Abstract
The product of adenovirus type 5 (Ad5) gene IX, protein IX (pIX), is a multifunctional protein that stabilizes the viral capsid and has transcriptional activity. We show that pIX also contributes to the Ad5-induced reorganization of the host-cell nuclear ultrastructure: pIX induces the formation of specific and dynamic nuclear inclusions, and the host promyelocytic leukaemia (PML) protein, which is the main structural organizer of PML bodies, is stably relocated and confined within the pIX-induced inclusions late in infection. Our results suggest that Ad5 has evolved a unique strategy that leads to the sustained neutralization of PML bodies throughout infection, thereby ensuring optimal viral proliferation.
Introduction
The product of the adenovirus type 5 (Ad5) intermediate gene IX, protein IX (pIX) is known to contribute to capsid stability (Furcinitti et al., 1989). We have shown that pIX also functions as a transcriptional activator (Lutz et al., 1997). In addition, accumulation of pIX, whether expressed alone or during infection, induces the formation of specific nuclear structures, which are known as clear amorphous (c.a.) inclusions because of their lower density, as observed by electron microscopy (EM; Rosa-Calatrava et al., 2001).
To characterize the functions of c.a. inclusions, we followed their formation and fate during the remodelling of infected nuclei. Although neither viral transcripts nor capsid proteins were detected in these inclusions, the cellular promyelocytic leukaemia (PML) protein (de Thé et al., 1991) has been found inside them late in infection. PML is the structural organizer of host nuclear domains, known as ND10 or PML bodies (Maul et al. 2000), which are thought to be involved in the regulation of several cellular processes, including cell growth, differentiation (Wang et al., 1998) and apoptosis (Quignon et al., 1998; Zhong et al., 2000). ND10 bodies have also been shown to participate in cellular antiviral processes (Chee et al., 2003). Several viruses, including Ad5, have adapted to counteract these functions by disrupting ND10 bodies (Everett, 2001). In the case of Ad5, the viral E4 open reading frame 3 (ORF3) product induces the redistribution of PML to viral 'fibrous-like' structures during the early phase of infection (Carvalho et al., 1995). Our results show that pIX takes part in this Ad5-mediated neutralization of ND10 bodies by stably relocating the PML protein within c.a. inclusions during the late phase of infection, and thereby contributing to optimal viral proliferation.
Results and Discussion
The coiled-coil domain of pIX and clear amorphous inclusions
As shown previously (Rosa-Calatrava et al., 2001), recombinant pIX induces specific nuclear inclusions independently from other viral proteins. In the context of viral infection, immunofluorescence staining also showed a 'speckled' pattern of pIX distribution throughout the entire nucleus at 18 h post-infection (Fig. 1A). These structures grew progressively in size (reaching up to 1 μm in diameter) and diminished in number due to their fusion late in infection while relocating towards the nuclear periphery (Fig. 1B). When cells were infected with Ad5 (IX/V117D), a virus with a mutation that disrupts the pIX carboxy-terminal coiled-coil domain, such inclusions were no longer detected. Instead, immunofluorescence labelling showed a scattered distribution of this pIX variant in both the cytoplasm and nucleus at all times post-infection (Fig. 1C).
Figure 1.
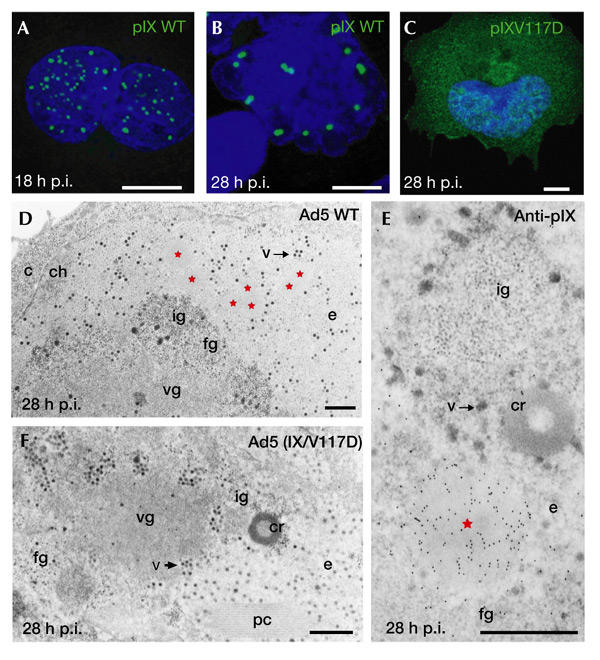
Adenovirus 5 protein IX induces specific, dynamic nuclear inclusions. (A–C) Immunofluorescence staining of protein IX (pIX). A549 cells were infected with wild-type (WT) adenovirus type 5 (Ad5) for 18 h (A) and 28 h (B), or with Ad5 (IX/V117D) for 28 h (C). (D–F) Electron microsopy (EM) analysis. At 28 h after infection of A549 cells with wild-type Ad5, the pIX inclusions (red stars) appear as clear amorphous (c.a.) inclusions (D). At the same time, no c.a. inclusions are detected in the nucleus of Ad5 (IX/V117D)-infected cells (F). Immunoelectron microscopy labelling of pIX gives a strong and specific labelling of c.a. inclusions (E). c, cytoplasm; ch, chromatin; cr, compact ring; e, perinuclear translucent area; fg, fibrilogranular region; ig, interchromatin granules; pc, protein crystal; p.i., post-infection; v, virus; vg, viral genome-storage site. Scale bars, 10 μm (A–C) and 1 μm (D–F).
