells of female adults. Figure 1A–D shows that flies carrying both the yolk–GAL4 and UAS–Vpu transgenes express the Vpu proteins in female adult fat-body cells. As is commonly seen in human cells (C. Besnard-Guérin, personal communication), western blot analysis showed that Vpu2/6 is expressed at higher levels than Vpu and that Vpu migrates more slowly than Vpu2/6 (Fig. 1E, lanes 1 and 3). This difference in gel migration between Vpu and Vpu2/6 was abrogated when protein extracts were treated with alkaline phosphatase (Fig. 1E, lane 2). Therefore, in Drosophila cells, as in human cells, Vpu but not Vpu2/6 is efficiently phosphorylated on Ser 52 and Ser 56. Our data indicate that Vpu and Vpu2/6 are properly expressed and modified in vivo in Drosophila fat-body cells, which do not show any detectable defects, indicating that Vpu expression does not affect the physiology of fat-body cells (Fig. 1C; and data not shown).
Figure 1.

Viral protein U is expressed and phosphorylated in fat-body cells of transgenic adult flies. Expression of viral protein U (Vpu) was detected in the fat-body of UAS–Vpu; yolk–GAL4 females by immunofluorescence (IF) using an anti-Vpu serum (A–C, visible light; B–D, ultraviolet light). (A,B) UAS–Vpu flies do not express Vpu in the absence of the yolk–GAL4 driver. A few fat-body cells are indicated with an arrow and have a normal form. (E) Protein extracts prepared from flies expressing Vpu–HA (haemagglutinin; lanes 1 and 2) and Vpu2/6–HA (lanes 3 and 4) were incubated in the presence (lanes 2 and 4) or absence (lanes 1 and 3) of calf intestinal alkaline phosphatase (CIP) before separation by SDS–polyacrylamide gel electrophoresis and analysis by western blotting using an anti-HA antibody.
Vpu affects Toll-dependent Drosophila immune responses
We investigated the effect of Vpu expression on the ability of Drosophila to resist infection by various microbes. Figure 2A shows that flies expressing Vpu resist infection by the Gram-negative bacteria Erwinia carotovora at the same level as do wild-type flies. Figure 2B–D, however, shows that flies expressing Vpu, but not Vpu2/6, are more susceptible to fungal infections than are wild-type flies. This effect is seen after natural infection by the entomopathogenic fungus Beauveria bassiana and after direct injection of either Aspergillus fumigatus or Neurospora crassa spores. This phenotype is similar to, although slightly weaker than, the fungal susceptibility generated by a null mutation in spatzle (spz), which encodes the putative ligand of the Toll receptor (Lemaitre et al., 1996). These results suggest that Vpu interferes with the Drosophila antifungal immune response.
Figure 2.
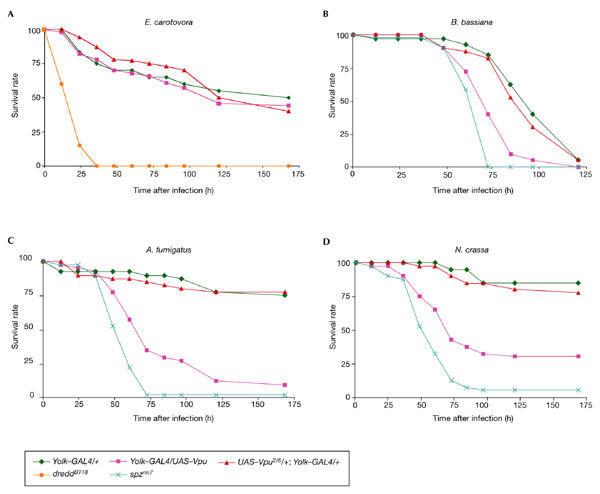
Directed expression of viral protein U makes flies highly susceptible to fungal infections. Directed expression of viral protein U (Vpu) but not Vpu2/6 makes flies susceptible to fungal infection (B–D) but does not affect resistance to Gram-negative bacterial infection (A). Female adults were pricked with a needle that was previously dipped into (A) a pellet of Erwinia carotovora, or concentrated solutions of spores of (C) Aspergillus fumigatus or (D) Neurospora crassa, or were naturally infected with Beauveria bassiana (B). The spatzle (spz) gene is required to resist fungal infections (B–D), whereas mutations in dredd make flies highly susceptible to Gram-negative bacterial infection (A).
