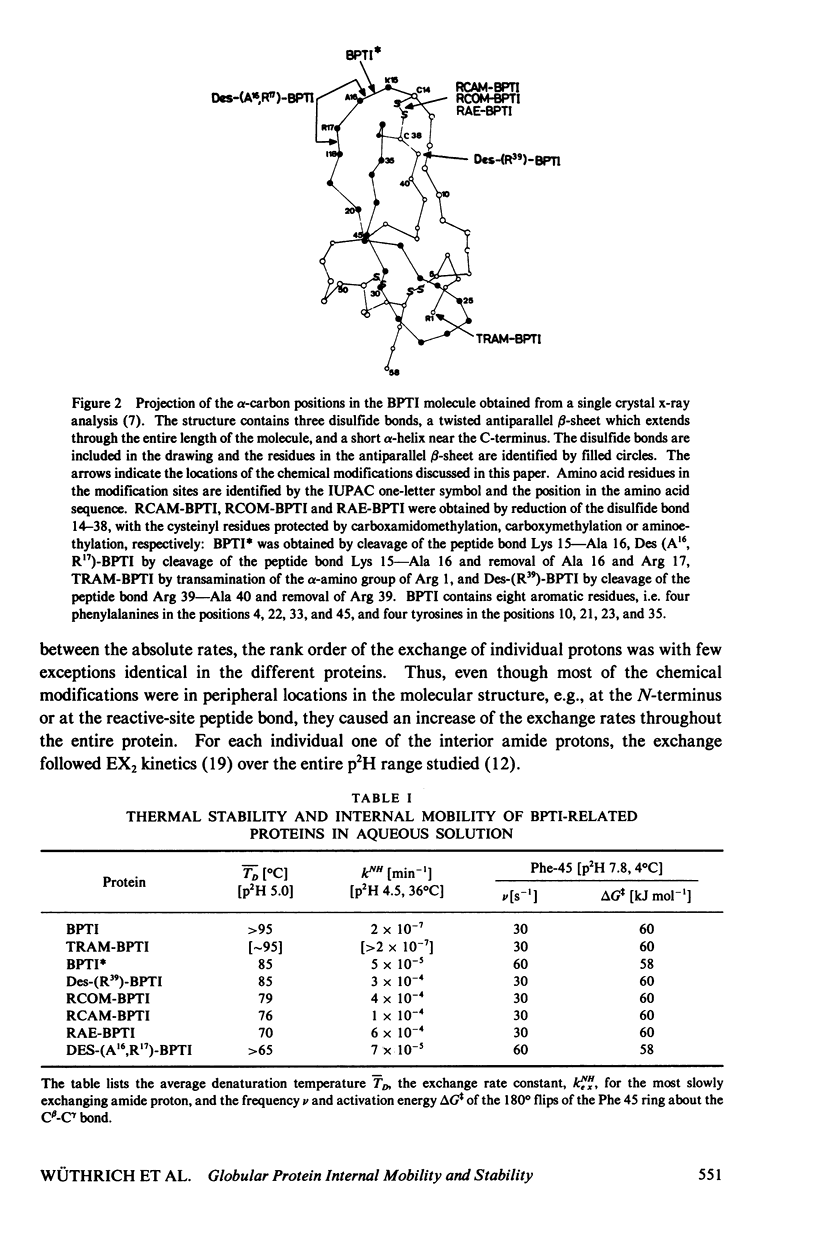
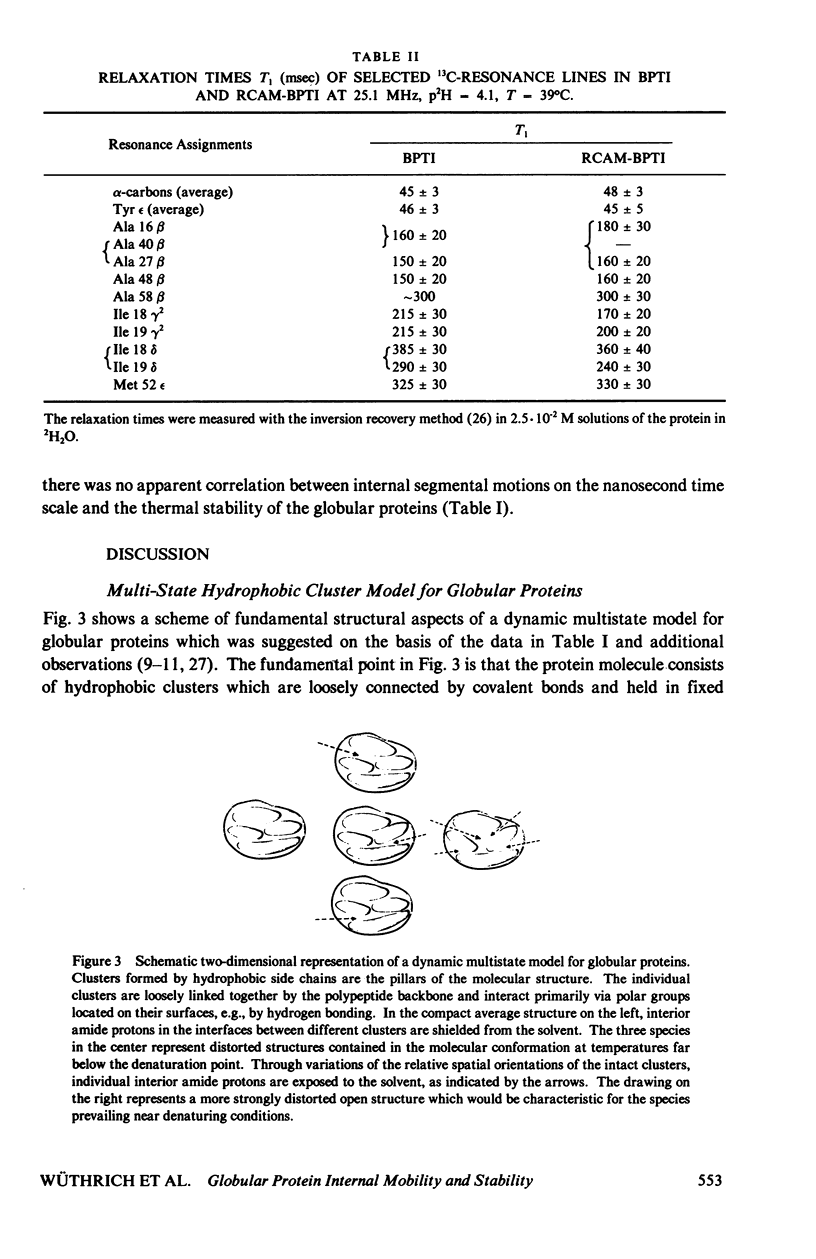
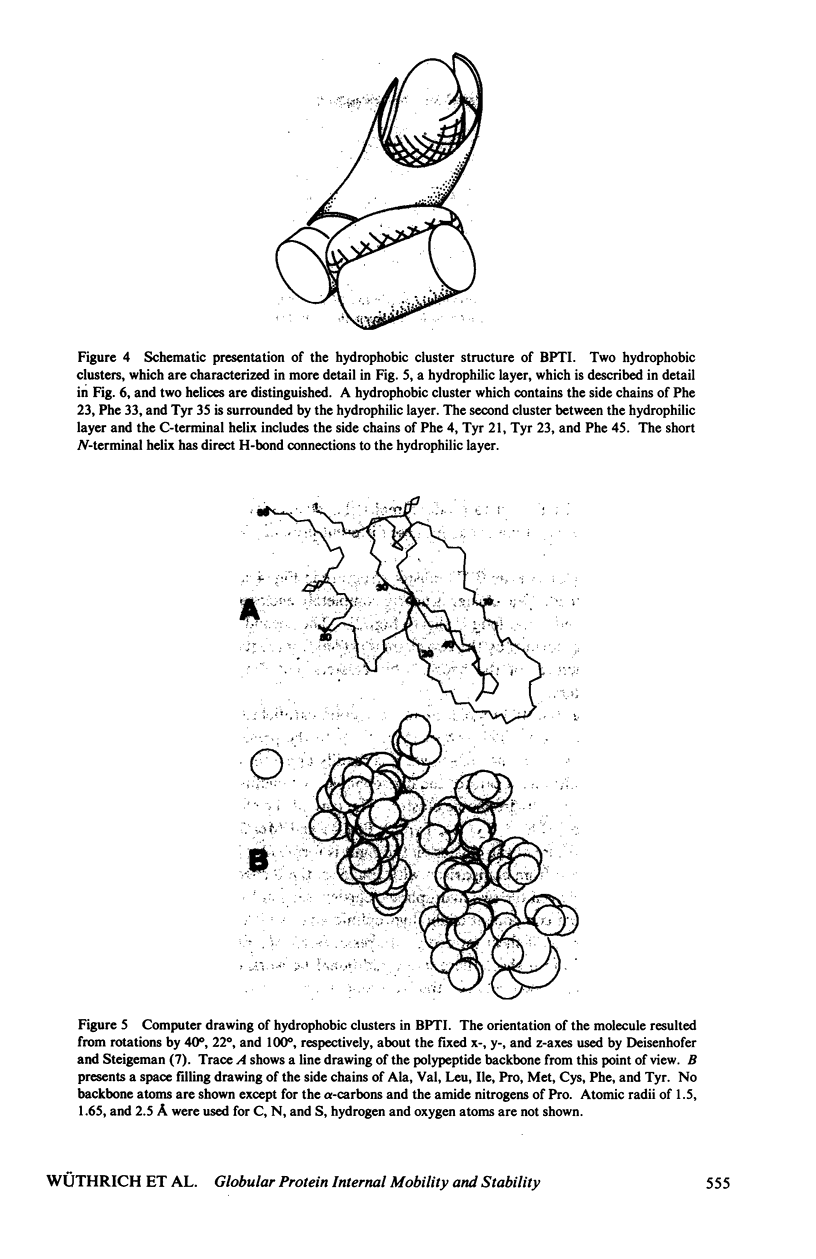
Abstract
The recent work is surveyed which leads to the suggestions that the conformation of globular proteins in solution corresponds to a dynamic ensemble of rapidly interconverting spatial structures, that clusters of hydrophobic amino acid side chains have an important role in the architecture of protein molecules, and that mechanistic aspects of protein denaturation can be correlated with internal mobility seen in the native conformation. These conclusions resulted originally from high resolution 1H nuclear magnetic resonance (NMR) studies of aromatic ring mobility, exchange of interior amide protons and thermal denaturation of the basic pancreatic trypsin inhibitor and a group of related proteins. Various new approaches to further characterize proteins in solution have now been taken and preliminary data are presented. These include computer graphics to outline hydrophobic clusters in globular protein structures, high resolution 1H-NMR experiments at variable hydrostatic pressure and 13C-NMR relaxation measurements. At the present early stage of these new investigations it appears that the hydrophobic cluster model for globular proteins is compatible with the data obtained.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown L. R., De Marco A., Richarz R., Wagner G., Wüthrich K. The influence of a single salt bridge on static and dynamic features of the globular solution conformation of the basic pancreatic trypsin inhibitor. 1H and 13C nuclear-magnetic-resonance studies of the native and the transaminated inhibitor. Eur J Biochem. 1978 Jul 17;88(1):87–95. doi: 10.1111/j.1432-1033.1978.tb12425.x. [DOI] [PubMed] [Google Scholar]