Using EM, the pIX-induced structures appeared as c.a. inclusions, which were pushed progressively toward the nuclear periphery (the electron-translucent area) due to the extensive accumulation of viral material late in infection (Fig. 1D). As revealed by immunoelectron microscopy (IEM), these c.a. inclusions were highly and specifically decorated with pIX antibodies. With the exception of the fibrilogranular network, the translucent area and virions, no other cellular or Ad-induced structures were labelled (Fig. 1E). When cells were infected with the Ad5 (IX/V117D) mutant, apart from the absence of c.a. inclusions, no other difference from infection with wild-type Ad5 was seen by EM (Fig. 1F). Together, these results show further the specific contribution of pIX to the formation of nuclear c.a. inclusions and confirm the strong requirement of its coiled-coil domain for their formation during infection.
No basic viral functions in pIX inclusions
In addition to its ability to form nuclear inclusions, pIX is a transcriptional activator. However, these two properties are separable: first, mutants have been characterized that separately retain the transactivating function or the capacity to form inclusions (Rosa-Calatrava et al., 2001); second, c.a. inclusions from Ad5-infected cells are devoid of viral DNA (see supplementary information online) and transcripts (Fig. 2A); third, no components of the splicing machinery were identified in c.a. inclusions (see supplementary information online); finally, pIX inclusions were progressively consigned to the transcriptionally silent nuclear translucent area late in infection (see above). These results indicate that c.a. inclusions are involved neither in transcriptional nor post-transcriptional processes during infection.
Figure 2.
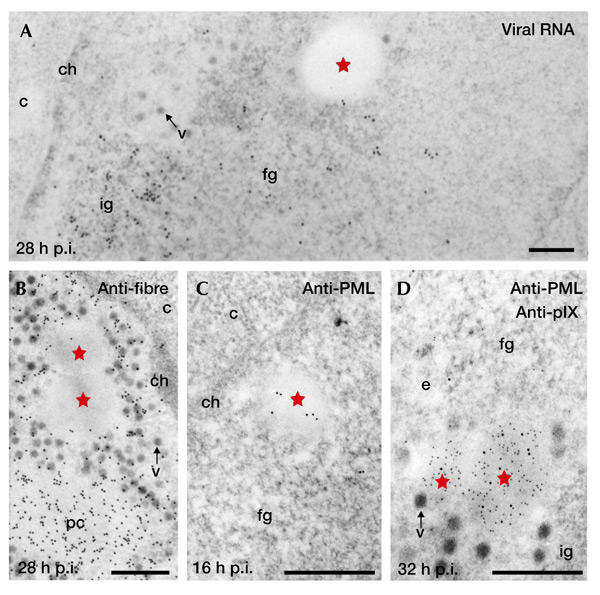
Protein IX inclusions are devoid of viral RNA and capsid proteins but contain promyelocytic leukaemia protein. (A) Localization by electron microscopy (EM) in situ hybridization of viral RNA using viral genomic probes. The clear amorphous (c.a.) inclusions (red stars) are devoid of gold particles, whereas the clusters of interchromatin granules (ig) and/or the fibrillogranular (fg) network are labelled. (B) Immunoelectron microscopy (IEM) labelling of viral fibre protein shows that c.a. inclusions are not labelled, whereas the protein crystals (pc) and most of the viruses (v) are strongly stained. (C) IEM labelling of promyelocytic leukaemia protein (PML) and (D) double IEM labelling of PML and protein IX (pIX; polyclonal anti-PML: 10-nm gold particles; monoclonal anti-pIX: 5-nm gold particles). At 16 h post-infection (p.i.), gold particles indicating the localization of PML are scattered over the nascent pIX-induced, small, irregularly shaped c.a. inclusion. At 32 h p.i., the PML protein colocalizes with pIX within the c.a. inclusions, whereas the fibrillogranular network (fg), the cluster of interchromatin granules (ig) and the electron translucent area (e) are devoid of PML staining. c, cytoplasm; ch, chromatin; v, virus. Scale bars, 1 μm.