In Drosophila, resistance to fungal infection is mediated by the Toll pathway, which controls the expression of genes that encode peptides with antifungal activities, such as Drosomycin (Drs) and Metchnikowin (Mtk; Lemaitre et al., 1996; Levashina et al., 1998). Consequently, we used northern blots to monitor the expression levels of these two genes in flies that express Vpu or Vpu2/6 after natural infection with B. bassiana. Figure 3A shows that natural infection by B. bassiana induced the expression of Drs and Mtk in wild-type flies and that their expression was abolished in spz mutants. The induction of the two antifungal-peptide genes, however, was severely impaired in flies expressing Vpu, but not in flies expressing Vpu2/6 (Fig. 3A). Resistance to Gram-negative bacterial infections is mediated by the Imd pathway, which controls the expression of a range of antibacterial peptide genes, such as Diptericin (Dpt). Figure 3B shows that Gram-negative bacterial infection induces the expression of Dpt in wild-type flies and this expression is abolished in dredd mutants. The induction of the Dpt gene was not affected in Vpu-expressing flies (Fig. 3B). These results show that expressing Vpu in Drosophila fat-body cells specifically reduces the induction of antifungal-peptide genes, but not antibacterial genes, resulting in susceptibility to fungal infection but not to Gram-negative bacterial infection. This result strongly suggests that Vpu does not affect general fat-body physiology, but specifically inhibits the Toll pathway. Next, we tested whether Vpu blocks the expression of the Drs gene in flies that carry the dominant Toll receptor mutation Tl10B, which constitutively activates the Toll pathway, thereby generating constitutive Drs and Mtk expression in the absence of infection (Lemaitre et al., 1996). Figure 3C shows that Vpu expression completely blocks the Drs and Mtk expression induced by the Tl10B mutation, whereas Vpu2/6 expression only affects the Tl10B phenotype slightly. This suggests a crucial role of Ser 52 and Ser 56 phosphorylation; however, the slight inhibition of the Toll pathway seen with Vpu2/6 suggests that Vpu has some effects that are independent of Ser 52 and Ser 56 phosphorylation. We conclude that Vpu does not affect the Imd pathway but specifically inhibits the Toll pathway by acting on a component of the cascade that functions downstream of the Toll receptor.
Figure 3.
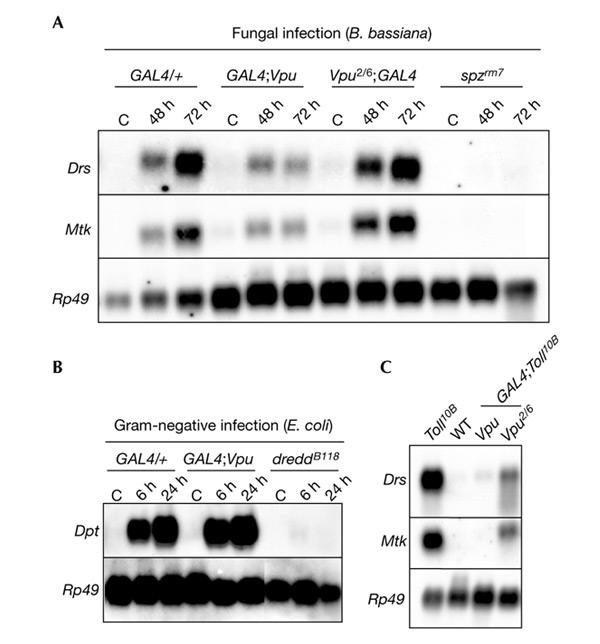
Viral protein U interferes with the Drosophila Toll pathway but not with the Imd pathway. (A) Northern blot analysis of Drosomycin (Drs) and Metchnikowin (Mtk) expression in flies expressing Vpu or Vpu2/6 collected 48 h or 72 h after natural infection by the fungus Beauveria bassiana. Expression of Vpu, but not Vpu2/6, affects Drs and Mtk induction after fungal infection, as does the spatzle mutation spzrm7. (B) Northern blot analysis of Diptericin (Dpt) expression in flies expressing Vpu collected 6 h and 24 h after injection of Gram-negative bacteria (Escherichia coli). Vpu expression did not affect Dpt expression, whereas the dredd mutation strongly affected its induction. (C) Northern blot analysis of Drs and Mtk expression in female adults expressing Vpu or Vpu2/6 in a wild-type (WT) or Toll10B mutant background. Vpu expression blocks the Toll10B-mediated constitutive expression of Drs and Mtk. C, control (uninfected flies); Rp49, Ribosomal protein 49.
