Short abstract
Surgeons and patients seeking improved treatment often forget that a new technique is not necessarily a better one
New surgical technology that offers the promise of improved patient care is attractive. Intrigued, and with an intuitive certainty, surgeons—cheered on by their patients—may adopt new technologies, despite little evidence of either their efficacy or their superiority over existing procedures. The argument that randomised clinical trials of surgical procedures are unethical because the new treatment is better than alternative treatment or no treatment is based on presumption more than fact, and arguments to the contrary are compelling.1 Surgical procedures that are later found to be ineffective waste resources and endanger lives. Understanding why such procedures come to be offered as treatment can inform us—whether we are well intended perpetrators or unsuspecting patients.
Impetus for change
New medical technology may come in the form of a drug, a device, a procedure, a technique, or a process of care. In surgery, innovation is generally either a new procedure that uses existing devices or drugs, such as chymopapain for lumbar disc disorders, or an existing procedure that uses new devices, such as those for spinal fusion.
Factors that determine the adoption and diffusion of a new technology fall into two categories: characteristics of the technology itself (box 1) and contextual factors that promote it (box 2). Surgeons are attracted to the new technology if it can be passively observed, easily and quickly learnt, and added to their existing practice with minimal disruption. If the potential contribution to their practice is sufficiently great, surgeons are more likely to invest time and effort and tolerate disruption of their routine to gain the competitive advantage that a new technology offers.
Social theory
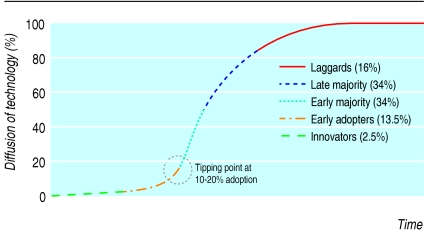
Social observers have advanced pivotal theories regarding the adoption and diffusion of technologies. Everett Rogers described an S-curve portraying the diffusion of innovations and identified characteristics that act as drivers or barriers.2 The diffusion of an innovation comprises five stages: the launch by innovators followed in successive stages by early adopters, the early majority, the late majority, and, finally, the laggards (figure). Malcolm Gladwell3 explains social change as the result of circumstances in which ideas, products, messages, and behaviours spread like viruses through “word-of-mouth epidemics” that are set in motion by three types of individuals: “mavens,” who gather information and pass it on to others; “connectors,” who are sociable and bring people together; and “salesmen,” who have a talent for persuasion.3 The speed of diffusion accelerates to a peak (the tipping point), which occurs on average at 20% adoption.
Box 1: Characteristics of new technology adopted by surgeons
Procedure is compatible with current practice and can be adequately supported in the available facilities
Surgeons can observe the procedure being done
Procedure can be offered to patients for a trial period before it is fully adopted
Procedure is a simple modification of an existing procedure or can be easily learnt by attending surgeons
Volume of cases presenting to the hospital and the expected demand from patients justify surgeons learning the procedure
Procedure will appeal to patients
Box 2: Critical dynamics in adoption and diffusion of new technology
Patients' demand for the technology (personal)
Low cost to surgeons of learning and using the procedure (professional)
Manufacturer's aggressive promotion of the technology (commercial)
Magnitude of benefit perceived by each stakeholder
Below I describe four examples of implementation reflecting these theories and those of other important observers.4-9 I focus on neurosurgery, because of my personal experience, although comparable examples are found in all branches of surgery, both conventional and endovascular. As we tend to learn more from failures than from successes, I first describe two surgical technologies that, after wide diffusion, were discredited.
Discredited technologies
In 1964, Lyman Smith reported that injecting the enzyme chymopapain into an intervertebral disc relieved pain caused by herniation of the lumbar disc.10 By 1974, 17 000 patients were having chymopapain chemonucleolysis each year. In 1989, the American Medical Association's diagnostic and therapeutic technology assessment group questioned the efficacy of the procedure and raised concerns about its safety, citing rare but serious complications.11 Their evaluation showed that, compared with placebo or no treatment, chymopapain was effective in only selected patients. In addition, when it was used by less experienced surgeons some patients incurred serious complications, including anaphylaxis and damage to the spinal cord.11 Although these complications were rare, widespread attention in the media to the drawbacks of chymopapain chemonucleolysis and pressure from an informed public hastened general abandonment of the procedure by patients as well as surgeons. Some centres still use chymopapain as an interim treatment between exercise and surgery for patients with herniated discs, but it has been relegated to a niche procedure sustained by a small group of advocates.