As pIX stabilizes interactions in the Ad5 capsid, we analysed whether major capsid proteins colocalize with c.a. inclusions. These were devoid of fibre proteins (Fig. 2B) and penton base (see supplementary information online), and were weakly labelled with anti-hexon antibodies (see supplementary information online). As expected, all three antibodies used gave an intense labelling of the viruses and protein crystals.
It seems, therefore, that the function of pIX inclusions is completely unlinked to the fundamental viral processes of DNA transcription, RNA maturation and virion assembly.
Ad5 infection relocates PML in clear amorphous inclusions
Previous IEM analyses showed the presence of PML in what were then unidentified viral nuclear inclusions during the late phase of Ad5 infection (Puvion-Dutilleul et al., 1995). It was later shown that these viral inclusions correspond to those induced by pIX. PML was, in fact, associated with all c.a. inclusions from the first stage of their formation (Fig. 2C) until late in infection, as confirmed by double IEM (32 h post-infection; Fig. 2D). Furthermore, ND10 was not detected during the whole late period of infection (Fig. 2D; and see below).
We followed the time course of PML redistribution during Ad5 infection by immunofluorescence staining (Fig. 3A). PML bodies were disrupted by the Ad5 E4 ORF3 product, which redistributed PML into a meshwork of fibrous tracks early in infection (Fig. 3Ab). At later times (16 h post-infection), the pIX inclusions colocalized most often with these tracks or were found in close proximity to them (Fig. 3Ac). On pIX accumulation (24 h post-infection), this colocalization intensified (Fig. 3Ad–f), whereas the E4 ORF3-induced tracks vanished progressively to finally become almost undetectable (Fig. 3Ag–l). At 32 h post-infection, an almost complete loss of PML staining occurred, whether monoclonal (Fig. 3Ag–i) or polyclonal (Fig. 3Aj–l; the same as in IEM experiments) antibodies were used.
Figure 3.
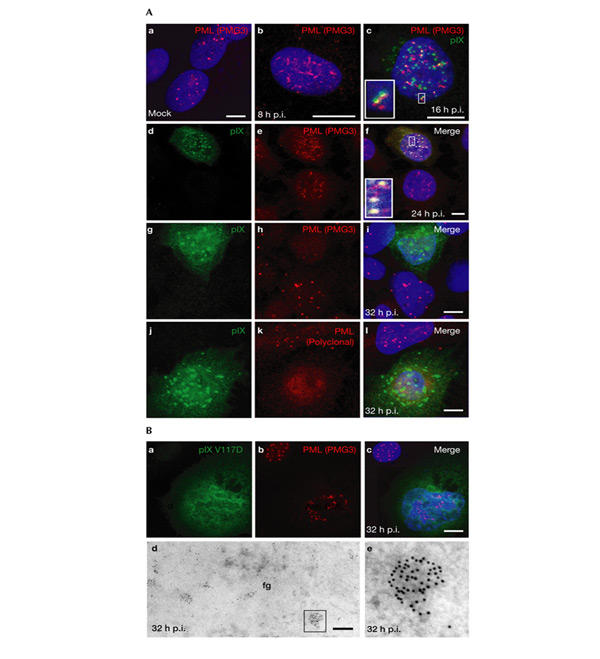
Promyelocytic leukaemia protein detection is impaired late after infection with wild-type adenovirus type 5 but not with adenovirus type 5 (IX/V117D). (A) Immunofluorescence staining of protein IX (pIX) and promyelocytic leukaemia protein (PML) in mock-infected A549 cells (Aa) or in A549 cells infected with wild-type adenovirus type 5 (Ad5) (Ab–Al). (Ac), (Af), (Ai) and (Al) are the merged confocal images obtained with both fluorochromes separately. Insets in (Ac) and (Af) correspond to enlarged details. Nuclear domain 10 (ND10) bodies of uninfected nuclei are visible in the upper left part of (Af), and in the lower and upper parts of the panels (Ai) and (Al), respectively. A nucleus at a stage of infection similar to that shown in (Ab) is visible in the lower part of (Af). (Ba–Bc) Ad5 (IX/V117D)-infected A549 cells were immunofluorescence stained as described above. An uninfected nucleus is visible at the top left corners. (Bd,Be) Immuno-EM labelling of PML. PML is scattered within the fibrillogranular network (fg) or accumulates homogenously in nodules ((Bd); and detail in (Be)), resembling primary immature ND10 bodies. Scale bars, 10 μm in (A, Ba–c), 0.5 μm in (Bd,e). p.i., post-infection; PMG3, an anti-PML antibody.