Vpu blocks the degradation of Cactus
In Drosophila, Toll activates an intracellular signalling pathway that is mediated by the adaptor molecules Tube and DmMyd88 and by the Pelle kinase. This results in the degradation of the I-κB-α homologue, Cactus, and the subsequent nuclear translocation of the NF-κB transcription factors Dorsal and Dif, which control Drs expression (Belvin & Anderson, 1996; Nicolas et al., 1998; Tauszig-Delamasure et al., 2002). We tested whether Vpu can inhibit the Cactus degradation that is induced in cell culture by a constitutively active form of the Pelle kinase that is encoded by a Torso–Pelle construct (Galindo et al., 1995; Silverman et al., 2000). Previous studies showed that in Drosophila S2 cells an activated form of Toll (TollΔLRR) induces a Drs–luciferase reporter gene (Tauszig et al., 2000). Figure 4A shows that expression of Vpu blocks activation of the Drs–luciferase reporter gene induced by the expression of TollΔLRR. This confirms that, as observed in vivo, the Toll pathway is a target of Vpu in S2 cells. Figure 4B shows that expression of Vpu (Fig. 4B, lane 3), but not Vpu2/6 (Fig. 4B, lane 4), prevents Cactus degradation induced by the expression of the Torso–Pelle construct (Fig. 4B, lanes 1 and 2). Our data show that Vpu inhibits Toll-mediated Cactus degradation.
Figure 4.
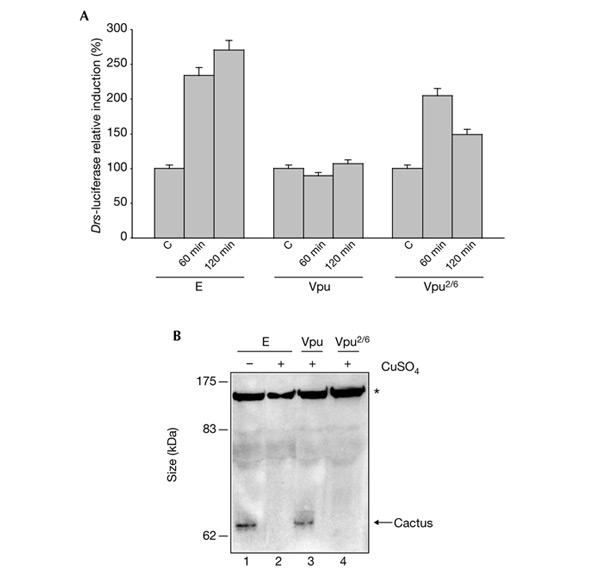
Viral protein U blocks the degradation of Cactus. (A) Viral protein U (Vpu) affects Drosomycin (Drs) expression in S2 cells. Cells were co-transfected with 1 μg of an expression vector that contains a copper-inducible TollΔLRR construction (Tauszig et al., 2000), plus 1 μg of an empty expression vector (E), a vector that contains Vpu, or a vector that contains Vpu2/6, and 0.1 μg of a reporter plasmid that encodes luciferase under the control of the Drs promoter. Forty-eight hours after transfection, CuSO4 (500 μM) was added to induce the expression of the TollΔLRR construct. After 60 or 120 min, the cells were harvested and luciferase activity was determined from cell extracts as described in the supplementary information online. C, control (no copper). (B) Vpu blocks Cactus degradation. S2 cells were stably transfected with a copper-inducible Torso–Pelle construct (Silverman et al., 2000). Cells were transfected with 1 μg of an empty expression vector (lanes 1 and 2) or with vectors that contain Vpu (lane 3) or Vpu2/6 (lane 4). Thirty-six hours after transfection, CuSO4 was added to induce the expression of the Torso–Pelle construct. Cellular protein extracts were prepared and were analysed on a western blot using an anti-Cactus antibody. A nonspecific band (indicated with an asterisk) was used as a loading control.
