Abstract
The R-SNARE VAMP4, which contains a dileucine motif, binds to the AP-1 (adaptor protein-1) subunit μ1a, but not μ1b, or the GGAs (Golgi-associated gamma ear containing ARF binding proteins). Serine 20 and leucines 25,26 are essential for this binding. AP-1 association with VAMP4 is enhanced when serine 30, in an acidic cluster, is phosphorylated by casein kinase 2. This phosphorylation-dependent modulation of AP-1 binding is mediated by PACS-1 (phosphofurin acidic cluster sorting protein). Ablation of both the dileucine motif and serine 30 results in a dramatic mislocalization of VAMP4 in the regulated secretory pathway in AtT20 cells. A dominant-negative PACS-1, which binds acidic clusters but not AP-1, also causes mislocalization of VAMP4. Our data support a model whereby phosphorylation-dependent recruitment of PACS-1 enhances AP-1 association to cargo, and suggest that efficient retrieval depends on the formation of a complex between cargo, such as VAMP4, AP-1 and PACS-1.
Introduction
Numerous transmembrane cargo proteins are sorted via tyrosine- or dileucine-based signals in their cytoplasmic tails that are recognized by cytosolic coat adaptor proteins (APs), which recruit vesicle formation machinery. Such adaptors include the heterotetrameric AP complexes as well as the Golgi-associated gamma ear containing ARF binding proteins (GGAs) (Boehm & Bonifacino, 2001; Boman, 2001). The association of adaptors with sorting signals in the cargo can be modulated by phosphorylation of the cargo. Casein kinase 2 (CK2) phosphorylation of serine residues in the mannose 6-phosphate receptors (MPRs), and the endopeptidase furin, plays a role in the high-affinity binding of AP-1 (Molloy et al., 1994; Mauxion et al., 1996). This phosphorylation-enhanced AP-1 binding is mediated by the adaptor protein PACS-1 (phosphofurin acidic cluster sorting protein) (Wan et al., 1998).
In contrast, little is known about the sorting and interaction of SNARE proteins (Jahn et al., 2003) with APs. Darsow et al. (1998) first identified a dileucine motif in the Q-SNARE Vam3p, a yeast vacuolar SNARE, which is essential for its AP-3-dependent sorting to the vacuole. Potential tyrosine and dileucine motifs occur in mammalian Q-SNAREs (syntaxin 6, 7, 8, 13) and R-SNAREs (VAMP4, 7). In particular, Watson & Pessin (2000) investigated a tyrosine motif in syntaxin 6 and showed that its deletion led to an accumulation of syntaxin 6 at the plasma membrane. Kasai et al. (2001) have identified dileucine motifs in both syntaxin 7 and 8, which are critical for their intracellular localization. Likewise, localization of VAMP7 (Ti-VAMP) to late endosomes has been shown to require binding to AP-3 (Martinez-Arca et al., 2003). Finally, a dileucine motif in VAMP4 has been shown to bind to AP-1 (Peden et al., 2001).
VAMP4 has been implicated in endosome to trans-Golgi network (TGN) vesicle trafficking (Mallard et al., 2002). In cells with a regulated secretory pathway such as AtT20 cells, VAMP4 is removed during granule maturation (Eaton et al., 2000), a process that involves homotypic fusion and membrane remodelling by clathrin coats (Tooze et al., 2001). These data are supported by the presence of VAMP4 and syntaxin 6 in immature secretory granules (ISGs) but not in mature secretory granules (MSGs) (Steegmaier et al., 1999; Wendler et al., 2001). Inspection of the VAMP4 cytoplasmic domain revealed an acidic cluster motif adjacent to the known dileucine motif. As acidic cluster motifs have been shown to modulate retrieval of other cargo from ISGs (Crump et al., 2001), we further investigated the mechanism of VAMP4 trafficking. We demonstrate that a phosphoserine in VAMP4 modulates AP-1 binding via PACS-1. Mutations in both AP-1 and PACS-1 binding sites of VAMP4 cause a dramatic change in the intracellular localization of VAMP4 to MSGs. From these data, we propose that the formation of a ternary complex (VAMP4, AP-1, PACS-1) is required for correct sorting of VAMP4. These results suggest that trafficking of a SNARE protein can be mediated by the recruitment of clathrin coats and regulated by phosphorylation, and may be essential for maturation of organelles such as ISGs.
Results and discussion
VAMP4 interacts with AP-1 but not AP-2, AP-3 or GGAs
To investigate the interaction of syntaxin 6 and VAMP4 with APs, we performed pull-down experiments with the cytosolic domain of VAMP4 or syntaxin 6 and cytosol (Fig. 1B). While VAMP4 recruited AP-1 from cytosol (Fig. 1B), it did not interact with AP-2 or AP-3 (data not shown). No interaction between syntaxin 6 and AP-1, or surprisingly AP-2, was detected (Fig. 1B), although it was shown that syntaxin 6 contains a tyrosine motif (Watson & Pessin, 2000).