In 1967, Gazi Yasargil reported the first case of “revascularisation” of the brain by anastomosis of the superficial temporal artery to the middle cerebral artery in patients with transient cerebral ischaemia or fixed ischaemic neurological deficits.12 Adoption and diffusion of the technology were rapid. The rationale was simple and easily explained to patients; learning to do the procedure was not difficult; and the potential population eligible for revascularisation was enormous. When an international randomised controlled trial investigated the procedure in 1985,13 it found no benefit for the patients enrolled. The procedure was rapidly abandoned by both patients and payers, although it is still used for carefully selected patients with unique indications.
Figure 1.
S-curve showing the five stages in adoption of innovations2
Beneficial technology adopted slowly
In 1982, Romodanov and Shcheglov14 reported the first large series in which they treated intracranial aneurysms by intra-aneurysmal obliteration using a detachable balloon. The technique proved unsatisfactory, but pushable platinum coils were then introduced for the endovascular treatment of selected intracranial cerebral aneurysms. The platinum coil was supplanted in 1991 by the detachable coil,15 which established the promise of endovascular approaches. Endovascular treatment, however, was used almost exclusively for aneurysms that could not be treated surgically and for patients treated before their aneurysm had ruptured.
Although endovascular techniques were less invasive, their adoption met two barriers: firstly, patients with aneurysms historically were referred to neurosurgeons, who referred to endovascular specialists only those patients for whom a surgical approach posed unacceptable risks; and, secondly, unfavourable outcomes of endovascular obliteration during the early phases of adoption were widely publicised and exploited to maintain direct surgical clipping as the established procedure for treating intracranial aneurysms. More than a decade later, endovascular management began to spread into medical practice for the following reasons:
The number of neurovascular surgeons (interventionalists) trained reached a critical mass to establish the new technology
Stroke units evolved to treat a range of cerebrovascular conditions
Technological advances in image guidance and the versatility of coils reduced the risks of rupture during endovascular procedures
A large multi-institutional randomised controlled trial comparing endovascular obliteration with microsurgical clipping established the equivalence of outcomes and the marginal advantages of the endovascular approach on outcomes such as morbidity and cost.16
Experts forecast the continued expansion of endovascular treatment and a sharply limited use of direct microsurgical approaches for repairing intracranial aneurysms by the end of the decade. A venerable surgical technology is about to disappear into the annals of history.
Surgical technology not yet evaluated
Any surgical technology that is avidly adopted and spreads rapidly without evidence of its comparative benefit runs the risk of being abandoned after objective examination. Instrumentation for spinal fusion has not been rigorously evaluated, and I believe that some of the current indications for its use will not be sustained and a yet to be reported randomised controlled trial will define clinical indications far more stringent than those used at present. The number of patients having spinal procedures is increasing, and the proportion of those who have some form of spinal fusion is increasing disproportionately. Spinal instrumentation is increasing by 20% a year in the United States, and the indications for operations using new instrumentation are often loosely characterised and flexible. The primary driver of spinal instrumentation is the industry that manufactures spinal devices, which runs workshops to teach surgeons how to use its products.
Each year, 200 new spinal surgeons trained to perform spinal fusion enter US practice, and in any specified region, the volume of spinal procedures correlates directly with the number of practising spinal surgeons. For some spinal surgeons the question when treating a patient with chronic pain of spinal origin often is not whether to fuse, but what kind of fusion to perform. As a component of health care, the rising cost of spinal fusion with instrumentation is unsustainable.
Steps in deciding to adopt a new surgical technology
Before adopting a new technology, surgeons and institutions should carefully consider the questions in box 3. At what stage on the S-curve should they decide to adopt and implement a technology? Reasons to be an early adopter may be the surgeon's image, the culture of the institution, or a willingness to take a risk. However, those who are more conservative and sceptical may change only under pressure during the late majority stage. These characteristics may ultimately determine the adoption or rejection of a new surgical technology, but the precondition that is often forgotten in the excitement that comes with change is certainty that the new technology will improve the quality of clinical care for patients. If this precondition is not satisfied, the technology should be abandoned: even a logical and scientifically sound approach is no substitute for proof in practice.