The fate of PML was different in the absence of pIX inclusions on infection with Ad5 (IX/V117D): late in infection, immunofluorescence staining showed that PML remained clearly detectable, with levels similar to those seen in uninfected cells (Fig. 3Bb,c). Moreover, several PML-containing nodules, similar to primary PML bodies (Lallemand-Breitenbach et al., 2001), were detected in the viral fibrillogranular network by IEM staining (Fig. 3Bd,e). In addition, a strong, diffuse nuclear PML background was detected, similar to that seen in uninfected cells.
The loss of PML immunofluorescence reactivity with wild-type Ad5 did not correspond to a degradation of the PML protein. As described above (Fig. 2D), PML remained clearly detectable by IEM labelling in the c.a. inclusions throughout infection. Furthermore, as suggested by western blot analysis (Fig. 4), the pattern and overall amounts of PML and its post-translationally modified isoforms were not significantly altered in Ad5-infected cells compared to uninfected cells, even late in infection, if adjusted to actin protein levels. Our observation that PML is not degraded during infection is in agreement with the results of Leppard & Everett (1999). The discrepancy between immunofluorescence and IEM responsiveness might therefore be because of the inaccessibility of PML to the antibodies inside intact c.a. inclusions (as in the case of immunofluorescence staining). Conversely, PML might become exposed at the section surface of inclusions in nuclear slices (as in the case of IEM).
Figure 4.
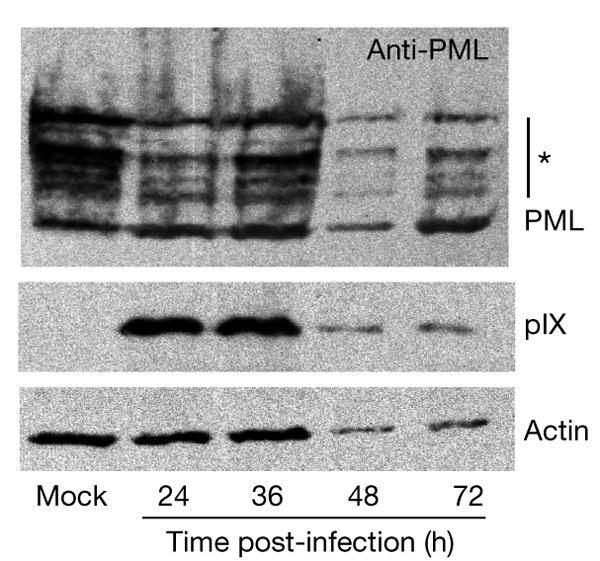
Adenovirus type 5 infection does not induce degradation of the promyelocytic leukaemia protein. A549 cells were treated with 1,000 units ml−1 of interferon-γ (IFN-γ) for 24 h, before mock infection and infection with wild-type adenovirus type 5 (20 infective units per cell). Whole-cell extracts were analysed by immunoblotting with polyclonal chicken antibodies against promyelocytic leukaemia protein (PML). Positions of PML and IFN-induced PML isoforms (asterisk) are indicated. The same blot was reprobed with polyclonal antibodies against protein IX (pIX) and actin, as indicated. The infection cycle, although retarded by IFN treatment, ends in cell death and lysis. The decrease in the actin, pIX and PML signals thus corresponds to the lysis of infected cells, and therefore to a loss of cellular material, late in infection.
The finding by Leppard & Everett (1999) that the pattern of PML isoforms is gradually modified is in contrast to the unchanged pattern seen under our experimental conditions. This difference might be either related to the fact that we have used interferon to increase PML expression or linked to the cell types used (A549 lung epithelial cells in our experiments and HEp2 epidermoid carcinoma cells in those of Leppard & Everett). In either case, it will be of interest to determine whether the sequestration of PML into the pIX inclusions contributes to the status of its post-translational modifications.
Our results show that late in Ad5 infection, concomitantly with pIX accumulation, PML is subject to a further redistribution from the early E4 ORF3-induced fibrous structures, leading to its progressive relocation and confinement within the c.a. inclusions. Moreover, the nucleoplasmic pool of PML seems to have the same fate. These observations might reflect a strategy to neutralize ND10 bodies and prevent their re-formation by a sequential redistribution and confinement of their chief organizer, PML, into virus-induced structures during the course of infection.