- Carter J. V., Knox D. G., Rosenberg A. Pressure effects on folded proteins in solution. Hydrogen exchange at elevated pressures. J Biol Chem. 1978 Mar 25;253(6):1947–1953. [PubMed] [Google Scholar]
- Dubs A., Wagner G., Wüthrich K. Individual assignments of amide proton resonances in the proton NMR spectrum of the basic pancreatic trypsin inhibitor. Biochim Biophys Acta. 1979 Mar 27;577(1):177–194. doi: 10.1016/0005-2795(79)90020-5. [DOI] [PubMed] [Google Scholar]
- Hetzel R., Wüthrich K., Deisenhofer J., Huber R. Dynamics of the aromatic amino acid residues in the globular conformation of the basic pancreatic trypsin inhibitor (BPTI). II. Semi-empirical energy calculations. Biophys Struct Mech. 1976 Aug 23;2(2):159–180. doi: 10.1007/BF00863707. [DOI] [PubMed] [Google Scholar]
- Hvidt A., Nielsen S. O. Hydrogen exchange in proteins. Adv Protein Chem. 1966;21:287–386. doi: 10.1016/s0065-3233(08)60129-1. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Kawato S., Ikegami A. A theory of fluorescence polarization decay in membranes. Biophys J. 1977 Dec;20(3):289–305. doi: 10.1016/S0006-3495(77)85550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinetti T. D., Snyder G. H., Sykes B. D. Nuclear magnetic resonance determination of intramolecular distances in bovine pancreatic trypsin inhibitor using nitrotyrosine chelation of lanthanides. Biochemistry. 1976 Oct 19;15(21):4600–4608. doi: 10.1021/bi00666a009. [DOI] [PubMed] [Google Scholar]