Box 3: Questions to ask before adopting new surgical technology
Will the technology improve the quality of clinical care?
If so, will key early adopters be able and willing to promote its rapid and successful adoption?
What is its likely rate of diffusion?
Are there incompatibilities with the social patterns and technologies that are already in place, and how can they be resolved?
Do we have the financial, human, and infrastructural resources required?
Have we identified and evaluated all known and potential barriers to adoption?
Conclusion
Use of new surgical technology has the potential to provide patients with the best possible care while reinforcing the professional vitality of the surgeon and the institution, boosting their image, and providing a competitive advantage. Conversely, that decision also has the potential to sully reputations, waste resources, and cause inadvertent harm to patients. Surgeons and institutions must guard against “going with the tide” in adopting a technology without solid evidence of its efficacy and superiority over alternatives. In the final analysis, a surgeon's skill and ability to perform a procedure well is unimportant, in fact irrelevant, if the procedure should not be done in the first place.
Summary points
Enthusiasm for new surgical technology has often outstripped evidence
Adoption of new techniques follows an S-shaped curve
Adoption before efficacy has been proved may waste resources and harm patients
Technology that is easy to use and applicable to large numbers of patients is most likely to be adopted without evidence
Contributors and sources: CBW is senior adviser for the Health Technology Center and senior fellow at the Institute for the Future in California. As an academic neurosurgeon, he became interested in the adoption of medical and surgical technologies and the importance of evidence based medicine and has lectured widely on the topic. Susan Eastwood edited the article.
Competing interests: None declared.
References
- 1.McCulloch P, Taylor I, Sasako M, Lovett B Griffin D. Randomised trials in surgery: problems and possible solutions. BMJ 2002;324: 1448-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers EM. Diffusion of innovations. 4th ed. New York: Free Press, 1995.
- 3.Gladwell M. The tipping point: how little things can make a big difference. New York: Little, Brown, 2000.
- 4.Christensen CM. The innovator's dilemma. Boston: Harvard Business School Press, 1997.
- 5.Cain M, Mittmann R. Diffusion of innovation in health care. Oakland: California HealthCare Foundation, 2002. www.iftf.org/docs/SR-778_Diffusion_of_Innovation_in_HC.pdf (accessed 1 Sep 2005).
- 6.Mittman R. The diffusion of new technologies: why do we always fall for the hype? Oakland: California HealthCare Foundation, 2004. www.ihealthbeat.org/index.cfm?Action=dspItem&itemID=100786 (accessed 19 Dec 2005).
- 7.McKinlay JB. From “promising report” to “standard procedure”: seven stages in the career of a medical innovation. Milbank Mem Fund Q Health Soc 1981;59: 374-411. [PubMed] [Google Scholar]
- 8.Chalmers TC. Randomization and coronary artery surgery. Ann Thorac Surg 1972;14: 323-7. [DOI] [PubMed] [Google Scholar]
- 9.McKinlay JB. Evaluating medical technology in the context of a fiscal crisis: the case of New Zealand. Milbank Mem Fund Q Health Soc 1980;58: 217-67. [PubMed] [Google Scholar]
- 10.Smith L. Enzyme dissolution of the nucleus pulposus in humans. JAMA 1964;187: 137-40. [DOI] [PubMed] [Google Scholar]
- 11.Diagnostic and Therapeutic Technology Assessment Group. Chemonucleolysis for herniated lumbar disk. JAMA 1989;262: 953-6. [PubMed] [Google Scholar]
- 12.Yasargil MG, Krayenbuhl HA, Jacobson JH 2nd. Microneurosurgical arterial reconstruction. Surgery 1970;67: 221-33. [PubMed] [Google Scholar]
- 13.The EC/IC Bypass Study Group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke: results of an international randomized trial. N Engl J Med 1985;313: 1191-200. [DOI] [PubMed] [Google Scholar]
- 14.Romodanov AP, Shcheglov VI. Intravascular occlusion of saccular aneurysms of the cerebral arteries by means of a detachable balloon catheter. Adv Tech Stand Neurosurg 1982;9: 25-49. [Google Scholar]
- 15.Guglielmi G, Vinuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: preliminary clinical experience. J Neurosurg 1991;75: 8-14. [DOI] [PubMed] [Google Scholar]
- 16.Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360: 1267-74. [DOI] [PubMed] [Google Scholar]