Recombinant pIX specifically assembles around PML bodies
We next examined the intrinsic effects of pIX on ND10 by overexpressing pIX in a non-viral context. As shown by immunofluorescence staining, pIX inclusions assembled systematically over or next to PML bodies (Fig. 5A). The persistent speckled pattern of PML distribution and of other ND10 components, such as Sp100 and small ubiquitin-related modifier 1 (SUMO1; Fig. 5Ab,c), colocalizing with the pIX inclusions, indicated that ND10 bodies were not disrupted and that there was neither nuclear redistribution nor degradation of their components. The fact that the ND10 bodies remained compact might explain their persistent recognition by the antibodies despite pIX accumulating over them.
Figure 5.
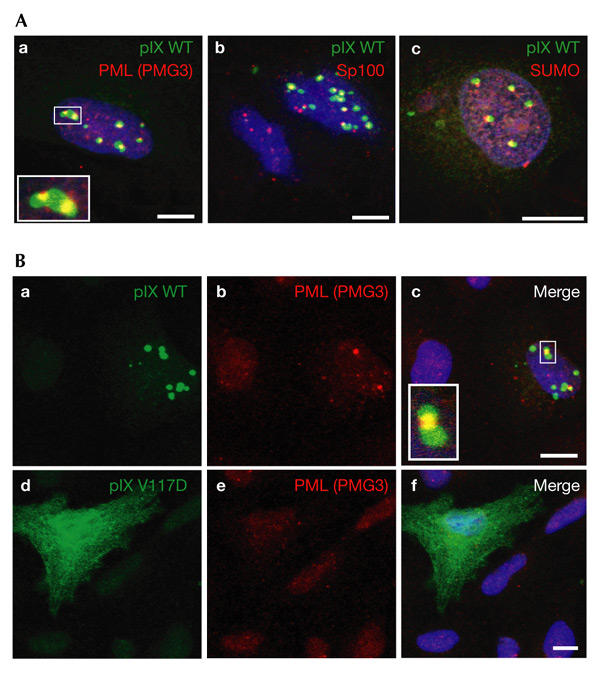
Protein IX inclusions systematically accumulate over ND10 bodies and protect them against arsenite-induced disruption. (A) Thirty-six hours after transfection with a plasmid expressing wild-type (WT) protein IX (pIX), A549 cells were reacted with monoclonal anti-PML (promyelocytic leukaemia protein) or anti-SUMO (small ubiquitin-like modifier) and polyclonal anti-pIX antibodies, except in (Ab), in which monoclonal anti-pIX and rabbit anti-Sp100 antibodies were used. The inset in (Aa) is an enlargement of part of the main image (framed). (B) Twelve hours after transfection with plasmids expressing wild-type pIX or pIX (V117D), A549 cells were treated for 36 h with 1 μM arsenite before being reacted with monoclonal anti-PML and rabbit polyclonal anti-pIX antibodies. The inset in (Bc) is an enlargement of part of the main image (framed). Scale bars, 10 μm. PMG3, an anti-PML antibody.
To assess the specificity of pIX accumulation over ND10 bodies, we investigated the effects of a set of pIX mutants (Rosa-Calatrava et al., 2001) on this colocalization (see supplementary information online). The integrity of the pIX coiled-coil domain was found to be essential for both pIX association with the nuclear matrix and its colocalization with ND10.
It has been shown previously that prolonged exposure to arsenite results in the disruption of ND10 bodies (Lallemand-Breitenbach et al., 2001). We therefore examined the effect of arsenite on ND10 over which pIX inclusions had pre-assembled. Cells were transfected with plasmids encoding wild-type or mutant pIX and were subsequently subjected to treatment with arsenite. Immunofluorescence staining showed that ND10 bodies were preserved, at least in part, when colocalized with pIX inclusions (Fig. 5Ba–c; and see supplementary information online), whereas they were readily disrupted in their absence (Fig. 5Bd–f). This suggests that ND10 bodies might be 'insulated' from their environment by the 'concrescence' of pIX around them. In addition, pIX inclusions might directly sequester factors that are implicated in the arsenite-induced disruption of ND10 bodies. We propose that a similar confinement of the PML protein may also occur late in infection, as suggested by the irreversible disappearance of both ND10 and the nucleoplasmic pool of PML, and by the inaccessibility of PML to antibodies (Fig. 3).
