Abstract
The fibulins are a family of proteins that are associated with basement membranes and elastic extracellular matrix fibres. This review summarizes findings from studies of animal models of fibulin deficiency, human fibulin gene mutations, human tumours and injury models that have advanced our understanding of the normal and pathological roles of members of this formerly obscure family.
Introduction
In little more than a decade since the discovery of the first fibulin (Argraves et al., 1989), a six-member family of extracellular-matrix (ECM) proteins has emerged (Table 1; Fig. 1). The fibulins are minimally defined as having a series of epidermal growth factor (EGF)-like modules, followed by a carboxy-terminal fibulin-type module (Fig. 2). It is evident that the fibulins are an ancient family of proteins, which are highly conserved in species as evolutionarily distant as worms and humans. Fibulins have a diverse array of protein ligands (Timpl et al., 2003; and see supplementary information online). As a consequence of these widespread interactions, fibulins are hypothesized to function as intramolecular bridges that stabilize the organization of supramolecular ECM structures, such as elastic fibres and basement membranes. Indeed, the family name originates from the Latin word fibula, which means clasp or buckle. The biophysical features of the fibulins have been well described in a recent article (Timpl et al., 2003). Here, we place into perspective findings from many types of studies, including DNA microarray and gene-targeting experiments, that collectively provide new insights into the functions of the fibulins under physiological and pathological conditions.
Table 1.
Fibulin family nomenclature
| Name | Synonymous names | Gene symbol | Human chromosome location | References |
|---|---|---|---|---|
| Fibulin 1 | BM90 | FBLN1 | 22q13.31 | (Argraves et al., 1990; Kluge et al., 1990) |
| Fibulin 2 | – | FBLN2 | 3p24-p25 | (Pan et al., 1993) |
| Fibulin 3 | S15, T16, EFEMP1 | FBNL | 2p16 | (Tran et al., 1997) |
| Fibulin 4 | MBP1, EFEMP2, UPH1, H411 | EFEMP2 | 11q13 | (Gallagher et al., 1999; Giltay et al., 1999) |
| Fibulin 5 | DANCE, EVEC, UP50 | FBLN5 | 14q32.1 | (Kowal et al., 1999; Nakamura et al., 1999) |
| Fibulin 6 | Hemicentin, him4 | FBLN6 | 1q25.3 | (Vogel & Hedgecock, 2001) |
Figure 1.
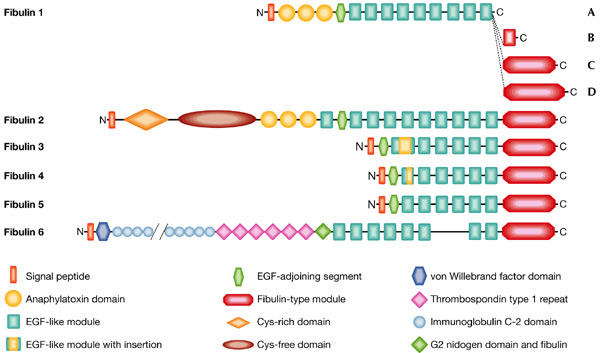
Modular structures of the fibulins. The most recent addition to the family, fibulin 6 (hemicentin), was originally identified in the nematode (Vogel & Hedgecock, 2001), with orthologues in other species (human, mouse and rat) having now been identified. Nine of the 48 immunoglobulin domains in fibulin 6 are shown (double slashes indicate where the omitted domains occur). Alternative splice variants are known for fibulins 1–4, albeit only variants for fibulin 1 (designated A–D) are displayed.
Figure 2.
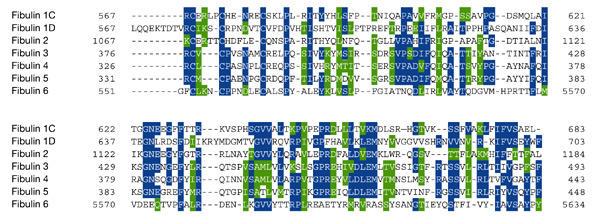
Alignment of fibulin-type module sequences from the human fibulins. The carboxy-terminal regions of the fibulins were aligned using ClustalW 1.82, and Boxshade 3.21 was used to highlight conserved amino acids. Identical residues are indicated in the blue background and chemically similar residues with green shading. The GenBank accession numbers for the sequences depicted are as follows: fibulin 1C, CAA37772.1; fibulin 1D, AAB17099.1; fibulin 2, CAA57876.1; fibulin 3, NP_004096.2; fibulin 4, CAA10791.2; fibulin 5, NP_006320.2; fibulin 6, NP_114141.1.