Previous studies have shown that Cactus is phosphorylated on specific serine residues of the conserved DSGXXS motif that is present in the amino-terminal part of the Cactus protein (Reach et al., 1996; Fernandez et al., 2001). In mammals, the regulatory domain required for human I-κB-α degradation also contains a DSGXXS motif, suggesting a common mechanism for I-κB-α and Cactus degradation (Fernandez et al., 2001; Karin & Ben-Neriah, 2000). Bour and colleagues (2001) recently proposed that Vpu, by binding to β-TrCP through its phosphorylated DSGXXS motif, interferes with the I-κB-α/β-TrCP interaction. Our in vivo data that show that Vpu, through its phosphorylated DSGXXS motif, can interfere with degradation of Cactus in Drosophila, which supports this hypothesis. We propose that Vpu, by binding to a homologue of β-TrCP in Drosophila, inhibits Cactus degradation.
Slimb is not involved in Toll pathway activation in adults
Slimb is the closest Drosophila homologue of β-TrCP and regulates protein degradation in several signalling pathways (Jiang & Struhl, 1998). It has been shown recently that Slimb is part of a ubiquitin–proteasome pathway that represses the Imd pathway (Khush et al., 2002). A null mutation in the slimb gene (slimbm) is lethal (Grima et al., 2002). We rescued the slimbm mutation during development with a heat-shock (hs)–slimb transgene, as described in Grima et al. (2002), and analysed the response to fungal infection of adult flies in which the Slimb protein is depleted. Figure 5 shows that in slimbm mutant flies the Drs gene is expressed after fungal infection at even higher levels than in the wild-type flies. We found that Dpt is expressed constitutively at a low level in unnchallenged slimbm mutant flies (data not shown), as previously shown (Khush et al., 2002). Although Vpu expression during larval stages does induce a slimb-mutant-like phenotype during wing and leg development (B.L.-B., unpublished data), Vpu expression does not have the same effect as the slimbm mutation on Dpt expression (Fig. 3B). Altogether, our results show that Slimb is not required for proper Toll pathway function in the adult stage.
Figure 5.
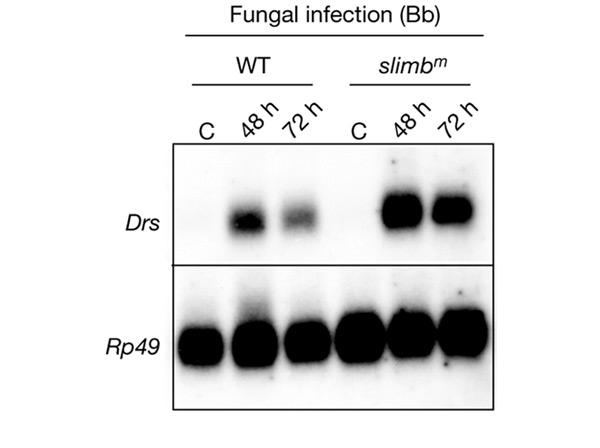
Slimb is not involved in the activation of the Toll pathway after fungal infection at the adult stage. Northern blot analysis of Drosomycin (Drs) expression in wild-type (WT) flies or slimbm flies collected 48 h and 72 h after infection with the fungus Beauveria bassiana (Bb). The slimbm mutation does not affect Drs induction after fungal infection. The slimbm mutant has been described elsewhere (Grima et al., 2002). C, control (uninfected flies); Rp49, Ribosomal protein 49.