Figure 1.
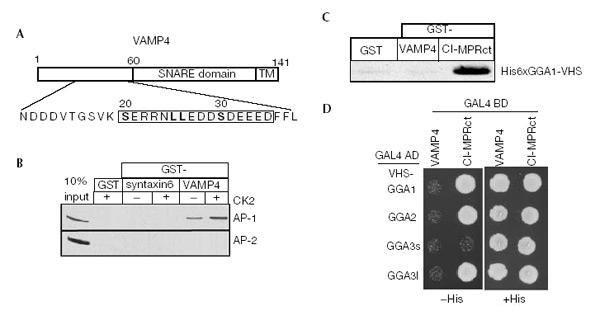
Binding of VAMP4 to AP-1 is modulated by CK2. (A) VAMP4 contains a dileucine motif surrounded by two acidic clusters (boxed). (B) GST-syntaxin 6 or -VAMP4 were pretreated with or without CK2, bound to glutathione beads and incubated with BAMC. AP-1 binds GST-VAMP4, but not syntaxin 6, and binding is enhanced by CK2 phosphorylation. AP-2 does not bind either syntaxin 6 or VAMP4. (C) Immobilized his-GGA-VHS domains were incubated with GST-CI-MPR or -VAMP4. GGA1 bound to the CI-MPR, while no binding of GGA1, 2 or 3-VHS to VAMP4 was detected (data not shown). (D) Yeast was co-transformed with VAMP42–60 and the GGA1–3-VHS domain, and histidine-dependent growth was assessed. The GGAs do not bind to VAMP42–60, while specific GGA interaction with CI-MPR was detected.
As the dileucine motif of VAMP4 is surrounded by two stretches of charged amino acids, including potential CK2 phosphorylation sites (Fig. 1A), we investigated whether binding of VAMP4 to AP-1 is affected by incubation with CK2. Consistently, the interaction between VAMP4 and AP-1 is enhanced when VAMP4 is pretreated with CK2 (Fig. 1B).
The VHS domain of the GGA proteins binds to acidic dileucine motifs found in the MPRs (Puertollano et al., 2001; Zhu et al., 2001) as well as Vps10p, the receptor for carboxypeptidase Y (Dennes et al., 2002). Since VAMP4 contains an acidic dileucine motif similar to CI-MPR (Fig. 1A), we tested for interaction between VAMP4 and the VHS domains of the GGAs. Using pull-down assays (Fig. 1C) and yeast two-hybrid screens (Fig. 1D), we could not detect any interaction between VAMP4 and VHS-GGA 1, 2 and 3.
VAMP4 interacts with the μ1a subunit
To determine whether the interaction between VAMP4 and AP-1 depends on the dileucine motif of VAMP4, we mutated GST-VAMP4 replacing leucine 25 and 26 with valine (LL/VV). These mutations abolished both the CK2-dependent and -independent interaction with AP-1 (Fig. 2A), confirming that AP-1 binding is indeed mediated by the dileucine motif of VAMP4.
Figure 2.
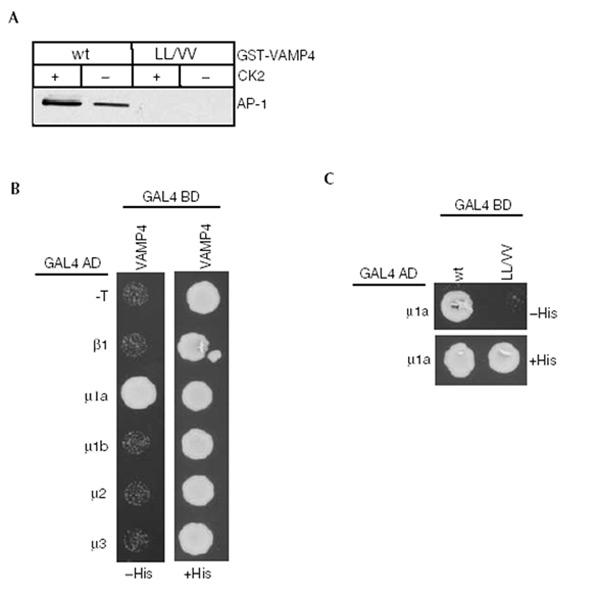
VAMP4 binds exclusively to the μ1a subunit of AP-1 via its dileucine motif. (A) GST-VAMP4 or the LL/VV mutant were pretreated with or without CK2, bound to glutathione beads, incubated with BAMC and processed as in Fig. 1B. Mutations in the dileucine motif abolish AP-1 binding. (B) Yeast was co-transformed with VAMP42–60 and adaptins. A direct interaction of VAMP42–60 with the μ1a subunit of AP-1, but not with other adaptins, was observed. (C) Mutation of the dileucines in VAMP42–60 abolishes μ1a interaction.