Fibulins in elastic fibre biology
There is substantial evidence that implicates the fibulins in both elastic matrix fibre assembly and function. Fibulins 1, 2 and 5 all bind to tropoelastin (Nakamura et al., 2002; Sasaki et al., 1999; Yanagisawa et al., 2002). Early in development, fibulin 1 is associated with ECM fibres that contain both elastin and the microfibril-associated proteins fibrillins 1 and 2 (Visconti et al., 2003). The expression of fibulin 1 during murine lung development is coordinately expressed with tropoelastin and another elastin-associated microfibril protein, latent TGF-β-binding protein 2 (LTBP2; Mariani et al., 2002). Fibulin 1 also colocalizes with elastin in the core of mature elastin-containing fibres in skin and blood vessels (Roark et al., 1995). Unlike fibulin 1, fibulin 2 is found at the interface between microfibrils and the elastin core (Reinhardt et al., 1996). The ability of fibulin 2 to bind elastin and fibrillin 1 may indicate that it anchors fibrillin-containing microfibrils to elastin fibres. Fibulin 5 binding to both integrins and elastin implicates it as a connector of elastin fibres to cells (Nakamura et al., 1999). The interaction of these fibres with the cell surface might be an integral part of elastic fibre assembly, as for other ECM fibres, such as fibronectin (FN). In support of this, mice deficient in fibulin 5 have defective assemblages of elastic fibres (Nakamura et al., 2002; Yanagisawa et al., 2002).
Fibulins in cardiovascular biology
Fibulins 1 and 2 are highly expressed during cardiac valvuloseptal formation. Both are produced by migratory cardiac mesenchymal cells that have transdifferentiated from endocardial cells (Bouchey et al., 1996; Tsuda et al., 2001; Zhang et al., 1995). In developing and adult heart valves, fibulins 1 and 2 are prominently expressed and fibulin 4 is moderately expressed (Giltay et al., 1999; Zhang et al., 1995). Relatively little fibulin 3 is found in adult heart valves (Giltay et al., 1999). The fact that fibulin 1 deficiency does not result in overt valvuloseptal defects (Kostka et al., 2001) could indicate compensation by other fibulins.
Fibulins are prominently expressed in blood vessels. During development, fibulin 1 is expressed by the primordial vascular smooth muscle cells (VSMCs) that associate with the ventral endothelium of the dorsal aortae (Hungerford et al., 1997). Primordial VSMCs of the developing aortic-arch vessels also synthesize fibulin 2. In addition, fibulin 2 is expressed by coronary endothelial cells (ECs) that originate from epicardial cells, but it is not expressed by capillary ECs (Tsuda et al., 2001). In adult blood vessels, pronounced fibulin 1 deposition occurs in the matrix that surrounds VSMCs and in the elastic laminae of arteries (Roark et al., 1995). Fibulin 3 expression is prominent in some capillaries, but not in large blood vessels (Giltay et al., 1999). Fibulin 3 is highly expressed in human umbilical vein ECs (on the basis of a GeneAtlas analysis; http://expression.gnf.org), but its expression is repressed during in vitro human capillary tube formation (Bell et al., 2001). Fibulin 4 is found in the medial layers of large veins and arteries and in some small capillaries (Giltay et al., 1999). Fibulin 5 seems to be restricted to the arterial vasculature and is expressed predominantly by VSMCs of developing arteries and at low levels by VSMCs of adult blood vessels (Kowal et al., 1999). ECs also express fibulin 5, especially the pulmonary artery endothelium (Jean et al., 2002; Kowal et al., 1999). Fibulin 1 is not generally expressed in ECs (Roark et al., 1995).
Knockout animal models and heritable diseases in humans
The importance of the fibulins in development and disease has been highlighted by gene-targeting experiments in animal models and the identification of spontaneous mutations in humans. Fibulin 1 deficiency in mice causes extensive haemorrhaging and perinatal death (Kostka et al., 2001). The bleeding observed in this case was not due to defective coagulation, but rather to abnormal EC morphology that included hypertrophy, peculiar apical processes and increased intracellular vacuoles (Kostka et al., 2001). In humans, a type of synpolydactyly (congenital malformation of the hand) involves a chromosomal translocation between the fibulin 1 gene and C12orf2 (Debeer et al., 2002). A haploinsufficiency in the level of fibulin 1D is hypothesized to account for the limb abnormalities seen (Debeer et al., 2002). In this regard, mutation of fibrillin 2, a fibulin 1-associated protein (Visconti et al., 2003), also leads to syndactyly (Chaudhry et al., 2001).
