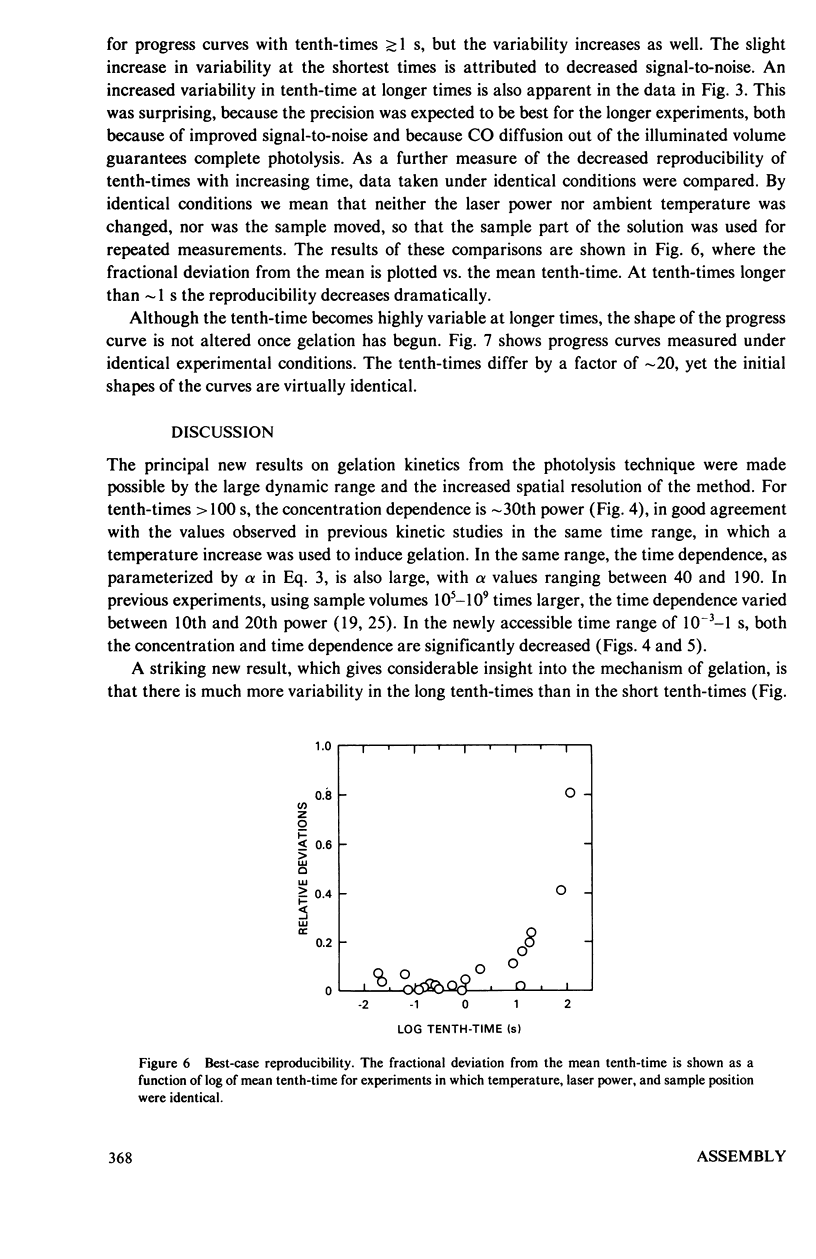
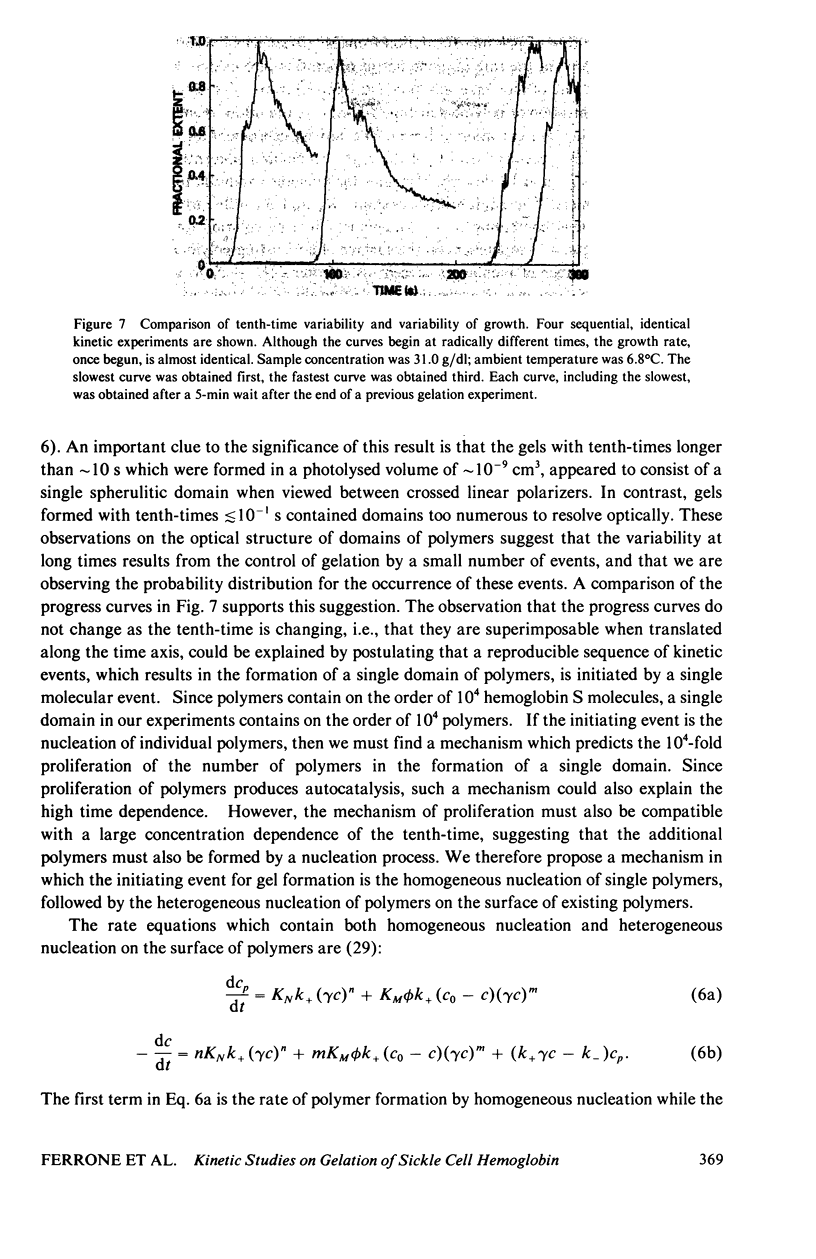
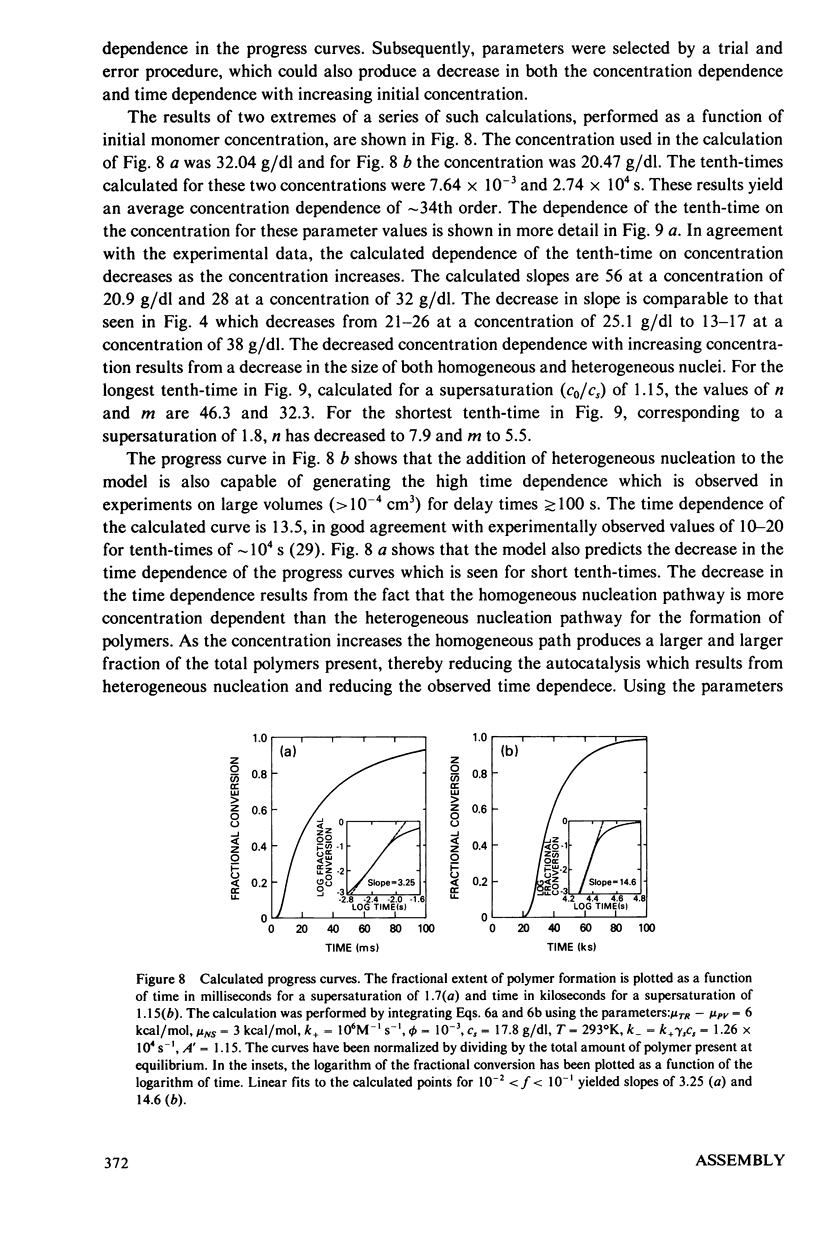
Abstract
The kinetics of deoxyhemoglobin S gelation have been investigated using photolytic dissociation of the carbon monoxide complex to initiate the process. Measurements over a wide range of times, 10(-3)-10(4) show that both the concentration dependence of the tenth-time (i.e., the time required to complete one-tenth the reaction) and the time dependence of the process decrease as gelation speeds up. In slowly gelling samples, where single domains of polymers are formed in the small sample volumes employed with this technique (1-2 x 10(-9) cm3), there is a marked increase in the variability of the tenth-times. These results are explained by a mechanism in which gelation is initiated by homogeneous nucleation of polymers in the bulk solution phase, followed by heterogeneous nucleation on the surface of existing polymers. At the lowest concentrations, homogeneous nucleation is so improbable that stochastic behavior is observed in the small sample volumes, and heterogeneous nucleation is the dominant pathway for polymer formation, thereby accounting for the high time dependence. At the highest concentrations homogeneous nucleation becomes much more probable, and the time dependence decreases. The decrease in concentration dependence of the tenth-time with increasing concentration results from a decrease in size of both the homogeneous and heterogeneous critical nuclei. The model rationalizes the major observations on the kinetics of gelation of deoxyhemoglobin S, and is readily testable by further experiments.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briehl R. W. Gelation of sickle cell hemoglobin. IV. Phase transitions in hemoglobin S gels: separate measures of aggregation and solution--gel equilibrium. J Mol Biol. 1978 Aug 25;123(4):521–538. doi: 10.1016/0022-2836(78)90205-x. [DOI] [PubMed] [Google Scholar]
