Abstract
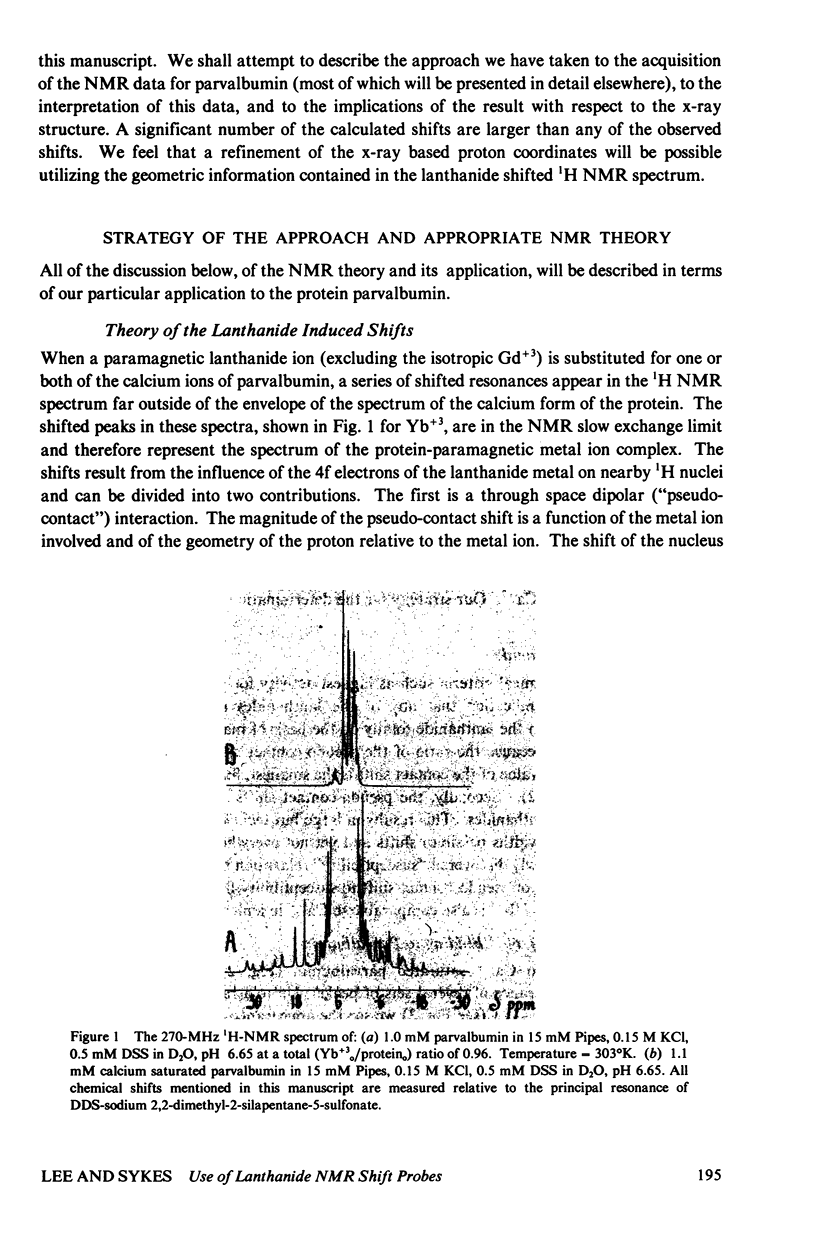
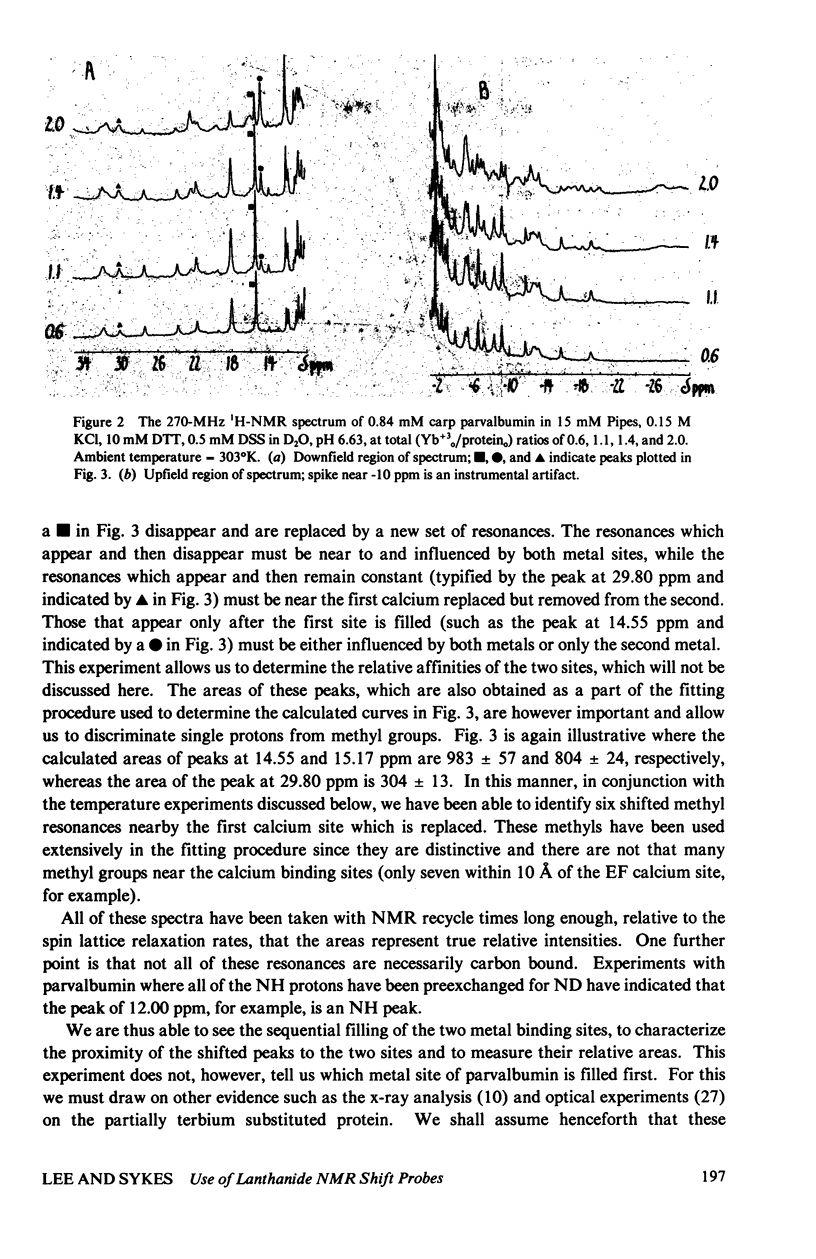
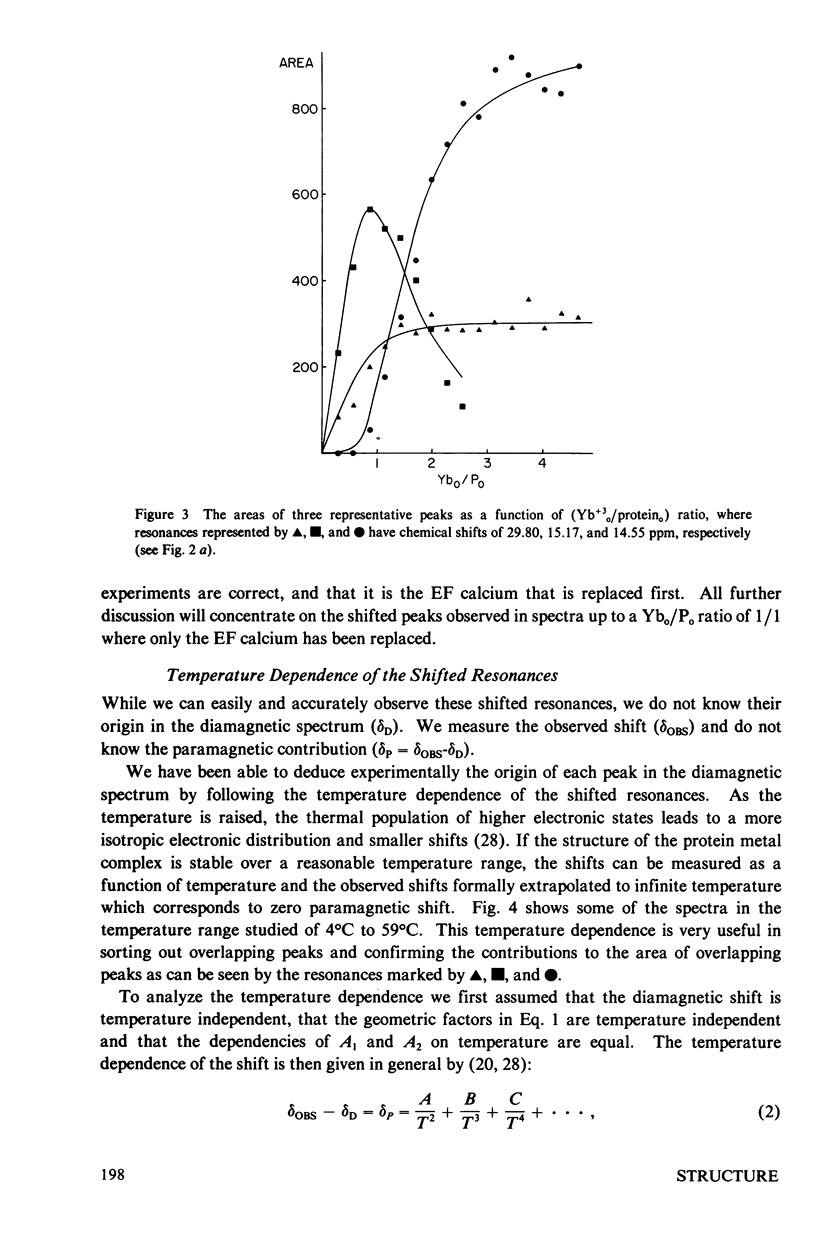
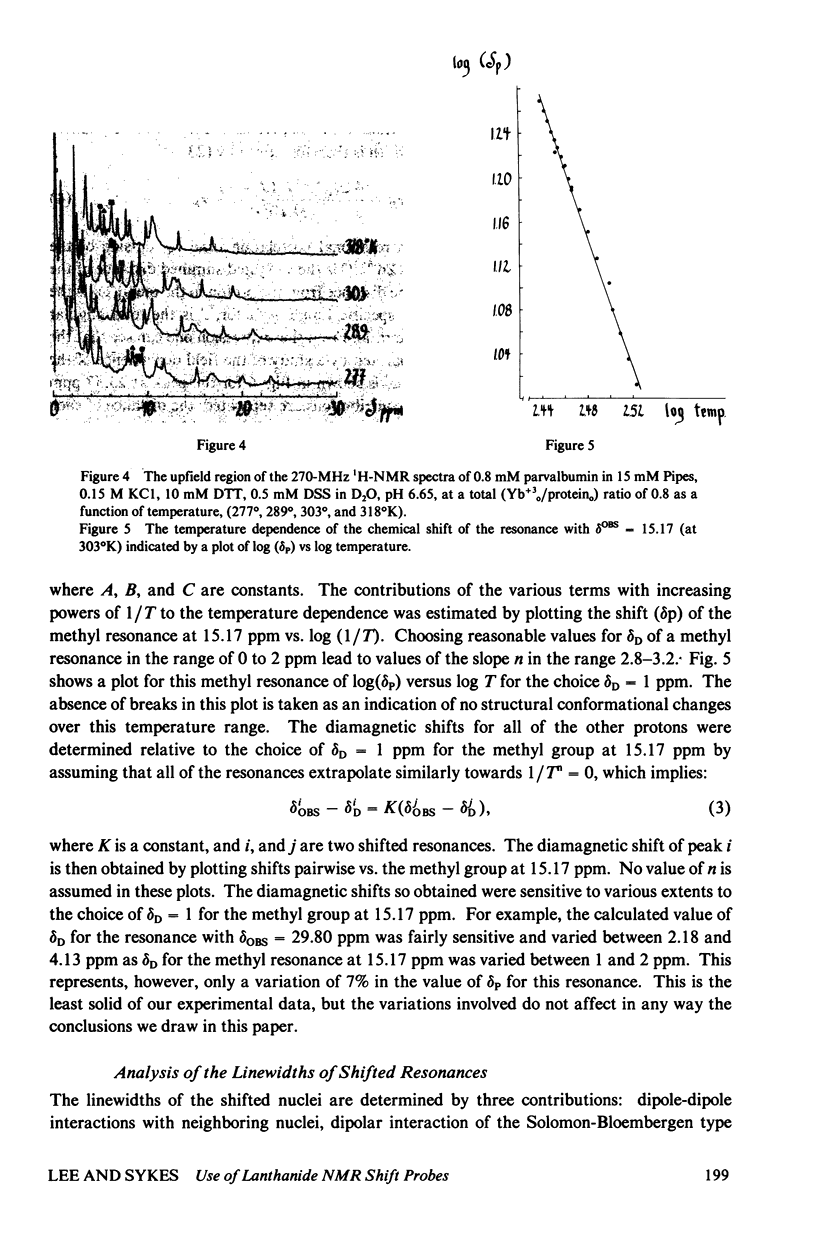
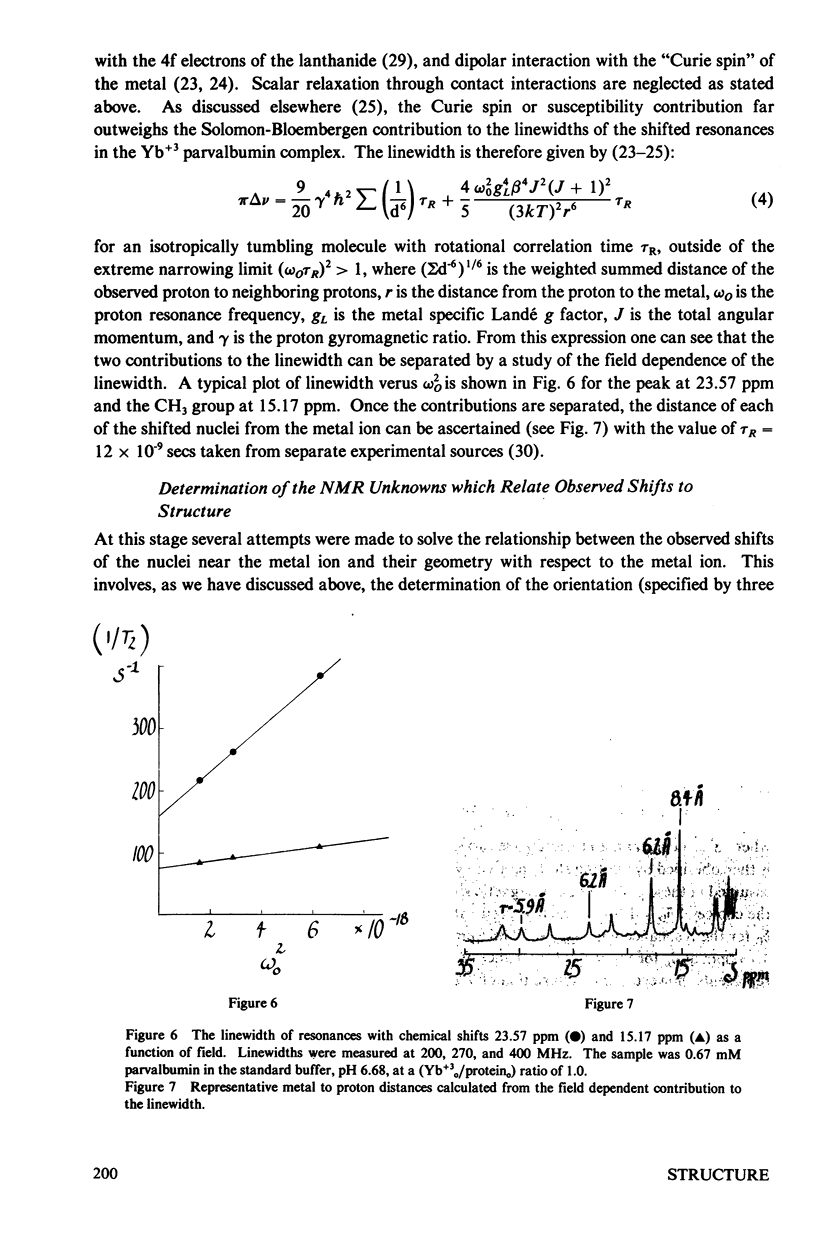
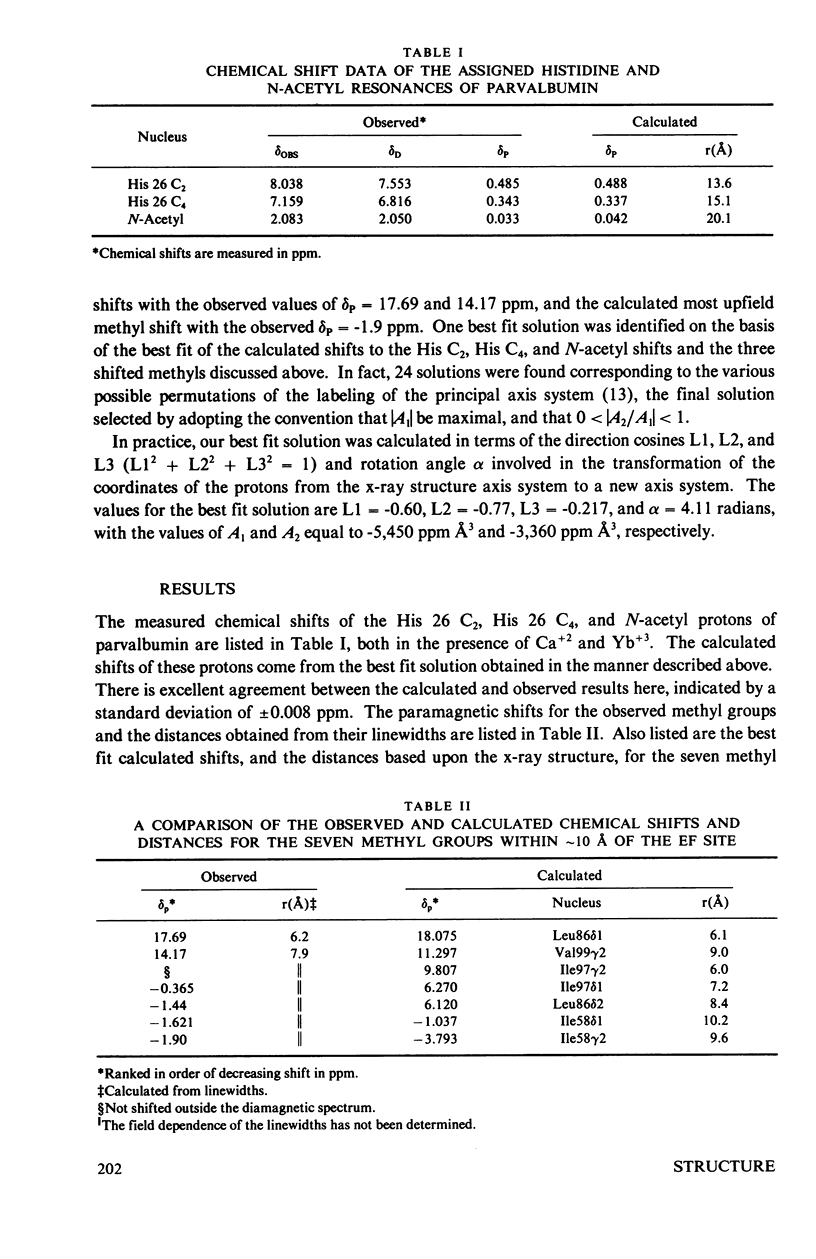
The homologous sequences observed for many calcium binding proteins such as parvalbumin, troponin C, the myosin light chains, and calmodulin has lead to the hypothesis that these proteins have homologous structures at the level of their calcium binding sites. This paper discusses the development of a nuclear magnetic resonance (NMR) technique which will enable us to test this structural hypothesis in solution. The technique involves the substitution of a paramagnetic lanthanide ion for the calcium ion which results in lanthanide induced shifts and broadening in the 1H NMR spectrum of the protein. These shifts are sensitive monitors of the precise geometrical orientation of each proton nucleus relative to the metal. The values of several parameters