Several recent findings indicate the involvement of fibulins in inherited eye disorders. Fibulins 1 and 4 are candidate genes for retinopathies that map to chromosomes 22 and 11, respectively (Weigell-Weber et al., 2003). A mutation (Arg345Trp) in the fibulin 3 gene has been linked to Malattia Leventinese (ML), a macular dystrophy (Stone et al., 1999). During the development of this disease, as well as in age-related macular degeneration (AMD), an amorphous material known as drusen accumulates between the retinal-pigment epithelium (RPE) and the Bruch's membrane. In ML, fibulin 3 is not found in the drusen, but accumulates within cells of the RPE (Marmorstein et al., 2002). In AMD, which has no associated fibulin 3 mutation, fibulin 3 nonetheless accumulates between the RPE and the drusen (Marmorstein et al., 2002). The expression of fibulins 3 and 1 are also elevated in a murine retinopathy model that primarily involves degeneration of rod photoreceptors (Kennan et al., 2002). Ectopic expression of fibulin 1 also disrupts Xenopus eye morphogenesis (Grammer et al., 2000).
Knockout experiments emphasize the essential role that fibulin 5 has in elastic fibre assembly. Mice deficient in the expression of fibulin 5, an elastin-binding protein, are viable but show symptoms of defective elastic fibre formation, including a tortuous aorta, severe emphysema and loose skin (cutis laxa; Nakamura et al., 2002; Yanagisawa et al., 2002). In humans, homozygosity for a missense mutation in fibulin 5 is also associated with a severe form of cutis laxa (Loeys et al., 2002) and a scarcity of elastic fibres.
Nematodes deficient in hemicentin (a homologue of fibulin 6) display defective cell–cell and cell–matrix interactions (Vogel & Hedgecock, 2001). Uterine and intestinal cells fail to affix stably to the body wall, and cells of the vas deferens fail to join the cloaca. There is also a failure in the assembly of hemidesmosomes and intermediate filaments in the epidermis.
Fibulins and cancer
Human fibrosarcoma tumour cell lines show a trend towards a reduction or absence of fibulin 1D expression (Qing et al., 1997). Fibrosarcoma cells that express fibulin 1D show reduced growth in vivo, as well as a lowered growth capacity in soft agar and a reduced ability to invade reconstituted basement membranes (Qing et al., 1997). Similarly, the ectopic expression of fibulin 1D inhibits the motility of breast carcinoma cells on FN (Twal et al., 2001). The motility suppressive effects of fibulin 1D are attributed to a reduction in the cell adhesion and migration-promoting activity of FN (Twal et al., 2001). Ectopic expression of fibulin 1D also inhibits transformation by the papillomavirus E6 gene (Du et al., 2002). The mechanism by which fibulin 1D regulates E6-mediated oncogenic activities might relate to the fact that these two proteins interact (Du et al., 2002). These findings support the conclusion that fibulin 1D acts as a tumour suppressor.
Elevated expression of fibulin 1 is associated with human breast tumours (Forti et al., 2002; Greene et al., 2003). Also suggestive of fibulin 1 overexpression in breast carcinoma is the fact that breast-cancer patients produce antibodies against fibulin 1 (Forti et al., 2002). These observations seem paradoxical in light of the evidence that fibulin 1D is a tumour suppressor. An explanation may come from findings that there is a trend towards increased expression of fibulin 1C compared with the D variant in ovarian carcinomas (Moll et al., 2002). Levels of fibulin 1 splice variants have not been quantified in breast cancer but if fibulin 1C levels are elevated in breast tumours as in ovarian tumours, it would suggest that fibulin 1C opposes the action of fibulin 1D and promotes tumorigenesis. It is also possible that humoral immunity to fibulin 1 in breast cancer reflects the breakdown of fibulin 1D and concomitant loss of tumour suppression. In support of this, increased levels of fibulin 1 fragments have been reported in human breast tumours (Greene et al., 2003). Furthermore, findings from DNA microarray studies of lung adenocarcinomas show that fibulins 1 and 2 are consistently associated with matrix metalloproteinase 2 expression, a protein that promotes tumour invasion and metastasis (Creighton & Hanash, 2003).
Fibulin 2 has been identified as one of 64 overexpressed metastasis-associated genes in solid tumours of diverse types (Ramaswamy et al., 2003). Fibulin 4 expression is elevated in human colon tumours (Gallagher et al., 2001), whereas cancers in other tissues tend to show downregulation of fibulin 5 (Schiemann et al., 2002). In contrast to the motility suppressive effects of fibulin 1D on fibrosarcoma cells, overexpression of fibulin 5 increases fibrosarcoma cell migration (Schiemann et al., 2002).