- Dykes G., Crepeau R. H., Edelstein S. J. Three-dimensional reconstruction of the fibres of sickle cell haemoglobin. Nature. 1978 Apr 6;272(5653):506–510. doi: 10.1038/272506a0. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J., Ross P. D., Tschudin R. G., Becker E. D. Comparison of sickle cell hemoglobin gelation kinetics measured by NMR and optical methods. Biochem Biophys Res Commun. 1976 Mar 22;69(2):538–547. doi: 10.1016/0006-291x(76)90554-4. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Perutz M. F., Bertles J. F., Döbler J. Structure of sickled erythrocytes and of sickle-cell hemoglobin fibers. Proc Natl Acad Sci U S A. 1973 Mar;70(3):718–722. doi: 10.1073/pnas.70.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrell R. L., Crepeau R. H., Edelstein S. J. Cross-sectional views of hemoglobin S fibers by electron microscopy and computer modeling. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1140–1144. doi: 10.1073/pnas.76.3.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. W., Bensusan H. B. The kinetics of the sol-gel transformation of deoxyhemoglobin S by continuous monitoring of viscosity. J Lab Clin Med. 1975 Oct;86(4):564–575. [PubMed] [Google Scholar]
- Hofrichter J., Hendricker D. G., Eaton W. A. Structure of hemoglobin S fibers: optical determination of the molecular orientation in sickled erythrocytes. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3604–3608. doi: 10.1073/pnas.70.12.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J. Ligand binding and the gelation of sickle cell hemoglobin. J Mol Biol. 1979 Mar 5;128(3):335–369. doi: 10.1016/0022-2836(79)90092-5. [DOI] [PubMed] [Google Scholar]
