Abstract
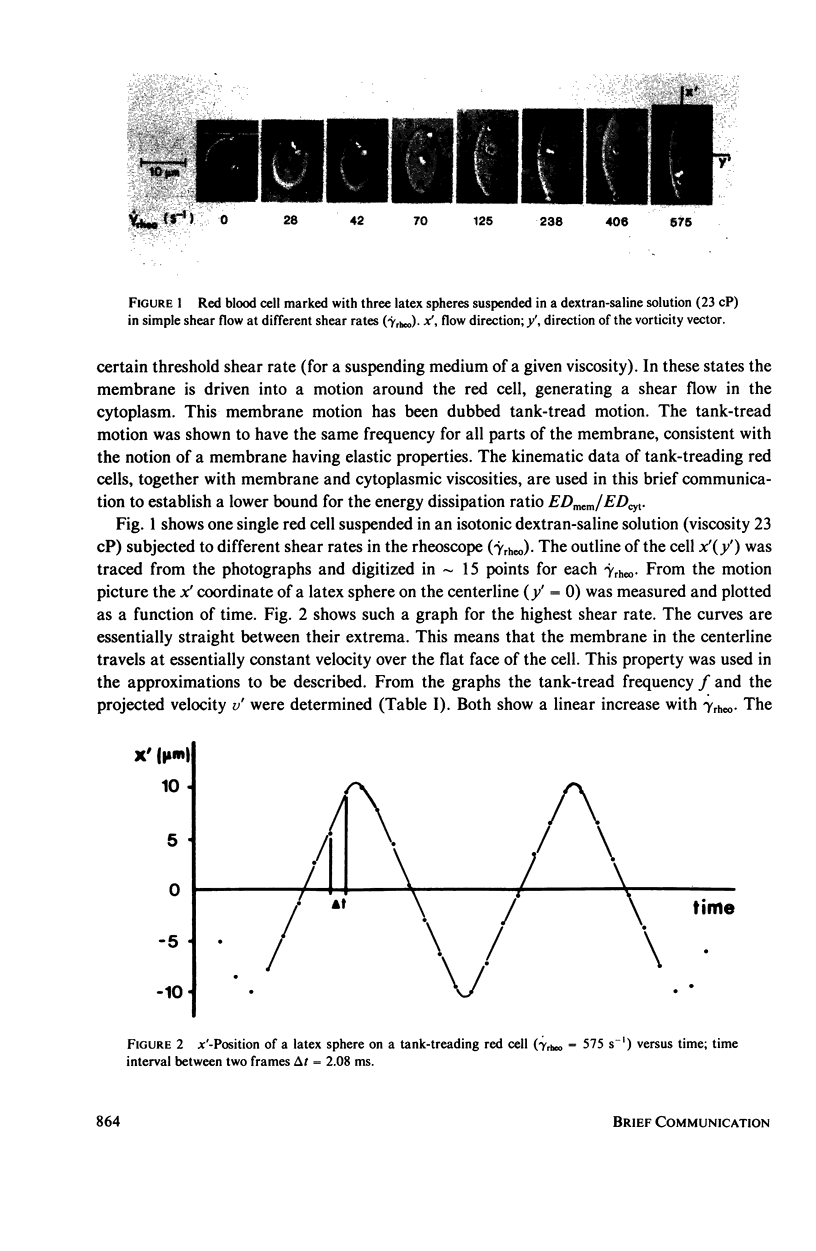
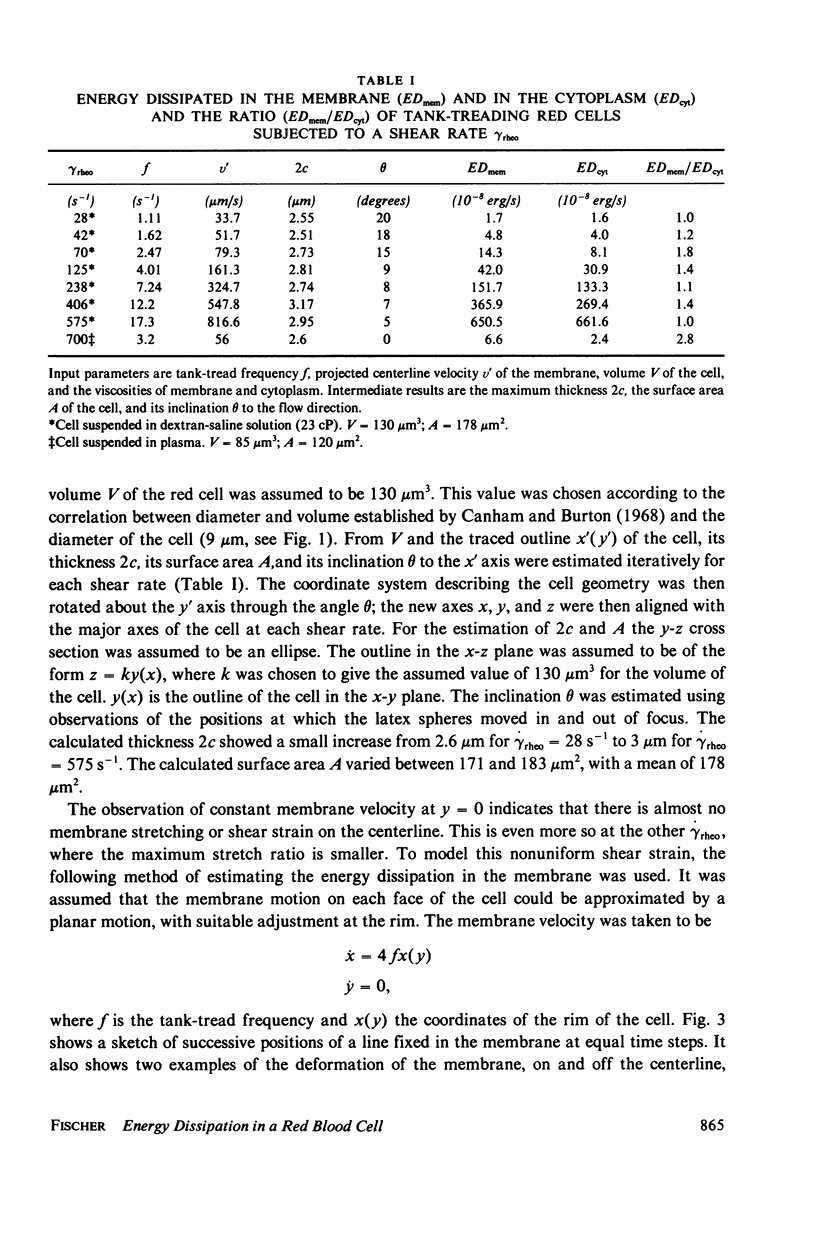
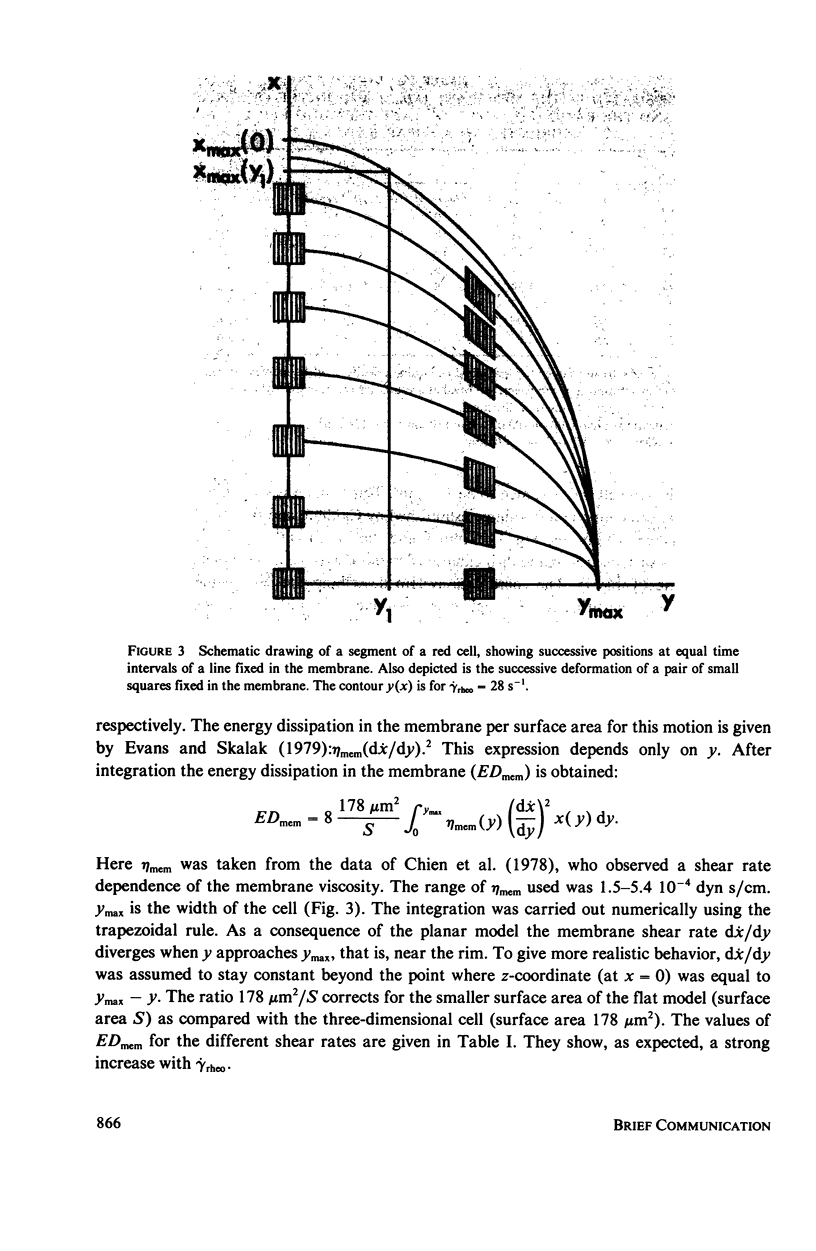
The energy dissipation in the membrane (ED mem) and in the cytoplasm (ED cyt) of tank-treading human red blood cells is estimated. The tank-tread motion of the membrane occurs when the cells in a sheared suspension assume a steady-state of orientation (Fischer et al., 1978, Science [Wash. D. C.], 202:894). The kinematic data used are from red cells suspended either in a dextran-saline solution at a low hematocrit, or in plasma at a hematocrit of 45%. The viscosities of the cytoplasm and the membrane are taken from the literature. The cell in dextran was subjected to seven different shear rates. Both ED mem and ED cyt showed a strong increase with shear rate. Their ratio, however, was always of the order of 1. From this value and the value which was given by Hochmuth et al. (1979, Biophys. J., 26:101) for a shape recovery of a red cell, it is concluded that the range of ED mem/ED cyt for all possible geometries is 1-100.