Fibulins in injury
The expression of several of the fibulins is induced in response to injury. Fibulin 1 expression is increased in a murine model of cardiomyopathy that is caused by increased Gi-receptor signalling (Redfern et al., 2000). In sun-damaged skin elastosis, fibulin 2 deposition in association with elastic fibres is greatly increased (Hunzelmann et al., 2001). Fibulin 2 expression is also increased in the early phase of streptozotocin-induced diabetic glomerulosclerosis (Wada et al., 2001). In elastase-induced emphysema in mice, fibulin 5 expression is increased in the alveolar wall (Kuang et al., 2003). The expression of fibulin 5, which is low in adult arteries, is activated in medial and neointimal VSMCs in response to vascular injury (Kowal et al., 1999), as well as in lung vasculature in response to hyperoxia (Jean et al., 2002) and in atherosclerotic plaques (Kowal et al., 1999). Transforming growth factor-β (TGF-β), which has a key role in vascular injury response, stimulates fibulin 5 expression (Schiemann et al., 2002). Overexpression of fibulin 5 enhances basal and TGF-β-mediated activation of p38 mitogen-activated protein kinase and ERK1/ERK2 (Schiemann et al., 2002). A similar profile of protein kinase activation has also been observed in response to fibulin 1 stimuli (Twal et al., 2001). Overexpression of fibulins 5 and 3 increase fibroblast DNA synthesis (Lecka-Czernik et al., 1996; Schiemann et al., 2002). Overexpression of fibulin 4 in macrophages also promotes DNA synthesis (Heine et al., 1999), and fibulin 4 expression is augmented in macrophages by lipopolysaccharide treatment, which suggests a role in response to sepsis (Heine et al., 1999).
A recent study has shown that patients with unstable angina pectoris and acute myocardial infarction have significantly reduced levels of plasma fibulin 1 (Kawata et al., 2001). This has led to speculation that plasma fibulin 1 may be transferred to or consumed in or around the atherosclerotic lesion. Indeed, fibulin 1 is incorporated into fibrin clots that are associated with atherosclerotic lesions (Tran et al., 1995). The significance of fibulin 1 in the development of atherosclerosis is not yet known, but plasma fibulin 1 could be important as a risk factor for cardiovascular diseases and atherosclerosis progression.
Regulation of fibulin expression
Information is gradually emerging concerning the mechanisms that regulate the expression of the fibulins during development or disease. Evidence indicates that steroids regulate the expression of fibulins 1, 2 and 3. Oestradiol stimulates fibulin 1C expression in ovarian tumour cells (Clinton et al., 1996; Hayashido et al., 1998), and dexamethasone increases fibulin 1C expression in human eye trabecular meshwork cells (Ishibashi et al., 2002). Progesterone has been shown to stimulate the expression of fibulins 1 and 2 in human endometrial stromal cells (Okada et al., 2003). In a mouse Wilms' tumour model, the expression of fibulin 2, but not fibulin 1, is increased by dexamethasone (Talts et al., 1995). Glucocorticoids downregulate the expression of fibulins 1 and 2 in bone marrow stroma (Gu et al., 2001) and oestrogen represses the expression of fibulin 3 in MCF7 breast cancer cells (Hayashi et al., 2003).
Sp transcription factors are important in fibulin 1 expression (Castoldi & Chu, 2002; Grassel et al., 1999). Fibulin 1 transcription is activated by the ubiquitous Sp1 and Sp3, but not by the more tissue-restricted Sp4 (Castoldi & Chu, 2002). Fibulin 2 may be similarly regulated (Grassel et al., 1999). The fibulin 2 gene also contains two consensus cAMP-negative response elements. Interaction of cAMP-activated liver X receptor-α with these enhancer elements results in increased fibulin 2 expression (Anderson et al., 2003).
Future directions
The studies cited herein implicate the fibulins in an array of physiological and pathological processes and open many new avenues for investigation. For example, the finding that mutation of the fibulin 5 gene is linked to cutis laxa highlights the possibility that mutations in genes for the other family members might contribute to disorders that involve elastic fibres. Given the circumstantial evidence that fibulin 1C and D variants might have opposing effects on tumorigenesis, studies that directly test this possibility and evaluate the expression of fibulin 1 variants in human tumours are warranted. The finding that plasma fibulin 1 levels are reduced in coronary heart disease patients raises questions as to the molecular basis for this and whether this protein is a useful diagnostic serum marker. Just as our understanding of the function of fibulins 1 and 5 has benefited from the study of mice that are genetically deficient in the expression of these proteins, similar benefit can be expected from the creation and study of mice that are deficient in the other fibulin family members.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/4-7400033-s1.pdf).