Discussion
We have shown that expressing the HIV-1 accessory protein Vpu in Drosophila fat-body cells leads to immunocompromised flies: Vpu inhibits the Toll signalling pathway but not the Imd pathway. Consequently, flies expressing Vpu do not express antifungal-peptide genes and are highly susceptible to fungal infection, but have normal sensitivity to Gram-negative bacterial infections. Vpu blocks the Toll pathway by interfering with Cactus degradation. We also show that Slimb, the closest Drosophila homologue to β-TrCP, is not required for Toll pathway activity in the adult stage. The latter result contradicts previous studies that showed that Slimb is required for Toll-dependent activation of twist and snail in Drosophila embryos (Spencer et al., 1999). The requirement of Slimb for Cactus degradation during dorsoventral patterning is, however, controversial, and Fernandez and co-workers (2001) suggested that Slimb functions early during embryogenesis and its effect on the Toll pathway might thus be indirect. Therefore, the target of Vpu in the Cactus-degradation machinery remains to be identified. Our study suggests that one of the functions of Vpu is to inhibit NF-κB-mediated innate immune defences. The similarities seen between Vpu inhibition of I-κB-α and Cactus degradation through its phosphorylated DSGXXS motif underline the value of Drosophila as a model for determining the functions of Vpu in regulating innate immune responses. Furthermore, our results shed light on some differences between the molecular players in the pathways that regulate NF-κB in Drosophila and mammals. Recent reports indicate that β-TrCP also regulates the proteolysis and/or constitutive processing of the NF-κB1/p105 precursor of p50 (Heissmeyer et al., 2001; Lang et al., 2003), as well as the inducible processing of the NF-κB2/p100 precursor of p52 (Fong & Sun, 2002). In both cases, a phosphorylation motif similar to DSGXXS is required for β-TrCP-mediated processing of these Rel compound proteins. Interestingly, this motif is not present in Relish, the third Drosophila Rel protein, which is a homologue of p100 and p105 (Dushay et al., 1996). Indeed, Relish requires caspase cleavage, but not proteasomal partial degradation, for its activation (Stöven et al., 2000, 2003). These marked differences presumably explain why Vpu has no effect on the Imd pathway that regulates Relish activity in response to Gram-negative bacterial infection. Thus, the comparison between the differential effects of Vpu in all NF-κB activation pathways, both in Drosophila and mammals, might also help in the understanding of the mechanisms involved in the regulation of innate immunity in both types of organisms.
Taking into account the crucial contribution of the innate immune response both to fighting HIV infection and activating the adaptive immune response, it is clearly advantageous for HIV to produce a protein that interferes with innate immunity pathways. Recently, TLR3 and TLR4 have been implicated in antiviral responses (Alexopoulou et al., 2001; Doyle et al., 2002). Therefore, Vpu may inhibit the innate antiviral response activated by these two receptors, facilitating the proliferation of HIV-1 inside the host. It will be worthwhile to determine whether Vpu specifically interferes with the TLR/NF-κB pathway in vertebrates, as it does with the TNF-α pathway.
Finally, our study provides an opportunity for using powerful enhancer and suppressor genetic screens in flies to identify other host factors that interact with Vpu and, thereby, to gain further insight into how this protein manipulates basic cellular processes such as the degradation pathway.
Methods
Fly stocks.
Vpu2/6 is a mutant form of Vpu, in which Ser 52 and Ser 56 have been replaced by asparagine residues. Complementary DNAs encoding Vpu and Vpu2/6 originate from an HIV-Lai isolate. They were subcloned into a pUAST plasmid vector with and without a carboxy-terminal haemagglutinin (HA) tag. The same insertions, UAS–Vpu2/6 (X chromosome) and UAS–Vpu (third chromosome), were used in all experiments in this study. UAS–Vpu–HA and UAS–Vpu2/6–HA were used in calf intestinal phosphatase experiments. CantonS was used as a wild-type strain. Other strains used have been described elsewhere (Leulier et al., 2000; Grima et al., 2002).
Survival assays and northern blot analysis.
Survival assays and northern blot analysis were performed as described in Leulier et al. (2000). Forty flies were used for survival analysis and the ribosomal protein rp49 probe was used as a loading control in northern blots.
Immunofluorescence, cell culture and transfection, protein extractions, western blot analysis, calf intestinal phosphatase experiments and reporter assays.