Impairing pIX inclusions reduces viral yield
To evaluate directly the contribution of the pIX inclusions to infection progression, we compared the virus yields reached after parallel infections with wild-type Ad5 and the Ad5 (IX/V117D) mutant. This mutation, which abolishes the formation of c.a. inclusions, does not affect pIX capsid integration (Rosa-Calatrava et al., 2001), thermal stability (see supplementary information online) or viral maturation (data not shown) of corresponding virions. Consequently, both viruses showed similar levels of infectivity (see supplementary information online). However, as shown in Fig. 6A, the overall production of the mutant virus progeny was significantly reduced compared with that of the wild-type virus. Moreover, mutant recovery was not improved on prolonged infection.
Figure 6.
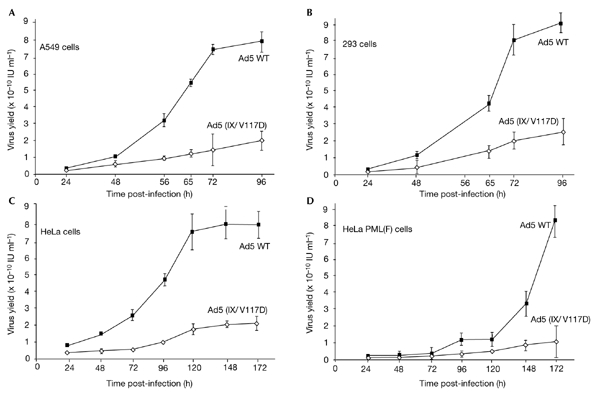
The absence of protein IX inclusions and/or overexpression of promyelocytic leukaemia protein both reduce viral growth rates and limit virus accumulation. A549 cells (A), 293 cells (B) HeLa cells (C) and HeLa cells overexpressing F-epitope-tagged PML protein (PML(F); D) were infected with wild-type (WT) adenovirus type 5 (Ad5) or Ad5 (IX/V117D) viruses (three particles per cell) and harvested at various times post-infection. Virions were released by freeze-thawing and were titrated on 293 cells. Average values (with error bars) of triplicate infections were plotted. IU, infective units.
As the V117D mutation also impaired pIX transcriptional activity (Rosa-Calatrava et al., 2001), at least part of the limitation in viral yield might be due to the retarded accumulation of viral late proteins in the absence of functional pIX. However, this was not the case, as almost wild-type levels of viral proteins were seen in the mutant-infected cells late in infection (data not shown). Furthermore, when the time course of virus production was measured in 293 cells, the recovery of the mutant virus still remained clearly below that of wild-type Ad5, despite the constitutive supply of the Ad5 E1a transcription activator by these cells (Fig. 6B).
If the lack of PML sequestration by the V117D pIX variant is directly responsible for the reduced growth of the mutant virus, then overexpression of PML should have a more dramatic effect on mutant growth than on wild-type virus recovery. To investigate this possibility, the production of wild-type Ad5 or Ad5 (IX/V117D) in either normal cells or cells overexpressing F-epitope-tagged PML protein was compared. Consistent with our hypothesis, maximal recovery of wild-type Ad5 was still reached, although clearly retarded, in the 'high PML' context, whereas the yield of mutant virus was reduced even further in these cells (Fig. 6C,D). These results, which show the contribution of pIX inclusions to maximal viral proliferation, confirm that they do so by neutralizing PML to prevent ND10 re-formation.
We conclude, therefore, that during Ad5 infection, the PML protein is sequentially relocated in two specific virus-induced structures (Fig. 7): starting at 6–8 h post-infection, the E4 ORF3 product functions as an early 'disrupter' by redistributing the PML protein from ND10 bodies into 'fibrous-like' track structures; after the beginning of viral DNA replication, pIX, which acts as a late 'PML neutralizer', drives the progressive and stable confinement of both nucleoplasmic and track-associated PML into the c.a. inclusions, thereby preventing the reassembly of PML bodies late in infection and ensuring optimal viral proliferation. In this respect, the construction and characterization of an Ad5 double-mutant, defective in both E4 ORF3-induced disruption of ND10 and pIX-induced sequestration of PML, will be useful.
Figure 7.
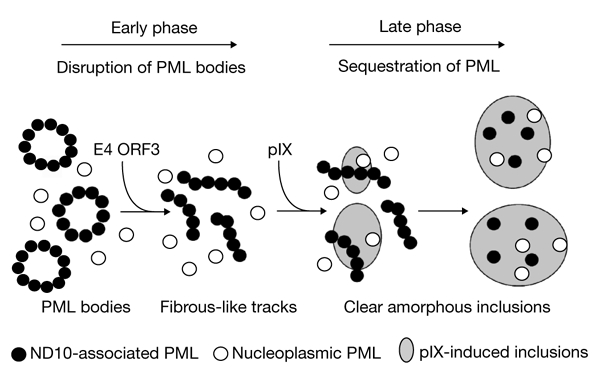
The adenovirus type 5 strategy for initiating and maintaining PML-body neutralization. Early in infection, adenovirus type 5 (Ad5) E4 open reading frame 3 (ORF3) induces the disruption of nuclear domain 10 (ND10) bodies by redistributing the PML protein into viral 'fibrous-like' tracks. After the onset of viral DNA replication, proten IX (pIX) accumulates and targets PML, progressively sequestering it within clear amorphous inclusions late in infection. PML, promyelocytic leukaemia protein.