Supplementary Material
Supplementary information
Acknowledgments
This work was supported by National Institutes of Health grant HL52813 (W.S.A.) and grants from the Irish Cancer Society, Enterprise Ireland, European Commission and the Association for International Cancer Research (W.M.G.). L.M.G. was supported by a PhD studentship from the Irish Cancer Society.
References
- Anderson L.M., Choe S.E., Yukhananov R.Y., Hopfner R.L., Church G.M., Pratt R.E. & Dzau V.J. ( 2003) Identification of a novel set of genes regulated by a unique liver X receptor-α-mediated transcription mechanism. J. Biol. Chem., 278, 15252–15260. [DOI] [PubMed] [Google Scholar]
- Argraves W.S., Dickerson K., Burgess W.H. & Ruoslahti E. ( 1989) Fibulin, a novel protein that interacts with the fibronectin receptor β subunit cytoplasmic domain. Cell, 58, 623–629. [DOI] [PubMed] [Google Scholar]
- Argraves W.S., Tran H., Burgess W.H. & Dickerson K. ( 1990) Fibulin is an extracellular matrix and plasma glycoprotein with repeated domain structure. J. Cell Biol., 111, 3155–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S.E., Mavila A., Salazar R., Bayless K.J., Kanagala S., Maxwell S.A. & Davis G.E. ( 2001) Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J. Cell Sci., 114, 2755–2773. [DOI] [PubMed] [Google Scholar]
- Bouchey D., Argraves W.S. & Little C.D. ( 1996) Fibulin-1, vitronectin, and fibronectin expression during avian cardiac valve and septa development. Anat. Rec., 244, 540–551. [DOI] [PubMed] [Google Scholar]
- Castoldi M. & Chu M.L. ( 2002) Structural and functional characterization of the human and mouse fibulin-1 gene promoters: role of Sp1 and Sp3. Biochem. J., 362, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry S.S., Gazzard J., Baldock C., Dixon J., Rock M.J., Skinner G.C., Steel K.P., Kielty C.M. & Dixon M.J. ( 2001) Mutation of the gene encoding fibrillin-2 results in syndactyly in mice. Hum. Mol. Genet., 10, 835–843. [DOI] [PubMed] [Google Scholar]
- Clinton G.M., Rougeot C., Derancourt J., Roger P., Defrenne A., Godyna S., Argraves W.S. & Rochefort H. ( 1996) Estrogens increase the expression of fibulin-1, an extracellular matrix protein secreted by human ovarian cancer cells. Proc. Natl Acad. Sci. USA, 93, 316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton C. & Hanash S. ( 2003) Expression of matrix metalloproteinase 9 (MMP-9/gelatinase B) in adenocarcinomas strongly correlated with expression of immune response genes. In Silico Biol., 3, 0026 [epub ahead of print]. [PubMed] [Google Scholar]
- Debeer P., Schoenmakers E.F., Twal W.O., Argraves W.S., De Smet L., Fryns J.P. & Van De Ven W.J. ( 2002) The fibulin-1 gene (FBLN1) is disrupted in a t(12;22) associated with a complex type of synpolydactyly. J. Med. Genet., 39, 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M., Fan X., Hong E. & Chen J.J. ( 2002) Interaction of oncogenic papillomavirus E6 proteins with fibulin-1. Biochem. Biophys. Res. Commun., 296, 962–969. [DOI] [PubMed] [Google Scholar]
- Forti S., Scanlan M.J., Invernizzi A., Castiglioni F., Pupa S., Agresti R., Fontanelli R., Morelli D., Old L.J., Pupa S.M. & Menard S. ( 2002) Identification of breast cancer-restricted antigens by antibody screening of SKBR3 cDNA library using a preselected patient's serum. Breast Cancer Res. Treat., 73, 245–256. [DOI] [PubMed] [Google Scholar]
- Gallagher W.M., Argentini M., Sierra V., Bracco L., Debussche L. & Conseiller E. ( 1999) MBP1: a novel mutant p53-specific protein partner with oncogenic properties. Oncogene, 18, 3608–3616. [DOI] [PubMed] [Google Scholar]
- Gallagher W.M., Greene L.M., Ryan M.P., Sierra V., Berger A., Laurent-Puig P. & Conseiller E. ( 2001) Human fibulin-4: analysis of its biosynthetic processing and mRNA expression in normal and tumour tissues. FEBS Lett., 489, 59–66. [DOI] [PubMed] [Google Scholar]