See supplementary information online.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/4-embor936-s1.pdf).
Supplementary Material
Supplementary Information
Acknowledgments
The authors would like to thank R. Khush and E. De Gregorio for help, discussions and critical reading of the manuscript, S. Netter for help with the transgenic lines, R. Steward and S. Wasserman for Cactus antibodies, J.-L. Imler for plasmids, N. Silverman for the Torso–Pelle S2 cell line, J.-L. Becker for S2 cells and C. Besnard-Guérin for communication of unpublished results. This work was supported by Agence Nationale de Recherche sur le Sida (ANRS), Sidaction, Comité de Paris de la Ligue Contre le Cancer and Agence de Recherche sur le Cancer (ARC). C.M. was supported by Sidaction and ANRS.
References
- Akari H., Bour S., Kao S., Adachi A. & Strebel K. ( 2001) The human immunodeficiency virus type 1 accessory protein Vpu induces apoptosis by suppressing the nuclear factor κB-dependent expression of antiapoptotic factors. J. Exp. Med., 194, 1299–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulou L., Holt A.C., Medzhitov R. & Flavell R.A. ( 2001) Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature, 413, 732–738. [DOI] [PubMed] [Google Scholar]
- Belvin M.P. & Anderson K.V. ( 1996) A conserved signaling pathway: the Drosophila toll–dorsal pathway. Annu. Rev. Cell Dev. Biol., 12, 393–416. [DOI] [PubMed] [Google Scholar]
- Bour S., Perrin C., Akari H. & Strebel K. ( 2001) The human immunodeficiency virus type 1 Vpu protein inhibits NF-κB activation by interfering with βTrCP-mediated degradation of IκB. J. Biol. Chem., 276, 15920–15928. [DOI] [PubMed] [Google Scholar]
- Doyle S. et al. ( 2002) IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity, 17, 251–263. [DOI] [PubMed] [Google Scholar]
- Dushay M., Asling B. & Hultmark D. ( 1996) Origins of immunity: Relish, a compound Rel-like gene in the antibacterial defense of Drosophila. Proc. Natl Acad. Sci. USA, 93, 10343–10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman M. & Malim M.H. ( 1998) HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science, 280, 1880–1884. [DOI] [PubMed] [Google Scholar]
- Fernandez N.Q., Grosshans J., Goltz J.S. & Stein D. ( 2001) Separable and redundant regulatory determinants in Cactus mediate its dorsal group dependent degradation. Development, 128, 2963–2974. [DOI] [PubMed] [Google Scholar]
- Fong A. & Sun S.C. ( 2002) Genetic evidence for the essential role of β-transducin repeat-containing protein in the inducible processing of NF-κB2/p100. J. Biol. Chem., 277, 22111–22114. [DOI] [PubMed] [Google Scholar]
- Galindo R.L., Edwards D.N., Gillespie S.K. & Wasserman S.A. ( 1995) Interaction of the pelle kinase with the membrane-associated protein tube is required for transduction of the dorsoventral signal in Drosophila embryos. Development, 121, 2209–2218. [DOI] [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Chelot E., Papin C., Limbourg-Bouchon B. & Rouyer F. ( 2002) The F-box protein slimb controls the levels of clock proteins period and timeless. Nature, 420, 178–182. [DOI] [PubMed] [Google Scholar]
- Heissmeyer V., Krappmann D., Hatada E.N. & Scheidereit C. ( 2001) Shared pathways of I-κB kinase-induced SCF(β-TrCP)-mediated ubiquitination and degradation for the NF-κB precursor p105 and I-κB-α. Mol. Cell. Biol., 21, 1024–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D. ( 2003) Drosophila immunity: paths and patterns. Curr. Opin. Immunol., 15, 12–19. [DOI] [PubMed] [Google Scholar]
- Jiang J. & Struhl G. ( 1998) Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature, 391, 493–496. [DOI] [PubMed] [Google Scholar]
- Karin M. & Ben-Neriah Y. ( 2000) Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol., 18, 621–663. [DOI] [PubMed] [Google Scholar]