in the equation relating the NMR shifts to the structure are however known as priori. We have attempted to determine these parameters, the orientation and principal elements of the magnetic susceptibility tensor of the protein bound metal, by studying the lanthanide induced shifts for the protein parvalbumin whose structure has been determined by x-ray crystallographic techniques. The interaction of the lanthanide ytterbium with parvalbumin results in high resolution NMR spectra exhibiting a series of resonances with shifts spread over the range 32 to -19 ppm. The orientation and principal elements of the ytterbium magnetic susceptibility tensor have been determined using three assigned NMR resonances, the His-26 C2 and C4 protons and the amino terminal acetyl protons, and seven methyl groups; all with known geometry relative to the EF calcium binding site. The elucidation of these parameters has allowed us to compare the observed spectrum of the nuclei surrounding the EF calcium binding site of parvalbumin with that calculated from the x-ray structure. A significant number of the calculated shifts are larger than any of the observed shifts. We feel that a refinement of the x-ray based proton coordinates will be possible utilizing the geometric information contained in the lanthanide shifted NMR spectrum.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agresti D. G., Lenkinski R. E., Glickson J. D. Lanthanide induced NMR perturbations of HEW lysozyme: evidence for nonaxial symmetry. Biochem Biophys Res Commun. 1977 Jun 6;76(3):711–719. doi: 10.1016/0006-291x(77)91558-3. [DOI] [PubMed] [Google Scholar]
- Carver J. P., Barber B. H., Fuhr B. J. Magnetic resonance studies of concanavalin A: assignment of histidine resonances in 220 MHz proton spectrum of complexes with Co2+ and Zn2+. J Biol Chem. 1977 May 25;252(10):3141–3146. [PubMed] [Google Scholar]
- Collins J. H. Homology of myosin DTNB light chain with alkali light chains, troponin C and parvalbumin. Nature. 1976 Feb 26;259(5545):699–700. doi: 10.1038/259699a0. [DOI] [PubMed] [Google Scholar]