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canham P. B., Burton A. C. Distribution of size and shape in populations of normal human red cells. Circ Res. 1968 Mar;22(3):405–422. doi: 10.1161/01.res.22.3.405. [DOI] [PubMed] [Google Scholar]
- Chien S., Sung K. L., Skalak R., Usami S., Tözeren A. Theoretical and experimental studies on viscoelastic properties of erythrocyte membrane. Biophys J. 1978 Nov;24(2):463–487. doi: 10.1016/S0006-3495(78)85395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien S., Usami S., Bertles J. F. Abnormal rheology of oxygenated blood in sickle cell anemia. J Clin Invest. 1970 Apr;49(4):623–634. doi: 10.1172/JCI106273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cokelet G. R., Meiselman H. J. Rheological comparison of hemoglobin solutions and erythrocyte suspensions. Science. 1968 Oct 11;162(3850):275–277. doi: 10.1126/science.162.3850.275. [DOI] [PubMed] [Google Scholar]
- Dintenfass L. Internal viscosity of the red cell and a blood viscosity equation. Nature. 1968 Aug 31;219(5157):956–958. doi: 10.1038/219956a0. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Hochmuth R. M. Membrane viscoelasticity. Biophys J. 1976 Jan;16(1):1–11. doi: 10.1016/S0006-3495(76)85658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Skalak R. Mechanics and thermodynamics of biomembranes: part 1. CRC Crit Rev Bioeng. 1979 Oct;3(3):181–330. [PubMed] [Google Scholar]
- Fischer T. M., Stöhr-Lissen M., Schmid-Schönbein H. The red cell as a fluid droplet: tank tread-like motion of the human erythrocyte membrane in shear flow. Science. 1978 Nov 24;202(4370):894–896. doi: 10.1126/science.715448. [DOI] [PubMed] [Google Scholar]
- Hochmuth R. M., Worthy P. R., Evans E. A. Red cell extensional recovery and the determination of membrane viscosity. Biophys J. 1979 Apr;26(1):101–114. doi: 10.1016/S0006-3495(79)85238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin A., Desforges J. F. Effect of Heinz bodies on red cell deformability. Blood. 1972 May;39(5):658–665. [PubMed] [Google Scholar]