- Khush R.S., Cornwell W.D., Uram J.N. & Lemaitre B. ( 2002) A ubiquitin–proteasome pathway represses the Drosophila immune deficiency signaling cascade. Curr. Biol., 12, 1728–1737. [DOI] [PubMed] [Google Scholar]
- Kroll M., Margottin F., Kohl A., Renard P., Durand H., Concordet J.P., Bachelerie F., Arenzana-Seisdedos F. & Benarous R. ( 1999) Inducible degradation of IκB-α by the proteasome requires interaction with the F-box protein h-β-TrCP. J. Biol. Chem., 274, 7941–7945. [DOI] [PubMed] [Google Scholar]
- Lang V., Janzen J., Fischer G.Z., Soneji Y., Beinke S., Salmeron A., Allen H., Hay R.T., Ben-Neriah Y. & Ley S.C. ( 2003) β-TrCP-mediated proteolysis of NF-κB1 p105 requires phosphorylation of p105 serines 927 and 932. Mol. Cell. Biol., 23, 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B., Nicolas E., Michaut L., Reichhart J. & Hoffmann J. ( 1996) The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell, 86, 973–983. [DOI] [PubMed] [Google Scholar]
- Leulier F., Rodriguez A., Khush R.S., Abrams J.M. & Lemaitre B. ( 2000) The Drosophila caspase Dredd is required to resist Gram-negative bacterial infection. EMBO Rep., 1, 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levashina E., Ohresser S., Lemaitre B. & Imler J. ( 1998) Two distinct pathways can control expression of the Drosophila antimicrobial peptide metchnikowin. J. Mol. Biol., 278, 515–527. [DOI] [PubMed] [Google Scholar]
- Margottin F., Bour S.P., Durand H., Selig L., Benichou S., Richard V., Thomas D., Strebel K. & Benarous R. ( 1998) A novel human WD protein, h-β-TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell, 1, 565–574. [DOI] [PubMed] [Google Scholar]
- Nicolas E., Reichhart J.M., Hoffmann J.A. & Lemaitre B. ( 1998) In vivo regulation of the IκB homologue cactus during the immune response of Drosophila. J. Biol. Chem., 273, 10463–10469. [DOI] [PubMed] [Google Scholar]
- Reach M., Galindo R.L., Towb P., Allen J.L., Karin M. & Wasserman S.A. ( 1996) A gradient of cactus protein degradation establishes dorsoventral polarity in the Drosophila embryo. Dev. Biol., 180, 353–364. [DOI] [PubMed] [Google Scholar]
- Silverman N., Zhou J., Stöven S., Pandey N., Hultmark D. & Maniatis T. ( 2000) A Drosophila IκB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev., 14, 2461–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer E., Jiang J. & Chen Z.J. ( 1999) Signal-induced ubiquitination of IκB-α by the F-box protein Slimb/β-TrCP. Genes Dev., 13, 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöven S., Ando I., Kadalayil L., Engström Y. & Hultmark D. ( 2000) Activation of the Drosophila NF-κB factor Relish by rapid endoproteolytic cleavage. EMBO Rep., 1, 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöven S., Silverman N., Junell A., Hedengren-Olcott M., Erturk D., Engstrom Y., Maniatis T. & Hultmark D. ( 2003) Caspase-mediated processing of the Drosophila NF-κB factor Relish. Proc. Natl Acad. Sci. USA, 100, 5991–5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauszig S., Jouanguy E., Hoffmann J.A. & Imler J.L. ( 2000) Toll-related receptors and the control of antimicrobial peptide expression in Drosophila. Proc. Natl Acad. Sci. USA, 97, 10520–10525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauszig-Delamasure S., Bilak H., Capovilla M., Hoffmann J.A. & Imler J.L. ( 2002) Drosophila MyD88 is required for the response to fungal and Gram-positive bacterial infections. Nature Immunol., 3, 91–97. [DOI] [PubMed] [Google Scholar]
- Yaron A., Hatzubai A., Davis M., Lavon I., Amit S., Manning A.M., Andersen J.S., Mann M., Mercurio F. & Ben-Neriah Y. ( 1998) Identification of the receptor component of the IκB-α ubiquitin ligase. Nature, 396, 590–594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


