Summary
A new model for risk assessment may not only revolutionize the field of toxicology, but also have vast implications for risk assessment
We all know a daily glass of red wine reduces the risk of heart disease. However, drinking several bottles of wine a day would soon lead to liver disease, pancreatitis and other debilitating health problems. High doses of radiation cause radiation sickness, cancer and death. But what if low doses of radiation could reduce the risk of developing cancer? Traditional toxicological models of risk assessment assume that as the dose of a harmful substance increases, so too does the risk associated with it. In the absence of experimental evidence, this linear relationship is extrapolated to low doses. But evidence is growing that the relationship between low doses and risk may not always be linear after all; some toxic substances often have unexpected effects. Recognizing this toxicological oddity will not only have profound implications for toxicological and pharmacological research, but may also have a broad impact on the way in which science, regulatory agencies and the public perceive and respond to risk.
Two models have traditionally been used to describe the dose–response relationship. When assessing the risk of non-carcinogens, toxicologists use a model that assumes a linear relationship between dose and risk, which holds true down to a certain threshold. Below this threshold, no more adverse affects are observed, indicating that the exposure level is safe (threshold model, Fig. 1A). When assessing the risk of carcinogens, a more cautious model is used. The linear non-threshold (LNT) model assumes that some level of risk is always present, even at the lowest possible dose (Fig. 1B). Under this assumption, even one X-ray has the potential to cause cancer. These two
...hormesis was effectively ignored by the toxicology community until relatively recently
models constitute the backbone of risk assessment, and form the basis for evaluating chemicals and drugs, estimating risk, establishing risk-communication practices and setting environmental and occupational health standards.
Figure 1.
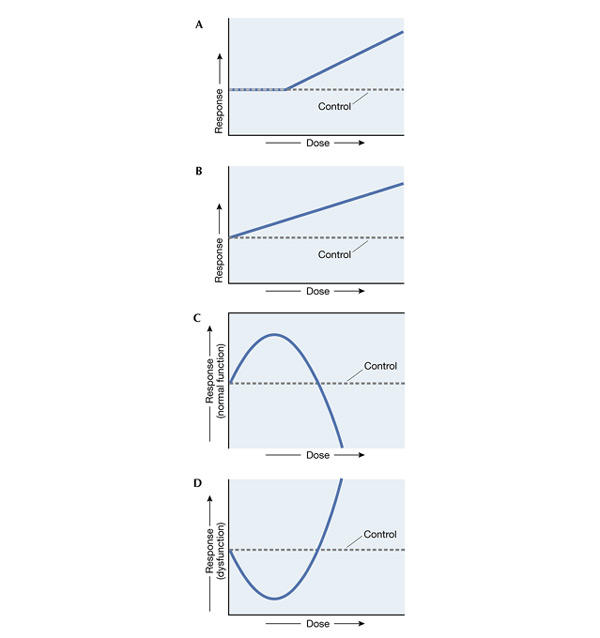
Dose–response relationships. (A) The threshold model, (B) the linear non-threshold (LNT) model, (C) the inverted U-shaped hormetic model and (D) the J-shaped hormetic model.
A third model rejects the standard assumption that effects at low doses can be extrapolated from data obtained from high doses, and instead describes the relationship as an inverted U- or J-shaped curve, depending on whether the substance causes a decrease in risk (as in growth or survival, Fig. 1C) or an increase (as in disease incidence, Fig. 1D). This combination of low-dose stimulation followed by high-dose inhibition is commonly termed 'hormesis', from the Greek word 'hormo' meaning 'to excite'. Edward Calabrese, Professor of Toxicology and Environmental Health Sciences at the University of Massachusetts (Amherst, MA, USA) and a strong advocate of the Ushaped dose–response curve, said, “I actually think there should be a paradigm shift and that the hormetic model should be the default model.”
Many common examples of hormesis can be found in our everyday lives. A modest intake of many vitamins and minerals is essential to our health, whereas excessively high doses can be damaging. Moderate alcohol consumption is now advocated, as is a moderate level of regular exercise; too much of either can cause harm to one's health. Psychologists have long recognized that mild forms of stress can promote mental and physical function, whereas extreme stress is more likely to cause mental anguish and physical ailments. However, the effects of low doses are not always beneficial; studies have shown that low doses of a tumour suppressor can actually promote tumour growth, and small amounts of various bactericides can promote bacterial colony growth. Contrary to the dose-response models now in place, risk is clearly not always linearly correlated to dose. As the Swiss physician Paracelsus noted more than 450 years ago, “the right dose differentiates a poison from a remedy.”