While it is well established that tyrosine motifs interact with the μ subunit of APs (Ohno et al., 1995; Owen & Evans, 1998), contradictory data exist as to which adaptor subunit interacts with dileucine motifs. We used the yeast two-hybrid system to determine which subunit of the AP-1 complex binds to VAMP4. VAMP42–60 showed strong interaction exclusively with μ1a but not μ1b or β1 (Fig. 2B), and in agreement with pull-down experiments, we did not detect an interaction with the μ2 or μ3a subunits. The LL/VV mutant showed no interaction confirming the role of the dileucine motif (Fig. 2C).
Our data show that VAMP4 interacts exclusively with the μ1a subunit via the dileucine-based motif. Furthermore, these data suggest that the highly homologous μ1b subunit cannot substitute for μ1a. While it has been speculated that μ1b can compensate for μ1a during early development (Meyer et al., 2000), this might not hold true for AP-1 binding to VAMP4.
Phosphorylation of serine 30 modulates AP-1 binding
To investigate further the CK2-dependent enhanced binding of AP-1 to VAMP4, we performed in vitro kinase assays to determine which residue in VAMP4 is a substrate for CK2 (Fig. 3). We focused on serine 20, and serine 30, present in a CK2 consensus site (Fig. 1A). We mutated each serine to alanine (S20A and S30A) and performed the kinase assay (Fig. 3A). S20A, as well as the LL/VV mutant, is phosphorylated as efficiently as wild type (wt). Mutation of S30 abolishes the phosphorylation of VAMP4 completely. As expected, a double (S20AS30A) mutant is not phosphorylated. We conclude that S30, within an acidic cluster, is the main CK2 phosphorylation site of VAMP4.
Figure 3.
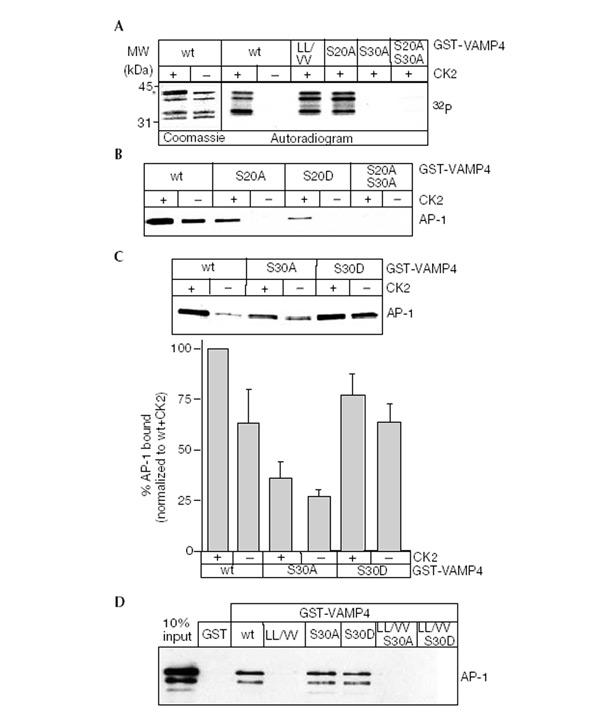
Serine 30 in VAMP4 is phosphorylated by CK2. (A) GST-VAMP4 or mutants were incubated with CK2 and [γ32P]-GTP. CK2 phosphorylates wt, LL/VV and S20A, but not S30A VAMP4. In the first two lanes, the asterisk indicates a band present in the CK2 preparation. (B, C) GST-VAMP4 or VAMP4 mutants were incubated and processed as in Fig. 1B. AP-1 binds to S20A only after CK2 phosphorylation. Efficient AP-1 binding occurs to S30D in a CK2-independent fashion, while the S30A mutant binds weakly. The lower panel shows the quantitation of the AP-1 binding to wt VAMP4 and S30A and S30D mutants in the presence or absence of CK2. (D) Reconstitution of the binary VAMP4/AP-1 complex. GST-VAMP4 proteins bound to glutathione beads were incubated with 1 μg of purified AP-1. wt VAMP4, S30A and S30D show equivalent amounts of AP-1 binding, while binding to LL/VV, LL/VV S30A and LL/VV S30 is abolished.