- Giltay R., Timpl R. & Kostka G. ( 1999) Sequence, recombinant expression and tissue localization of two novel extracellular matrix proteins, fibulin-3 and fibulin-4. Matrix Biol., 18, 469–480. [DOI] [PubMed] [Google Scholar]
- Grammer T.C., Liu K.J., Mariani F.V. & Harland R.M. ( 2000) Use of large-scale expression cloning screens in the Xenopus laevis tadpole to identify gene function. Dev. Biol., 228, 197–210. [DOI] [PubMed] [Google Scholar]
- Grassel S., Sicot F.X., Gotta S. & Chu M.L. ( 1999) Mouse fibulin-2 gene. Complete exon–intron organization and promoter characterization. Eur. J. Biochem., 263, 471–477. [DOI] [PubMed] [Google Scholar]
- Greene L.M., Twal W.O., Duffy M.J., McDermott E.W., Hill A.D., O'Higgins N.J., McCann A.H., Dervan P.A., Argraves W.S. & Gallagher W.M. ( 2003) Elevated expression and altered processing of fibulin-1 protein in human breast cancer. Br. J. Cancer., 88, 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y.C., Talts J.F., Gullberg D., Timpl R. & Ekblom M. ( 2001) Glucocorticoids down-regulate the extracellular matrix proteins fibronectin, fibulin-1 and fibulin-2 in bone marrow stroma. Eur. J. Haematol., 67, 176–184. [DOI] [PubMed] [Google Scholar]
- Hayashi S.I., Eguchi H., Tanimoto K., Yoshida T., Omoto Y., Inoue A., Yoshida N. & Yamaguchi Y. ( 2003) The expression and function of estrogen receptor α and β in human breast cancer and its clinical application. Endocr. Relat. Cancer, 10, 193–202. [DOI] [PubMed] [Google Scholar]
- Hayashido Y., Lucas A., Rougeot C., Godyna S., Argraves W.S. & Rochefort H. ( 1998) Estradiol and fibulin-1 inhibit motility of human ovarian- and breast-cancer cells induced by fibronectin. Int. J. Cancer, 75, 654–658. [DOI] [PubMed] [Google Scholar]
- Heine H., Delude R.L., Monks B.G., Espevik T. & Golenbock D.T. ( 1999) Bacterial lipopolysaccharide induces expression of the stress response genes hop and H411. J. Biol. Chem., 274, 21049–21055. [DOI] [PubMed] [Google Scholar]
- Hungerford J.E., Hoeffler J.P., Bowers C.W., Dahm L.M., Falchetto R., Shabanowitz J., Hunt D.F. & Little C.D. ( 1997) Identification of a novel marker for primordial smooth muscle and its differential expression pattern in contractile vs noncontractile cells. J. Cell Biol., 137, 925–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunzelmann N., Nischt R., Brenneisen P., Eickert A. & Krieg T. ( 2001) Increased deposition of fibulin-2 in solar elastosis and its colocalization with elastic fibres. Br. J. Dermatol., 145, 217–222. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Takagi Y., Mori K., Naruse S., Nishino H., Yue B.Y. & Kinoshita S. ( 2002) cDNA microarray analysis of gene expression changes induced by dexamethasone in cultured human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci., 43, 3691–3697. [PubMed] [Google Scholar]
- Jean J.C., Eruchalu I., Cao Y.X. & Joyce-Brady M. ( 2002) DANCE in developing and injured lung. Am. J. Physiol. Lung Cell Mol. Physiol., 282, L75–L82. [DOI] [PubMed] [Google Scholar]
- Kawata K., Tanaka A., Arai M., Argraves W.S. & Fukutake K. ( 2001) Alteration of plasma fibulin-1 concentrations in ischemic heart diseases. Jpn. J. Thromb. Hemostasis, 12, 126–132. [Google Scholar]
- Kennan A. et al. ( 2002) Identification of an IMPDH1 mutation in autosomal dominant retinitis pigmentosa (RP10) revealed following comparative microarray analysis of transcripts derived from retinas of wild-type and Rho(−/−) mice. Hum. Mol. Genet., 11, 547–557. [DOI] [PubMed] [Google Scholar]
- Kluge M., Mann K., Dziadek M. & Timpl R. ( 1990) Characterization of a novel calcium-binding 90-kDa glycoprotein (BM-90) shared by basement membranes and serum. Eur. J. Biochem., 193, 651–659. [DOI] [PubMed] [Google Scholar]