The concept of hormesis is not new and has been embraced by those studying epidemiology and molecular pharmacology, who were already aware of many chemicals that stimulate at low doses but inhibit at high doses (for example, aspirin and paracetamol). However, hormesis was effectively ignored by the toxicology community until relatively recently. As early as the late nineteenth century, several dose–response studies described low-dose stimulation followed by high-dose inhibition. But over the subsequent decades, a combination of factors relegated the hormetic model to scientific obscurity, to be replaced by the current risk-assessment models that are based on linear curves. In addition to having no strong advocates for hormesis, toxicologists were more interested in the upper end of the dose–response curves, where dose and risk are at their highest. Hormetic effects are difficult to measure and quantify without extensive studies using many animals, and so were not easily or frequently seen in experiments designed primarily to measure high-dose inhibition. To compound the problem, hormesis suffered from an association with the practice of homeopathy, whereby a disease is treated by administering remedies in doses so diluted that they might no longer contain even one molecule of the active ingredient. When no clear molecular mechanism could be found to support the concept of hormesis, the collective force of these obstacles led to its marginalization. Calabrese admits, “I think, in the beginning, people thought this was the toxicological version of cold fusion.”
Unlike the toxicology community, molecular pharmacologists have spent considerable time studying various dose responses, and have identified more than 30 receptor systems that show hormesis (see sidebar for a selected list of receptor systems displaying the hormetic biphasic response). The disparity between the two fields can be explained by their differing approaches to dose–response relationships: toxicology is concerned with the toxic effects of substances (commonly displayed at high dose), whereas pharmacology is primarily concerned with the therapeutic value of substances (often seen at small doses). John Doull, Professor Emeritus of Pharmacology and Toxicology at the University of Kansas Medical School (KS, USA) and one of the original authors of the leading toxicology textbook, Casarett & Doull's Toxicology: The Basic Science of Poisons (Klaassen, McGraw-Hill Professional, New York, USA; 2001), admits, “It is partly semantics and our reluctance to adopt new words” that have excluded hormesis from
If regulatory agencies can be convinced that hormetic effects need to be taken into account when establishing standards for health and the environment, this has the potential to alter dramatically the way in which risk is assessed and controlled
mainstream toxicology, and Toxicology, until only recently.
Molecular biology goes one step further than both toxicology and pharmacology by not only identifying the biological effect, but also investigating its fundamental causes. As Tony Stebbing, Honorary Fellow at the Plymouth Marine Laboratory (Plymouth, UK) said, “hormesis has much more to do with the underlying mechanism than the toxicant.” The discovery of molecular mechanisms to explain hormesis may encourage its more widespread acceptance. In the 'survival of the fittest', it follows that organisms should be best adapted to cope with optimal levels of dietary requirements (such as vitamins) and background levels of toxic substances (such as ionizing radiation or other carcinogens). Molecular biology has established that cells have sophisticated repair systems to cope with various types of damage. “Ecological criteria ultimately determine evolutionary change,” explained Peter Parsons, Emeritus Professor at the School of Genetics and Human Variation at La Trobe University (Victoria, Australia). “Organisms should evolve to survive best and live longest, or show highest fitness, in the habitats in which they most commonly occur.” This implies that the LNT model is invalid for all environmental agents, because organisms will have already developed a mechanism to cope with their effects.
The process of adaptive evolution explains how a similar hormetic reaction can be observed for such a wide range of agents that have no apparent shared physico-chemical properties. As hormetic effects have been observed for a broad range of species, substances and biological endpoints (such as growth, longevity, reproduction, immune response and many physiological and metabolic responses), there is no longer the expectation that one molecular mechanism can provide the explanation. As Calabrese pointed out, “There may be an overall strategy that cells have adopted that is played out through different tactics, and I think that the tactics are really the mechanisms that people are looking for.”box 1
More than 20 years ago, Stebbing proposed that the disturbance of homeostasis was the driving force behind the hormetic response. Organisms have homeostatic systems of biochemical and physiological control mechanisms that regulate internal processes and respond to external disturbances. These systems maintain metabolic equilibrium by over-compensating for any disruptions, such as those caused by external stress or damage. After the compensatory response neutralizes the problem and restores equilibrium, the fitness of the individual is optimized to respond to subsequent, more serious, challenges. However, high levels of stress or damage disrupt the organism beyond its limits of recovery, causing irreparable damage. Although the specific details of the mechanism may differ depending on how the organism is being challenged, the overall strategy is the same. “The underlying evolutionary process is selection for metabolic efficiency,” Parsons said.