To determine whether phosphorylation of VAMP4 influences the interaction with AP-1, we used wt VAMP4 or mutants that either lack (S20A or S30A) or mimic the phosphorylated state (S20D or S30D). Surprisingly, the binding of AP-1 to VAMP4 S20A or S20D was abolished completely, suggesting that S20 is an essential part of the dileucine motif (Fig. 3B). However, AP-1 could bind if either VAMP4 S20A or S20D were first phosphorylated by CK2, indicating that the interaction was now completely CK2 dependent. As the S20 mutants can only bind AP-1 if S30 is phosphorylated, we propose that the acidic cluster containing S30 modulates AP-1 binding. This is supported by the AP-1 binding behaviour of the S30 mutants (Fig. 3C). S30A still binds to AP-1, but binding is no longer influenced by phosphorylation, and is reduced to a level similar to the non-phosphorylated wt protein. S30D shows a strong interaction with AP-1, with or without CK2, comparable to the phosphorylated wt protein (Fig. 3B).
Two possible mechanisms could lead to enhanced binding of AP-1 to phosphorylated VAMP4. One is that the μ1 subunit binds with higher affinity to phosphorylated VAMP4. Alternatively, high-affinity binding of AP-1 to phosphorylated VAMP4 could be mediated by an additional protein. To differentiate between these possibilities, we reconstituted the binary complex with purified VAMP4 and AP-1 (Fig. 3D). We used the S30D mutant to study the role of phosphorylation to avoid any secondary effects caused by adding CK2. Purified AP-1 was found to bind equally to wt VAMP4, S30A or S30D mutants, while binding to the LL/VV, LL/VV S30A or LL/VV S30D mutants was abolished. These results are supported by yeast two-hybrid data where there was no difference in the interaction between the wt VAMP42–60, VAMP42–60 S30A or S30D and μ1a. Thus, phosphorylation of S30 does not influence directly AP-1 interaction with VAMP4, suggesting that an additional protein modulates AP-1 binding to phosphorylated VAMP4.
PACS-1 modulates AP-1 binding to phospho-VAMP4
PACS-1 is a candidate phosphorylation-dependent enhancer of AP-1 binding to the acidic cluster motif in VAMP4 (Crump et al., 2001). To determine whether VAMP4 interacts with PACS-1, we expressed HA-tagged PACS-1 in AtT20 cells and co-immuno-precipitated with antibodies to VAMP4. PACS-1 was specifically detected in a complex with VAMP4 in these cell lysates (Fig. 4A), and not in controls. In addition, pull-down assays with wt, LL/VV, S30A or S30D GST-VAMP4 proteins and purified PACS-1FBR (furin-binding region) demonstrated a direct interaction between VAMP4 and PACS-1 (Fig. 4B). Importantly, phosphorylated wt VAMP4, LL/VV and the S30D mutant exhibited enhanced association with PACS-1 compared with wt VAMP4 and the S30A mutant. Furthermore, this association does not require the dileucine motif, as it occurs with the LL/VV mutant. These data establish that PACS-1 association with VAMP4 is enhanced upon phosphorylation.
Figure 4.
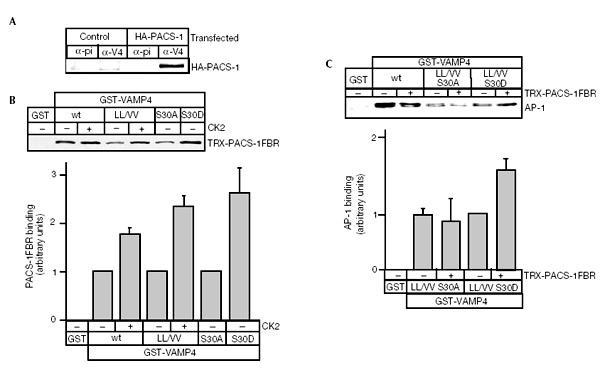
Phosphoserine 30 modulates AP-1 binding to VAMP4 via PACS-1. (A) AtT20 cells were infected with either wt or HA-PACS-1 vaccinia virus and used for immunoprecipitation with anti-VAMP4 antibody. HA-PACS-1 can be detected in the VAMP4 immunoprecipitates. (B) GST-VAMP4 was incubated with or without CK2, bound to glutathione beads and incubated with PACS-1. PACS-1 binding to wt or the LL/VV VAMP4 is enhanced by the presence of CK2; the S30D mutant shows a CK2-independent binding comparable to phosphorylated VAMP4. The lower panel shows quantitation of the PACS-1 binding to wt VAMP4 and mutants in the presence or absence of CK2. (C) Formation of the ternary VAMP4/PACS-1/AP-1 complex using GST-VAMP4, PACS-1 and purified AP-1. Addition of PACS-1 protein to the VAMP4 LL/VV S30D protein enhances binding of AP-1 compared to VAMP4 LL/VV S30A protein. Quantitation (lower panel) of AP-1 binding to the VAMP4/PACS-1 complex shows a PACS-1-dependent increase.