Speculation
Despite its location within region E1, gene IX is not transcribed before viral DNA replication (Lutz et al., 1997). One may wonder why pIX is not produced earlier in infection, which would allow the immediate neutralization of ND10 and associated anti-viral processes. ND10 disruption by E4 ORF3, by making all ND10-associated cellular functions and factors available to the viral machinery, might be more beneficial to the virus than their immediate blocking by pIX. By contrast, PML sequestration within the pIX inclusions late in infection would be well adapted to efficient virus growth. Thus, the resulting blockage of the re-formation of ND10 bodies might prevent their potential ability to titrate cellular and/or viral factors, which are essential, for example, in virion assembly, DNA packaging and virus release.
As well as PML, other cellular proteins (Sp100, double-stranded-RNA-dependent protein kinase and casein kinase II) have been found in pIX inclusions (Souquere-Besse et al., 2002). It is likely, therefore, that these inclusions contribute further to virus production by trapping cellular proteins, which may hamper the progression of the infectious cycle.
Clearly, the incidence, on host cells, of the pIX properties described here will have to be taken into account when designing Ad5-based vectors for therapeutic purposes (see supplementary information online).
Methods
Cells, viruses and plasmids.
A549, 293, HeLa and HeLa PML(F) (which stably overexpresses F-epitope-tagged PML protein; Muller et al., 1998) cells were infected with wild-type or mutated Ad5 at 1 or 20 infective units (IU) per cell. The V117D mutation (Rosa-Calatrava et al., 2001) was introduced into Ad5 by homologous recombination, generating the Ad5 (IX/V117D) virus. Viral growth, purification and titration were carried out as described in Legrand et al. (1999). Plasmids encoding wild-type pIX or its V117D derivative are described in Rosa-Calatrava et al. (2001).
Antibodies.
A monoclonal antibody (1G7) was raised against recombinant wild-type pIX (details available on request). Rabbit anti-pIX (Rosa-Calatrava et al., 2001), rabbit and chicken anti-PML antibodies, rabbit anti-Sp100 (Puvion-Dutilleul et al., 1995; Souquere-Besse et al., 2002), monoclonal anti-fibre (Legrand et al., 1999), anti-PML (PMG3; Stratagene) and anti-SUMO1 (Zymed) antibodies were used.
Transfections, cell extracts and immunoblot analysis.
A549 cells were transfected and scraped in SDS loading buffer, and proteins were separated by gel electrophoresis and detected by western blot analysis with specific antibodies using the ECL system (Amersham; Rosa-Calatrava et al., 2001).
Electron microscopy.
Infected A549 cells were harvested and fixed at various times post-infection. Ultrathin sections (80–100 nm) of embedded cells were collected on carbon-coated grids and stained as described in Puvion-Dutilleul et al. (1995). For IEM, primary and secondary antibodies (either goat anti-rabbit or anti-mouse IgG, conjugated to gold particles (5 or 10 nm in diameter)), were reacted as previously described in Puvion-Dutilleul et al. (1999). EM in situ hybridizations were performed using specific biotinylated viral polynucleotide probes, as previously described (Puvion-Dutilleul et al., 1994).
Immunofluorescence.
A549 cells were fixed and permeabilized (Rosa-Calatrava et al., 2001). After incubation for 1 h with primary antibodies, cells were washed and incubated with donkey Cy3-conjugated anti-mouse IgG and goat FITC-labelled anti-rabbit IgG (Sigma), or with donkey FITC-conjugated anti-mouse IgG and goat Cy3-labelled anti-rabbit IgG (Sigma). Nuclei were counterstained with Hoechst 33258. Cells were analysed with a confocal laser-scanning microscope.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/4-embor943-s1.pdf).
Supplementary Material
Supplementary information
Acknowledgments
We thank the IGBMC cell-culture staff, Y. Schlesinger, N. Messaddeq and S. Souquere-Besse for technical assistance, and A. Dejean for providing HeLa PML(F) cells. This work was supported by Transgene S.A. and by funds from the French Ministry of Science, the Centre National de la Recherche Scientifique, the University Louis Pasteur of Strasbourg and French charity associations: Association pour la Recherche sur le Cancer (grant numbers 9285, 5519 and 5701), Ligue Nationale Contre le Cancer and Fondation pour la Recherche Médicale.