- Kostka G., Giltay R., Bloch W., Addicks K., Timpl R., Fassler R. & Chu M.L. ( 2001) Perinatal lethality and endothelial cell abnormalities in several vessel compartments of fibulin-1-deficient mice. Mol. Cell. Biol., 21, 7025–7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal R.C., Richardson J.A., Miano J.M. & Olson E.N. ( 1999) EVEC, a novel epidermal growth factor-like repeat-containing protein upregulated in embryonic and diseased adult vasculature. Circ. Res., 84, 1166–1176. [DOI] [PubMed] [Google Scholar]
- Kuang P.P., Goldstein R.H., Liu Y., Rishikof D.C., Jean J.C. & Joyce-Brady M. ( 2003) Coordinate expression of fibulin-5/DANCE and elastin during lung injury repair. Am. J. Physiol. Lung Cell Mol. Physiol., 285, L1147–L1152. [DOI] [PubMed] [Google Scholar]
- Lecka-Czernik B., Moerman E.J., Jones R.A. & Goldstein S. ( 1996) Identification of gene sequences overexpressed in senescent and Werner syndrome human fibroblasts. Exp. Gerontol., 31, 159–174. [DOI] [PubMed] [Google Scholar]
- Loeys B., Van Maldergem L., Mortier G., Coucke P., Gerniers S., Naeyaert J.M. & De Paepe A. ( 2002) Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum. Mol. Genet., 11, 2113–2118. [DOI] [PubMed] [Google Scholar]
- Mariani T.J., Reed J.J. & Shapiro S.D. ( 2002) Expression profiling of the developing mouse lung: insights into the establishment of the extracellular matrix. Am. J. Respir. Cell Mol. Biol., 26, 541–548. [DOI] [PubMed] [Google Scholar]
- Marmorstein L.Y., Munier F.L., Arsenijevic Y., Schorderet D.F., McLaughlin P.J., Chung D., Traboulsi E. & Marmorstein A.D. ( 2002) Aberrant accumulation of EFEMP1 underlies drusen formation in Malattia Leventinese and age-related macular degeneration. Proc. Natl Acad. Sci. USA, 99, 13067–13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll F., Katsaros D., Lazennec G., Hellio N., Roger P., Giacalone P.L., Chalbos D., Maudelonde T., Rochefort H. & Pujol P. ( 2002) Estrogen induction and overexpression of fibulin-1C mRNA in ovarian cancer cells. Oncogene, 21, 1097–1107. [DOI] [PubMed] [Google Scholar]
- Nakamura T. et al. ( 1999) DANCE, a novel secreted RGD protein expressed in developing, atherosclerotic, and balloon-injured arteries. J. Biol. Chem., 274, 22476–22483. [DOI] [PubMed] [Google Scholar]
- Nakamura T. et al. ( 2002) Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature, 415, 171–175. [DOI] [PubMed] [Google Scholar]
- Okada H., Nakajima T., Yoshimura T., Yasuda K. & Kanzaki H. ( 2003) Microarray analysis of genes controlled by progesterone in human endometrial stromal cells in vitro. Gynecol. Endocrinol., 17, 271–280. [DOI] [PubMed] [Google Scholar]
- Pan T.C., Sasaki T., Zhang R.Z., Fassler R., Timpl R. & Chu M.L. ( 1993) Structure and expression of fibulin-2, a novel extracellular matrix protein with multiple EGF-like repeats and consensus motifs for calcium binding. J. Cell Biol., 123, 1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing J., Maher V.M., Tran H., Argraves W.S., Dunstan R.W. & McCormick J.J. ( 1997) Suppression of anchorage-independent growth and matrigel invasion and delayed tumor formation by elevated expression of fibulin-1D in human fibrosarcoma-derived cell lines. Oncogene, 15, 2159–2168. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S., Ross K.N., Lander E.S. & Golub T.R. ( 2003) A molecular signature of metastasis in primary solid tumors. Nature Genet., 33, 49–54. [DOI] [PubMed] [Google Scholar]
- Redfern C.H. et al. ( 2000) Conditional expression of a Gi-coupled receptor causes ventricular conduction delay and a lethal cardiomyopathy. Proc. Natl Acad. Sci. USA, 97, 4826–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D.P., Sasaki T., Dzamba B.J., Keene D.R., Chu M.L., Gohring W., Timpl R. & Sakai L.Y. ( 1996) Fibrillin-1 and fibulin-2 interact and are colocalized in some tissues. J. Biol. Chem., 271, 19489–19496. [DOI] [PubMed] [Google Scholar]