- Hofrichter J., Ross P. D., Eaton W. A. Kinetics and mechanism of deoxyhemoglobin S gelation: a new approach to understanding sickle cell disease. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4864–4868. doi: 10.1073/pnas.71.12.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J., Ross P. D., Eaton W. A. Supersaturation in sickle cell hemoglobin solutions. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3035–3039. doi: 10.1073/pnas.73.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs R., Jarosch H. S., Edelstein S. J. Polymorphism of sickle cell hemoglobin fibers. J Mol Biol. 1976 Apr 15;102(3):409–426. doi: 10.1016/0022-2836(76)90324-7. [DOI] [PubMed] [Google Scholar]
- Kowalczykowski S., Steinhardt J. Kinetics of hemoglobin S gelation followed by continuously sensitive low-shear viscosity. J Mol Biol. 1977 Sep 15;115(2):201–213. doi: 10.1016/0022-2836(77)90097-3. [DOI] [PubMed] [Google Scholar]
- Magdoff-Fairchild B., Chiu C. C. X-ray diffraction studies of fibers and crystals of deoxygenated sickle cell hemoglobin. Proc Natl Acad Sci U S A. 1979 Jan;76(1):223–226. doi: 10.1073/pnas.76.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdoff-Fairchild B., Poillon W. N., Li T., Bertles J. F. Thermodynamic studies of polymerization of deoxygenated sickle cell hemoglobin. Proc Natl Acad Sci U S A. 1976 Apr;73(4):990–994. doi: 10.1073/pnas.73.4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfa R., Steinhardt J. A temperature-dependent latent-period in the aggregation of sickle-cell deoxyhemoglobin. Biochem Biophys Res Commun. 1974 Aug 5;59(3):887–893. doi: 10.1016/s0006-291x(74)80062-8. [DOI] [PubMed] [Google Scholar]