To test if PACS-1 could mediate recruitment of AP-1 to phosphorylated VAMP4, we used the LL/VV S30A and LL/VV S30D VAMP4 mutants. This allowed us to separate phosphorylation-dependent binding from dileucine-dependent binding. Complex formation was assayed using GST-VAMP4 double mutants with PACS-1FBR and purified AP-1 (Fig. 4C). The presence of PACS-1 resulted in a significant increase in AP-1 binding to the LL/VV S30D mutant as compared to the LL/VV S30A mutant (Fig. 4C). This shows that AP-1 binding increases upon phosphorylation of VAMP4 and recruitment of PACS-1, and suggests that both PACS-1 and AP-1 might be required for the intracellular trafficking of VAMP4.
PACS-1 and AP-1 are required for sorting of VAMP4
To study the role of AP-1 and PACS-1 in VAMP4 sorting from ISGs, we employed two approaches. First, we expressed a dominant-negative form of PACS-1, PACS-1 admut, in AtT20 cells expressing wt VAMP4-Flag (Fig. 5). Expression of the PACS-1 admut protein, which still binds to acidic clusters but not to AP-1 (Crump et al., 2001), caused striking mislocalization of VAMP4 to the ACTH-positive tips containing MSG (Fig. 5C). Expression of the wt form of PACS-1 had no effect (Fig. 5B).
Figure 5.
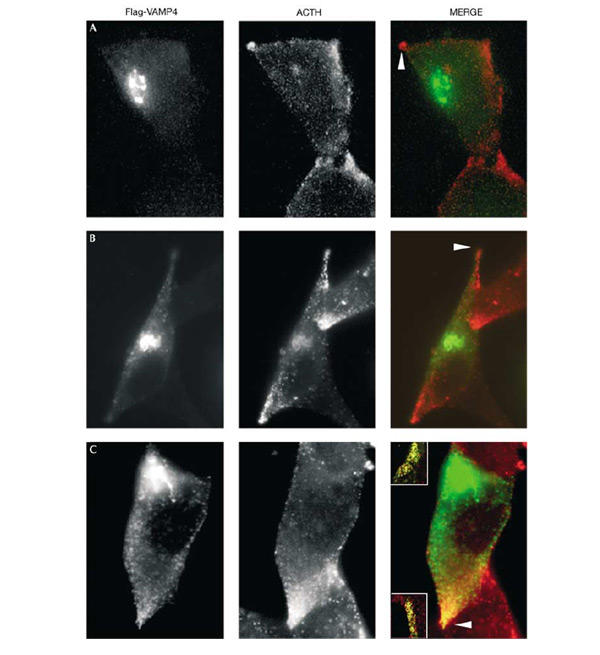
Expression of PACS-1 admut causes mislocalization of VAMP4. AtT20 cells were transfected with Flag-VAMP4 and after 60 h infected with either (A) wt, (B) HA-PACS-1 or (C) PACS-1 admut vaccinia virus. Flag-VAMP4 panels (green, detected with M2 ab) show the localization of VAMP4, ACTH panels (red, detected with anti-POMC ab) show the localization of POMC and ACTH, and MERGE panels show the merge of Flag and ACTH. Expression of PACS-1 admut (see the arrowhead in (C)) but not PACS-1 (see arrowheads in (A, B)) causes mislocalization of Flag-VAMP4 to the ACTH-positive MSGs in the tips of the cells. The insets in (C) show double labelling of VAMP4 and ACTH in two other tips.
Second, we mutated the AP-1 and PACS-1 binding sites in VAMP4. We expressed Flag-tagged wt VAMP4, LL/VV, S30A or the LL/VV S30A mutants in AtT20 cells. The intracellular localization of VAMP4 LL/VV or the S30A mutants largely resembled that of the wt protein (Fig. 6A–C), although there was a slight alteration in the perinuclear distribution of the LL/VV mutant compared to wt (Fig. 6A,B). In contrast, the VAMP4 double-mutant LL/VV S30A underwent a dramatic change and was now found in the tips of the AtT20 cells together with ACTH, a marker for MSGs (Fig. 6D).
Figure 6.