- McCammon J. A., Wolynes P. G., Karplus M. Picosecond dynamics of tyrosine side chains in proteins. Biochemistry. 1979 Mar 20;18(6):927–942. doi: 10.1021/bi00573a001. [DOI] [PubMed] [Google Scholar]
- Richarz R., Sehr P., Wagner G., Wüthrich K. Kinetics of the exchange of individual amide protons in the basic pancreatic trypsin inhibitor. J Mol Biol. 1979 May 5;130(1):19–30. doi: 10.1016/0022-2836(79)90549-7. [DOI] [PubMed] [Google Scholar]
- Richarz R., Wüthrich K. High-field 13C nuclear magnetic resonance studies at 90.5 MHz of the basic pancreatic trypsin inhibitor. Biochemistry. 1978 Jun 13;17(12):2263–2269. doi: 10.1021/bi00605a002. [DOI] [PubMed] [Google Scholar]
- Wagner G., DeMarco A., Wüthrich K. Dynamics of the aromatic amino acid residues in the globular conformation of the basic pancreatic trypsin inhibitor (BPTI). I. 1H NMR studies. Biophys Struct Mech. 1976 Aug 23;2(2):139–158. doi: 10.1007/BF00863706. [DOI] [PubMed] [Google Scholar]
- Wagner G., Kalb A. J., Wüthrich K. Conformational studies by 1H nuclear magnetic resonance of the basic pancreatic trypsin inhibitor after reduction of the disulfide bond between Cys-14 and Cys-38. Influence of charged protecting groups on the stability of the protein. Eur J Biochem. 1979 Apr 2;95(2):249–253. doi: 10.1111/j.1432-1033.1979.tb12960.x. [DOI] [PubMed] [Google Scholar]
- Wagner G., Tschesche H., Wüthrich K. The influence of localized chemical modifications of the basic pancreatic trypsin inhibitor on static and dynamic aspects of the molecular conformation in solution. Eur J Biochem. 1979 Apr 2;95(2):239–248. doi: 10.1111/j.1432-1033.1979.tb12959.x. [DOI] [PubMed] [Google Scholar]