- Moffat K., Gibson Q. H. The rates of polymerization and depolymerization of sickle cell hemoglobin. Biochem Biophys Res Commun. 1974 Nov 6;61(1):237–242. doi: 10.1016/0006-291x(74)90558-0. [DOI] [PubMed] [Google Scholar]
- Noguchi C. T., Schechter A. N. Effects of amino acids on gelation kinetics and solubility of sickle hemoglobin. Biochem Biophys Res Commun. 1977 Jan 24;74(2):637–642. doi: 10.1016/0006-291x(77)90350-3. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Briehl R. W., Minton A. P. Temperature dependence of nonideality in concentrated solutions of hemoglobin. Biopolymers. 1978 Sep;17(9):2285–2288. doi: 10.1002/bip.1978.360170920. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Hofrichter J., Eaton W. A. Calorimetric and optical characterization of sickle cell hemoglobin gelation. J Mol Biol. 1975 Aug 5;96(2):239–253. doi: 10.1016/0022-2836(75)90345-9. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Hofrichter J., Eaton W. A. Thermodynamics of gelation of sickle cell deoxyhemoglobin. J Mol Biol. 1977 Sep 15;115(2):111–134. doi: 10.1016/0022-2836(77)90093-6. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Minton A. P. Analysis of non-ideal behavior in concentrated hemoglobin solutions. J Mol Biol. 1977 May 25;112(3):437–452. doi: 10.1016/s0022-2836(77)80191-5. [DOI] [PubMed] [Google Scholar]
- Sunshine H. R., Hofrichter J., Eaton W. A. Gelation of sickle cell hemoglobin in mixtures with normal adult and fetal hemoglobins. J Mol Biol. 1979 Oct 9;133(4):435–467. doi: 10.1016/0022-2836(79)90402-9. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr Concerted formation of the gel of hemoglobin S. Proc Natl Acad Sci U S A. 1973 May;70(5):1506–1508. doi: 10.1073/pnas.70.5.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]


