Abstract
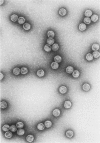
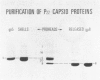
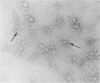
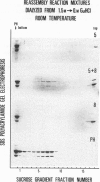
In the morphogenesis of double stranded DNA phages, a precursor protein shell empty of DNA is first assembled and then filled with DNA. The assembly of the correctly dimensioned precursor shell (procapsid) of Salmonella bacteriophage P22 requires the interaction of some 420 coat protein subunits with approximately 200 scaffolding protein subunits to form a double shelled particle with the scaffolding protein on the inside. In the course of DNA packaging, all of the scaffolding protein subunits exit from the procapsid and participate in further rounds of procapsid assembly (King and Casjens. 1974. Nature (Lond.). 251:112-119). To study the mechanism of shell assembly we have purified the coat and scaffolding protein subunits by selective dissociation of isolated procapsids. Both proteins can be obtained as soluble subunits in Tris buffer at near neutral pH. The coat protein sedimented in sucrose gradients as a roughly spherical monomer, while the scaffolding protein sedimented as if it were an elongated monomer. When the two proteins were mixed together in 1.5 M guanidine hydrochloride and dialyzed back to buffer at room temperature, procapsids formed which were very similar in morphology, sedimentation behavior, and protein composition to procapsids formed in vivo. Incubation of either protein alone under the same conditions did not yield any large structures. We interpret these results to mean that the assembly of the shell involves a switching of both proteins from their nonaggregating to their aggregating forms through their mutual interaction. The results are discussed in terms of the general problem of self-regulated assembly and the control of protein polymerization in morphogenesis.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asakura S. Polymerization of flagellin and polymorphism of flagella. Adv Biophys. 1970;1:99–155. [PubMed] [Google Scholar]
- Botstein D., Waddell C. H., King J. Mechanism of head assembly and DNA encapsulation in Salmonella phage p22. I. Genes, proteins, structures and DNA maturation. J Mol Biol. 1973 Nov 15;80(4):669–695. doi: 10.1016/0022-2836(73)90204-0. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. P22 morphogenesis. I: Catalytic scaffolding protein in capsid assembly. J Supramol Struct. 1974;2(2-4):202–224. doi: 10.1002/jss.400020215. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Casjens S. Molecular organization of the bacteriophage P22 coat protein shell. J Mol Biol. 1979 Jun 15;131(1):1–14. doi: 10.1016/0022-2836(79)90298-5. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Harrison S. C. DNA arrangement in isometric phage heads. Nature. 1977 Aug 18;268(5621):598–602. doi: 10.1038/268598a0. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., King J., Harrison S. C., Eiserling F. A. The structural organization of DNA packaged within the heads of T4 wild-type, isometric and giant bacteriophages. Cell. 1978 Jul;14(3):559–568. doi: 10.1016/0092-8674(78)90242-8. [DOI] [PubMed] [Google Scholar]
- Earnshaw W., Casjens S., Harrison S. C. Assembly of the head of bacteriophage P22: x-ray diffraction from heads, proheads and related structures. J Mol Biol. 1976 Jun 25;104(2):387–410. doi: 10.1016/0022-2836(76)90278-3. [DOI] [PubMed] [Google Scholar]
- Earnshaw W., King J. Structure of phage P22 coat protein aggregates formed in the absence of the scaffolding protein. J Mol Biol. 1978 Dec 25;126(4):721–747. doi: 10.1016/0022-2836(78)90017-7. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., King J. Genetic control of bacteriophage T4 baseplate morphogenesis. I. Sequential assembly of the major precursor, in vivo and in vitro. J Mol Biol. 1975 Dec 25;99(4):645–672. doi: 10.1016/s0022-2836(75)80178-1. [DOI] [PubMed] [Google Scholar]
- King J., Casjens S. Catalytic head assembling protein in virus morphogenesis. Nature. 1974 Sep 13;251(5471):112–119. doi: 10.1038/251112a0. [DOI] [PubMed] [Google Scholar]
- King J., Hall C., Casjens S. Control of the synthesis of phage P22 scaffolding protein is coupled to capsid assembly. Cell. 1978 Oct;15(2):551–560. doi: 10.1016/0092-8674(78)90023-5. [DOI] [PubMed] [Google Scholar]
- King J., Lenk E. V., Botstein D. Mechanism of head assembly and DNA encapsulation in Salmonella phage P22. II. Morphogenetic pathway. J Mol Biol. 1973 Nov 15;80(4):697–731. doi: 10.1016/0022-2836(73)90205-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murialdo H., Becker A. Head morphogenesis of complex double-stranded deoxyribonucleic acid bacteriophages. Microbiol Rev. 1978 Sep;42(3):529–576. doi: 10.1128/mr.42.3.529-576.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteete A. R., Jarvik V., Botstein D. Encapsulation of phage P22 DNA in vitro. Virology. 1979 Jun;95(2):550–564. doi: 10.1016/0042-6822(79)90508-7. [DOI] [PubMed] [Google Scholar]
- Schuster T. M., Scheele R. B., Adams M. L., Shire S. J., Steckert J. J., Potschka M. Studies on the mechanism of assembly of tobacco mosaic virus. Biophys J. 1980 Oct;32(1):313–329. doi: 10.1016/S0006-3495(80)84959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showe M. K., Black L. W. Assembly core of bacteriophage T4: an intermediate in head formation. Nat New Biol. 1973 Mar 21;242(116):70–75. doi: 10.1038/newbio242070a0. [DOI] [PubMed] [Google Scholar]
- Susskind M. M., Botstein D. Molecular genetics of bacteriophage P22. Microbiol Rev. 1978 Jun;42(2):385–413. doi: 10.1128/mr.42.2.385-413.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uratani Y., Asakura S., Imahori K. A circular dichroism study of Salmonella flagellin: evidence for conformational change on polymerization. J Mol Biol. 1972 Jun 14;67(1):85–98. doi: 10.1016/0022-2836(72)90388-9. [DOI] [PubMed] [Google Scholar]
- Wagenknecht T., Bloomfield V. A. In vitro polymerization of bacteriophage T4D tail core subunits. J Mol Biol. 1977 Nov 5;116(3):347–359. doi: 10.1016/0022-2836(77)90074-2. [DOI] [PubMed] [Google Scholar]
- van Driel R., Couture E. Assembly of bacteriophage T4 head-related strucutres. II. In vitro assembly of prehead-like structures. J Mol Biol. 1978 Aug 5;123(2):115–128. doi: 10.1016/0022-2836(78)90316-9. [DOI] [PubMed] [Google Scholar]
- van Driel R., Couture E. Assembly of the scaffolding core of bacteriophage T4 preheads. J Mol Biol. 1978 Aug 25;123(4):713–719. doi: 10.1016/0022-2836(78)90217-6. [DOI] [PubMed] [Google Scholar]