- Roark E.F., Keene D.R., Haudenschild C.C., Godyna S., Little C.D. & Argraves W.S. ( 1995) The association of human fibulin-1 with elastic fibers: an immunohistological, ultrastructural, and RNA study. J. Histochem. Cytochem., 43, 401–411. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Gohring W., Miosge N., Abrams W.R., Rosenbloom J. & Timpl R. ( 1999) Tropoelastin binding to fibulins, nidogen-2 and other extracellular matrix proteins. FEBS Lett., 460, 280–284. [DOI] [PubMed] [Google Scholar]
- Schiemann W.P., Blobe G.C., Kalume D.E., Pandey A. & Lodish H.F. ( 2002) Context-specific effects of fibulin-5 (DANCE/EVEC) on cell proliferation, motility, and invasion. Fibulin-5 is induced by transforming growth factor-β and affects protein kinase cascades. J. Biol. Chem., 277, 27367–27377. [DOI] [PubMed] [Google Scholar]
- Stone E.M. et al. ( 1999) A single EFEMP1 mutation associated with both Malattia Leventinese and Doyne honeycomb retinal dystrophy. Nature Genet., 22, 199–202. [DOI] [PubMed] [Google Scholar]
- Talts J.F., Weller A., Timpl R., Ekblom M. & Ekblom P. ( 1995) Regulation of mesenchymal extracellular matrix protein synthesis by transforming growth factor-β and glucocorticoids in tumor stroma. J. Cell Sci., 108, 2153–2162. [DOI] [PubMed] [Google Scholar]
- Timpl R., Sasaki T., Kostka G. & Chu M.L. ( 2003) Fibulins: a versatile family of extracellular matrix proteins. Nature Rev. Mol. Cell Biol., 4, 479–489. [DOI] [PubMed] [Google Scholar]
- Tran H., Tanaka A., Litvinovich S.V., Medved L.V., Haudenschild C.C. & Argraves W.S. ( 1995) The interaction of fibulin-1 with fibrinogen. A potential role in hemostasis and thrombosis. J. Biol. Chem., 270, 19458–19464. [DOI] [PubMed] [Google Scholar]
- Tran H., Mattei M., Godyna S. & Argraves W.S. ( 1997) Human fibulin-1D: molecular cloning, expression and similarity with S1-5 protein, a new member of the fibulin gene family. Matrix Biol., 15, 479–493. [DOI] [PubMed] [Google Scholar]
- Tsuda T., Wang H., Timpl R. & Chu M.L. ( 2001) Fibulin-2 expression marks transformed mesenchymal cells in developing cardiac valves, aortic arch vessels, and coronary vessels. Dev. Dyn., 222, 89–100. [DOI] [PubMed] [Google Scholar]
- Twal W.O., Czirok A., Hegedus B., Knaak C., Chintalapudi M.R., Okagawa H., Sugi, Y. & Argraves W.S. ( 2001) Fibulin-1 suppression of fibronectin-regulated cell adhesion and motility. J. Cell Sci., 114, 4587–4598. [DOI] [PubMed] [Google Scholar]
- Visconti R.P., Barth J.L., Keeley F.W. & Little C.D. ( 2003) Codistribution analysis of elastin and related fibrillar proteins in early vertebrate development. Matrix Biol., 22, 109–121. [DOI] [PubMed] [Google Scholar]
- Vogel B.E. & Hedgecock E.M. ( 2001) Hemicentin, a conserved extracellular member of the immunoglobulin superfamily, organizes epithelial and other cell attachments into oriented line-shaped junctions. Development, 128, 883–894. [DOI] [PubMed] [Google Scholar]
- Wada J., Zhang H., Tsuchiyama Y., Hiragushi K., Hida K., Shikata K., Kanwar Y.S. & Makino H. ( 2001) Gene expression profile in streptozotocin-induced diabetic mice kidneys undergoing glomerulosclerosis. Kidney Int., 59, 1363–1373. [DOI] [PubMed] [Google Scholar]
- Weigell-Weber M., Sarra G.M., Kotzot D., Sandkuijl L., Messmer E. & Hergersberg M. ( 2003) Genomewide homozygosity mapping and molecular analysis of a candidate gene located on 22q13 (fibulin-1) in a previously undescribed vitreoretinal dystrophy. Arch. Ophthalmol., 121, 1184–1188. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H., Davis E.C., Starcher B.C., Ouchi T., Yanagisawa M., Richardson J.A. & Olson E.N. ( 2002) Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature, 415, 168–171. [DOI] [PubMed] [Google Scholar]
- Zhang H.Y., Chu M.L., Pan T.C., Sasaki T., Timpl R. & Ekblom P. ( 1995) Extracellular matrix protein fibulin-2 is expressed in the embryonic endocardial cushion tissue and is a prominent component of valves in adult heart. Dev. Biol., 167, 18–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