- Collins J. H. Homology of myosin light chains, troponin-C and parvalbumins deduced from comparison of their amino acid sequences. Biochem Biophys Res Commun. 1974 May 7;58(1):301–308. doi: 10.1016/0006-291x(74)90927-9. [DOI] [PubMed] [Google Scholar]
- Darnall D. W., Birnbaum E. R. Rare earth metal ions as probes of calcium ion binding sites in proteins. Neodymium(3) acceleration of the activation of trypsinogen. J Biol Chem. 1970 Dec 10;245(23):6484–6486. [PubMed] [Google Scholar]
- Donato H., Jr, Martin R. B. Conformations of carp muscle calcium binding parvalbumin. Biochemistry. 1974 Oct 22;13(22):4575–4579. doi: 10.1021/bi00719a016. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H. Calcium-binding proteins. Annu Rev Biochem. 1976;45:239–266. doi: 10.1146/annurev.bi.45.070176.001323. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H., Nockolds C. E. Carp muscle calcium-binding protein. II. Structure determination and general description. J Biol Chem. 1973 May 10;248(9):3313–3326. [PubMed] [Google Scholar]
- Lenkinski R. E., Agresti D. G., Chen D. M., Glickson J. D. An analysis of the Co2+-induced nuclear magnetic resonance perturbations of hen egg white lysozyme. Biochemistry. 1978 Apr 18;17(8):1463–1468. doi: 10.1021/bi00601a016. [DOI] [PubMed] [Google Scholar]
- Marinetti T. D., Snyder G. H., Sykes B. D. Lanthanide interactions with nitrotyrosine. A specific binding site for nuclear magnetic resonance shift probes in proteins. J Am Chem Soc. 1975 Oct 29;97(22):6562–6570. doi: 10.1021/ja00855a046. [DOI] [PubMed] [Google Scholar]
- Marinetti T. D., Snyder G. H., Sykes B. D. Nitrotyrosine chelation of nuclear magnetic resonance shift probes in proteins: application to bovine pancreatic trypsin inhibitor. Biochemistry. 1977 Feb 22;16(4):647–653. doi: 10.1021/bi00623a015. [DOI] [PubMed] [Google Scholar]