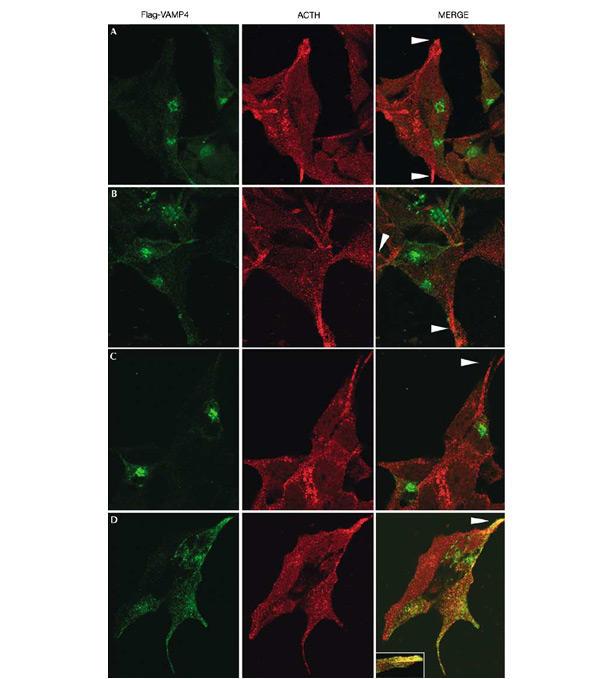
Sorting of VAMP4 in AtT20 cells requires both AP-1 and PACS-1 binding sites. The localization of transfected Flag-tagged (A) wt VAMP4, (B) VAMP4 LL/VV, (C) VAMP4 S30A or (D) VAMP4 LL/VV S30A was compared to endogenous ACTH in AtT20 cells. The left panels show the Flag tag (green, M2 ab), the centre panels show ACTH (red, POMC ab) and the right panels show the MERGE of the two images. The wt, LL/VV and S30A proteins remain predominantly in a perinuclear region, and are not found in the tips (arrowheads in (A–C)) with ACTH. Mutation of both the dileucine motif and the PACS-1 binding motif results in mislocalization of VAMP4 to ACTH-positive MSGs found in the tips of the cells (arrowhead in (D)). The inset in (C) MERGE shows double labelling of VAMP4 and ACTH in another tip.
We conclude that sorting of VAMP4 requires both the dileucine motif and phosphorylation of S30, and most importantly the complex of AP-1 and PACS-1. The order of recruitment remains to be established. Although VAMP4 can recruit AP-1, it remains to be investigated whether VAMP4 is sorted alone or together with its cognate SNARE binding partners. The phosphorylation-dependent recruitment of AP-1 via PACS-1 to VAMP4 may provide a mechanism by which ISG maturation could be regulated. Phosphorylation of VAMP4 may coordinate the activity of VAMP4, and ultimately control ISG maturation by the removal of the SNARE proteins required for homotypic fusion.
Materials and methods
In vitro phosphorylation.
In all, 2 μg of GST-VAMP4 purified with glutathione sepharose (Amersham Pharmacia Biotech) was incubated with 10 μCi [γ32P]GTP (Amersham Pharmacia Biotech) and 10 μU recombinant CK2α (Calbiochem) in binding buffer (25 mM Hepes–KOH (pH 7.2), 25 mM KCl, 2.5 mM MgOAc) with 0.5% Triton X-100, and protease inhibitor cocktail (Sigma), at 37 °C for 30 min. Samples were then processed for SDS–PAGE and autoradiography.
Pull-down experiments.
In all, 20 μg of GST-VAMP4 was incubated in 100 μl binding buffer with 1 mM GTP and 0.1 mU CK2 for 30 min at 37 °C in siliconized Eppendorf tubes. A volume of 100 μl of glutathione beads was added, and incubated for 1 h at 4 °C, washed and resuspended in 250 μl of 2 mg ml−1 bovine adrenal medulla cytosol (BAMC) in binding buffer and incubated rotating overnight at 4 °C. Beads were washed four times, and bound protein was subjected to SDS–PAGE analysed by immunoblotting. The γ subunit of AP-1, and the α subunit of AP-2 were detected using mAb 100/3 and 100/2, respectively (Sigma). Pull-down experiments using GST-CI-MPR, GST-VAMP4 and His6xGGA1-VHS were performed as described (Puertollano et al., 2001). Quantitation was made using at least three independent experiments.
Yeast two-hybrid analyses.
Yeast two-hybrid analysis was performed using Matchmaker3 (Clontech). VAMP4 amino-terminus (2–60 amino acids (aa)) was cloned into the bait vector pGADT7. μ1a, μ2 and μ3a in the prey vector pACT2 were provided by J.S. Bonifacino (National Institutes of Health, USA), and μ1b was a gift from C. Dillon (Cancer Research UK, London). β1 (from K. Nakayama, Tsukuba, Japan) was cloned into the pGADT7 vector. The VHS domains (Vps27p, Hrs and STAM) of human GGA1 (13–171 aa), GGA2 (29–188 aa) and GGA3s (12–171 aa) were amplified from plasmids provided by R. Kahn (Atlanta, USA) and cloned into pGADT7. The VHS domain of the rat GGA3l (12–171 aa) was amplified and cloned from a rat brain library (Clontech). CI-MPRct (2,395–2,482 aa) was amplified from plasmid pGEX CT87 (Dittié et al., 1999) and cloned into pGADT7. Rat PACS-1FBR (117–267 aa) was generated by PCR from a rat library brain.