The difficulty in identifying examples of and explanations for hormesis is due to the modest nature of the response, and the fundamental way in which dose–response relationships are measured. Studies now concentrate on the upper end of the dose scale, and aim to determine the level below which no more adverse effects are observed (the 'no observed adverse effects level', or NOAEL). For hormetic effects to be observed, studies need to be designed to include an adequate number of doses below this level. These doses need to be spaced sufficiently, and temporal measurements are required to distinguish between the initial disruption of homeostasis, the subsequent over-compensation response, and the re-establishment of homeostasis. Most importantly, animal models need to have a sufficiently high background level of the disease or condition being studied, so that the hormetic reduction of incidence can be quantified. These requirements, plus the need for reproducibility, make it much more difficult for hormetic effects to be studied and understood by classical toxicology experiments, and call for a significant change in the way these experiments are conducted.
Although, as Calabrese said, “The dose-response is the basic foundation of toxicology,” the study of hormetic behaviour is not a primary concern of toxicologists, who are more interested in the study of the toxic effects of substances. As the search for the physiological and biochemical basis for hormesis becomes more important, engaging other fields in the study of hormesis is crucial. Calabrese believes “Broad acceptance is going to come from the molecular community.” Like Doull, he admits that different fields “use different terminology” to describe similar phenomena. “There may be some synergism by people who are studying how organisms respond to low-level stressor agents that normally never see each other's work.” Inviting a wider scientific audience to examine hormesis might also encourage further discussion on its extensive implications. If regulatory agencies can be convinced that hormetic effects need to be taken into account when establishing standards for health and the environment, this has the potential to alter dramatically the way in which risk is assessed and controlled. It may be that the LNT model is discarded for cancer risk assessment, and a threshold is introduced, meaning that low levels of carcinogens would be considered non-harmful. This could then have repercussions on the levels of permissible water and air contaminants, and the extent to which hazardous wastes or chemical spills are cleaned up. Risk communication practices would have to change, which would then influence policy making and the legal definition of risk. Most likely, the public's perception of the dangers of chemicals is the biggest obstacle to overcome. Calabrese admits that people have “basically been afraid of this thing because of what they perceive are its implications.”
Understanding hormesis could improve the understanding of other biological phenomena, such as the ageing process, the course of bacterial infestations and insect outbreaks, or the relationship between stress and human performance
But the news is not all bad. Although the way in which chemicals and drugs are evaluated would have to change, this would hopefully improve human health and longevity. Understanding hormesis could improve the understanding of other biological phenomena, such as the ageing process, the course of bacterial infestations and insect outbreaks, or the relationship between stress and human performance. Ultimately, understanding hormesis should enhance the quality of life. “The only way there can be a paradigm shift is if you [...] show overwhelming evidence that your concept is actually superior,” Calabrese said. “If people think that the hormesis idea actually helps them explain things better than the current model, then it'll be successful.”
Receptor systems displaying hormetic dose–response relationships.
Adenosine
Adrenoreceptor
Bradykinin
Cholecystokinin (CCK)
Corticosterone
Dopamine
Endothelin
Epidermal growth factor
5-Hydroxytryptamine (5-HT)
Human chorionic gonadotropin
Muscarinic acetylcholine
Neuropeptides (for example, vasopressin)
Nitric oxide
N-methyl-D-aspartate (NMDA)
Oestrogen
Opioid
Platelet-derived growth factor
Prolactin
Prostaglandin
Somatostatin
Spermine
Testosterone
Transforming growth factor-β
Tumour necrosis factor-α
Adapted from Calabrese & Baldwin, Annu. Rev. Pharmacol. Toxicol., 43, 175–197; 2003