- Marinetti T. D., Snyder G. H., Sykes B. D. Nuclear magnetic resonance determination of intramolecular distances in bovine pancreatic trypsin inhibitor using nitrotyrosine chelation of lanthanides. Biochemistry. 1976 Oct 19;15(21):4600–4608. doi: 10.1021/bi00666a009. [DOI] [PubMed] [Google Scholar]
- Matthews B. W., Weaver L. H. Binding of lanthanide ions to thermolysin. Biochemistry. 1974 Apr 9;13(8):1719–1725. doi: 10.1021/bi00705a025. [DOI] [PubMed] [Google Scholar]
- McDonald C. C., Phillips W. D. Perturbation of the PMR spectrum of lysozyme by Co+2. Biochem Biophys Res Commun. 1969 Apr 10;35(1):43–51. doi: 10.1016/0006-291x(69)90480-x. [DOI] [PubMed] [Google Scholar]
- Moews P. C., Kretsinger R. H. Refinement of the structure of carp muscle calcium-binding parvalbumin by model building and difference Fourier analysis. J Mol Biol. 1975 Jan 15;91(2):201–225. doi: 10.1016/0022-2836(75)90160-6. [DOI] [PubMed] [Google Scholar]
- Nelson D. J., Opella S. J., Jardetzky O. 13C nuclear magnetic resonance study of molecular motions and conformational transitions in muscle calcium binding parvalbumins. Biochemistry. 1976 Dec 14;15(25):5552–5560. doi: 10.1021/bi00670a020. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B., Tenenhouse A. The role of cyclic AMP and calcium in cell activation. CRC Crit Rev Biochem. 1972 Feb;1(1):95–148. doi: 10.3109/10409237209102545. [DOI] [PubMed] [Google Scholar]
- Sowadski J., Cornick G., Kretsinger R. H. Terbium replacement of calcium in parvalbumin. J Mol Biol. 1978 Sep 5;124(1):123–132. doi: 10.1016/0022-2836(78)90151-1. [DOI] [PubMed] [Google Scholar]
- Stevens F. C., Walsh M., Ho H. C., Teo T. S., Wang J. H. Comparison of calcium-binding proteins. Bovine heart and brain protein activators of cyclic nucleotide phosphodiesterase and rabbit skeletal muscle troponin C. J Biol Chem. 1976 Aug 10;251(15):4495–4500. [PubMed] [Google Scholar]
- Weeds A. G., McLachlan A. D. Structural homology of myosin alkali light chains, troponin C and carp calcium binding protein. Nature. 1974 Dec 20;252(5485):646–649. doi: 10.1038/252646a0. [DOI] [PubMed] [Google Scholar]