VAMP4/AP-1 complex reconstitution assay.
In all, 10 μg of GST or GST-VAMP4 proteins were preincubated with 25 μl glutathione beads for 1 h at 4 °C in 200 μl GST binding buffer (150 mM NaCl, 50 mM Tris (pH 7.5), 2 mM MgCl2, 1% NP-40, 1 mM PMSF, 10 μg ml−1 leupeptin), supplemented with bovine serum albumin (BSA, 5 mg ml−1 final concentration) and incubated for an additional 30 min at 4 °C. Beads were washed twice in GST binding buffer, and 150 μl of AP-1 binding buffer plus 5 mg ml−1 BSA, 0.1 mM DTT, 1 mM PMSF, 10 μg ml−1 leupeptin, together with 1 μg purified AP-1, purified as described in Austin et al. (2000), was added and reactions incubated for 30 min rotating at room temperature (21°C). Beads were washed four times in GST binding buffer, and transferred to a new tube, resuspended in SDS–PAGE sample buffer and analysed by immunoblotting.
Binary and ternary complex reconstitution assay.
In all, 10 μg of GST or GST-VAMP4 proteins were preincubated with 25 μl glutathione beads for 1 h at 4 °C in 200 μl GST binding buffer and then incubated in PACS-1 binding buffer (100 mM NaCl, 35 mM Tris (pH 7.5), 2 mM MgCl2, 1% NP-40, 1 mM PMSF, 10 μg ml−1 leupeptin) containing 30 μg of TRX-PACS-1FBR, purified as described in Crump et al. (2001), for 1 h at 4 °C. BSA (5 mg ml−1) was added and incubated at 4 °C for 30 min. Beads were washed with PACS-1 binding buffer. Binary VAMP4/PACS-1 complexes were detected using the anti-Thiol mAb (Invitrogen). Ternary complexes were formed by further incubation with 150 μl AP-1 binding buffer with 5 mg ml−1 BSA and 1 μg purified AP-1 for 30 min rotating at RT. The samples were processed for immunoblots with mAb 100/3. Quantitation was performed using at least three independent experiments.
PACS-1 and PACS-1 admut expression and co-immunoprecipitation.
Vaccinia virus stocks were prepared from the wt WR, recombinant HA-PACS-1FBR and PACS-1 admut strains as described in Bresnahan et al. (1990). ATt20 cells (approximately 1×107 cells) were infected for 6 h, and fixed for indirect immunofluorescence or harvested in RIPA buffer (10 mM Tris–HCl (pH 7.6), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1% sodium deoxycholate) and incubated either with preimmune sera or a polyclonal anti-VAMP4 antisera (kindly provided by T. Galli, Paris). The immunoprecipitates were bound to protein A sepharose, washed and analysed by SDS–PAGE, followed by immunoblotting with anti-HA mAb.
Transfection and indirect immunofluorescence.
AtT20 cells were transfected with lipofectamine 2000 and Flag-tagged wt or mutant VAMP4. After 24–48 h the cells were fixed, or infected with wt, HA-PACS-1FBR or PACS-1 admut virus for 4 h and fixed. Cells were labelled with antibodies specific for the Flag epitope (mAb M2, Sigma) or POMC (Dittié et al., 1999). Primary antibodies were detected by either Alexa 488, Texas red or Cy3 labelled secondary antibodies, and analysed by confocal microscopy using Zeiss LSM 510 software.
Acknowledgments
We thank T. Galli (Paris) for the VAMP4 antibody, G. Schiavo, P. Parker, L.M. Sevilla and J. Tooze for critically reading the manuscript, H. Jefferies for help with the microscopy, and T. Porstmann for help with the GGA constructs. G.T. acknowledges support by National Institutes Health grants DK37274, AI48585 and AI49793.