References
- Carvalho T. et al. ( 1995) Targeting of adenovirus E1A and E4-ORF3 proteins to nuclear matrix-associated PML bodies. J. Cell Biol., 131, 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee A.V. et al. ( 2003) Promyelocytic leukemia protein mediates interferon-based anti-Herpes simplex virus 1 effects. J. Virol., 77, 7101–7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Thé H., Lavau C., Marchio A., Chomienne C., Degos L. & Dejean A. ( 1991) The PML–RAR α fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell, 66, 675–684. [DOI] [PubMed] [Google Scholar]
- Everett R.D. ( 2001) DNA viruses and viral proteins that interact with PML nuclear bodies. Oncogene, 20, 7266–7273. [DOI] [PubMed] [Google Scholar]
- Furcinitti P.S., van Oostrum J. & Burnett R.M. ( 1989) Adenovirus polypeptide IX revealed as capsid cement by difference images from electron microscopy and crystallography. EMBO J., 8, 3563–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand-Breitenbach V. et al. ( 2001) Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor α degradation. J. Exp. Med., 193, 1361–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand V., Spehner D., Schlesinger Y., Settelen N., Pavirani A. & Mehtali M. ( 1999) Fiberless recombinant adenoviruses: virus maturation and infectivity in the absence of fiber. J. Virol., 73, 907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppard K.N. & Everett R.D. ( 1999) The adenovirus type 5 E1b 55K and E4 Orf3 proteins associate in infected cells and affect ND10 components. J. Gen. Virol., 80, 997–1008. [DOI] [PubMed] [Google Scholar]
- Lutz P., Rosa-Calatrava M. & Kedinger C. ( 1997) The product of the adenovirus intermediate gene IX is a transcriptional activator. J. Virol., 71, 5102–5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul G.G., Negorev D., Bell P. & Ishov A.M. ( 2000) Properties and assembly mechanisms of ND10, PML bodies, or PODs. J. Struct. Biol., 129, 278–287. [DOI] [PubMed] [Google Scholar]
- Muller S. et al. ( 1998) Conjugation with ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus. EMBO J., 17, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvion-Dutilleul F., Bachellerie J.P., Visa N. & Puvion E. ( 1994) Rearrangements of intranuclear structures involved in RNA processing in response to adenovirus infection. J. Cell Sci., 107, 1457–1468. [DOI] [PubMed] [Google Scholar]
- Puvion-Dutilleul F., Chelbi-Alix M.K., Koken M., Quignon F., Puvion E. & de Thé H. ( 1995) Adenovirus infection induces rearrangements in the intranuclear distribution of the nuclear body-associated PML protein. Exp. Cell Res., 218, 9–16. [DOI] [PubMed] [Google Scholar]
- Puvion-Dutilleul F., Legrand V., Mehtali M., Chelbi-Alix M.K., de Thé H. & Puvion E. ( 1999) Deletion of the fiber gene induces the storage of hexon and penton base proteins in PML/Sp100-containing inclusions during adenovirus infection. Biol. Cell., 91, 617–628. [PubMed] [Google Scholar]
- Quignon F., De Bels F., Koken M., Feunteun J., Ameisen J.C. & de Thé H. ( 1998) PML induces a novel caspase-independent death. Nature Genet., 20, 259–265. [DOI] [PubMed] [Google Scholar]
- Rosa-Calatrava M., Grave L., Puvion-Dutilleul F., Chatton B. & Kedinger C. ( 2001) Functional analysis of adenovirus protein IX identifies domains involved in capsid stability, transcriptional activity, and nuclear reorganization. J. Virol., 77, 7131–7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souquere-Besse S. et al. ( 2002) Adenovirus infection targets the cellular protein kinase CK2 and RNA-activated protein kinase (PKR) into viral inclusions of the cell nucleus. Microsc. Res. Tech., 56, 465–478. [DOI] [PubMed] [Google Scholar]
- Wang Z.G. et al. ( 1998) Role of PML in cell growth and the retinoic acid pathway. Science, 279, 1547–1551. [DOI] [PubMed] [Google Scholar]
- Zhong S., Salomoni P., Ronchetti S., Guo A., Ruggero D. & Pandolfi P.P. ( 2000) Promyelocytic leukemia protein (PML) and daxx participate in a novel nuclear pathway for apoptosis. J. Exp. Med., 191, 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