- Wagner G., Wütherich K., Tschesche H. A 1H nuclear-magnetic-resonance study of the conformation and the molecular dynamics of the glycoprotein cow-colostrum trypsin inhibitor. Eur J Biochem. 1978 May;86(1):67–76. doi: 10.1111/j.1432-1033.1978.tb12285.x. [DOI] [PubMed] [Google Scholar]
- Wagner G., Wüthrich K. Correlation between the amide proton exchange rates and the denaturation temperatures in globular proteins related to the basic pancreatic trypsin inhibitor. J Mol Biol. 1979 May 5;130(1):31–37. doi: 10.1016/0022-2836(79)90550-3. [DOI] [PubMed] [Google Scholar]
- Wagner G., Wüthrich K. Dynamic model of globular protein conformations based on NMR studies in solution. Nature. 1978 Sep 21;275(5677):247–248. doi: 10.1038/275247a0. [DOI] [PubMed] [Google Scholar]
- Wagner G., Wüthrich K. Structural interpretation of the amide proton exchange in the basic pancreatic trypsin inhibitor and related proteins. J Mol Biol. 1979 Oct 15;134(1):75–94. doi: 10.1016/0022-2836(79)90414-5. [DOI] [PubMed] [Google Scholar]
- Wagner G., Wüthrich K., Tschesche H. A 1H nuclear-magnetic-resonance study of the solution conformation of the isoinhibitor K from Helix pomatia. Eur J Biochem. 1978 Sep 1;89(2):367–377. doi: 10.1111/j.1432-1033.1978.tb12538.x. [DOI] [PubMed] [Google Scholar]
- Wüthrich K., Wagner G. Nuclear magnetic resonance of labile protons in the basic pancreatic trypsin inhibitor. J Mol Biol. 1979 May 5;130(1):1–18. doi: 10.1016/0022-2836(79)90548-5. [DOI] [PubMed] [Google Scholar]