References
- Austin C., Hinners I. & Tooze S.A. ( 2000) Direct and GTP-dependent interaction of ADP-ribosylation factor 1 with clathrin adaptor protein AP-1 on immature secretory granules. J. Biol. Chem., 275, 21862–21869. [DOI] [PubMed] [Google Scholar]
- Boehm M. & Bonifacino J.S. ( 2001) Adaptins: the final recount. Mol. Biol. Cell, 12, 2907–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman A.L. ( 2001) GGA proteins: new players in the sorting game. J. Cell Sci., 114, 3413–3418. [DOI] [PubMed] [Google Scholar]
- Bresnahan P.A., Leduc R., Thomas L., Thorner J., Gibson H.L., Brake A.J., Barr P.J. & Thomas G. ( 1990) Human fur gene encodes a yeast KEX2-like endoprotease that cleaves pro-β-NGF in vivo. J. Cell Biol., 111, 2851–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump C.M., Xiang Y., Thomas L., Gu F., Austin C., Tooze S.A. & Thomas G. ( 2001) PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic. EMBO J., 20, 2191–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow T., Burd C.G. & Emr S.D. ( 1998) Acidic di-leucine motif essential for AP-3-dependent sorting and restriction of the functional specificity of the Vam3p vacuolar t-SNARE. J. Cell Biol., 142, 913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennes A., Madsen P., Nielsen M.S., Petersen C.M. & Pohlmann R. ( 2002) The yeast Vps10p cytoplasmic tail mediates lysosomal sorting in mammalian cells and interacts with human GGAs. J. Biol. Chem., 277, 12288–12293. [DOI] [PubMed] [Google Scholar]
- Dittié A.S., Klumperman J. & Tooze S.A. ( 1999) Differential distribution of mannose-6-phosphate receptors and furin in immature secretory granules. J. Cell Sci., 112, 3955–3966. [DOI] [PubMed] [Google Scholar]
- Eaton B.A., Haugwitz M., Lau D. & Moore H.P. ( 2000) Biogenesis of regulated exocytotic carriers in neuroendocrine cells. J. Neurosci., 20, 7334–7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., Lang T. & Sudhof T.C. ( 2003) Membrane fusion. Cell, 112, 519–533. [DOI] [PubMed] [Google Scholar]
- Kasai K. & Akagana K. ( 2001) Roles of the cytoplasmic and transmembrane domains of syntaxins in intracellular localization and trafficking. J. Cell Sci., 114, 3115–3124. [DOI] [PubMed] [Google Scholar]
- Mallard F. et al. ( 2002) Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J. Cell Biol., 156, 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arca S. et al. ( 2003) A dual mechanism controlling the localization and function of exocytic v-SNAREs. Proc. Natl Acad. Sci. USA, 100, 9011–9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauxion F., Le Borgne R., Munier-Lehmann H. & Hoflack B. ( 1996) A casein kinase II phosphorylation site in the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor determines the high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J. Biol. Chem., 271, 2171–2178. [DOI] [PubMed] [Google Scholar]
- Meyer C., Zizioli D., Lausmann S., Eskelinen E.-L., Hamann J., Saftig P., von Figura K. & Schu P. ( 2000) μ1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J., 19, 2193–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy S.S., Thomas L., VanSlyke J.K., Stenberg P.E. & Thomas G. ( 1994) Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J., 13, 18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H. et al. ( 1995) Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science, 269, 1872–1875. [DOI] [PubMed] [Google Scholar]
- Owen D.J. & Evans P.R. ( 1998) A structural explanation for the recognition of tyrosine-based endocytotic signals. Science, 282, 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden A.A., Park G.Y. & Scheller R.H. ( 2001) The di-leucine motif of vesicle-associated membrane protein 4 is required for its localization and AP-1 binding. J. Biol. Chem., 276, 49183–49187. [DOI] [PubMed] [Google Scholar]
- Puertollano R., Aguilar R.C., Gorshkova I., Crouch R.J. & Bonifacino J.S. ( 2001) Sorting of mannose-6-phosphate receptors mediated by the GGAs. Science, 292, 1712–1716. [DOI] [PubMed] [Google Scholar]
- Steegmaier M., Klumperman J., Foletti D.L., Yoo J.-S. & Scheller R.H. ( 1999) Vesicle-associated membrane protein 4 is implicated in trans-Golgi network vesicle trafficking. Mol. Biol. Cell, 10, 1957–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze S.A., Martens G.J.M. & Huttner W.B. ( 2001) Secretory ganule biogenesis: rafting to the SNARE. Trends Cell Biol., 11, 116–122. [DOI] [PubMed] [Google Scholar]
- Wan L., Molloy S.S., Thomas L., Liu G., Xiang Y., Rybak L. & Thomas G. ( 1998) PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell, 94, 205–216. [DOI] [PubMed] [Google Scholar]
- Watson R.T. & Pessin J.E. ( 2000) Functional cooperation of two independent targeting domains in syntaxin 6 is required for its efficient localization in the trans-Golgi network of 3T3L1 adipocytes. J. Biol. Chem., 275, 1261–1268. [DOI] [PubMed] [Google Scholar]
- Wendler F., Page L., Urbé S. & Tooze S.A. ( 2001) Homotypic fusion of immature secretory granules during maturation requires syntaxin 6. Mol. Biol. Cell, 12, 1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Doray B., Poussu A., Lehto V.P. & Kornfeld S. ( 2001) Binding of GGA2 to the lysosomal enzyme sorting motif of the mannose-6-phosphate receptor. Science, 292, 1716–1718. [DOI] [PubMed] [Google Scholar]


