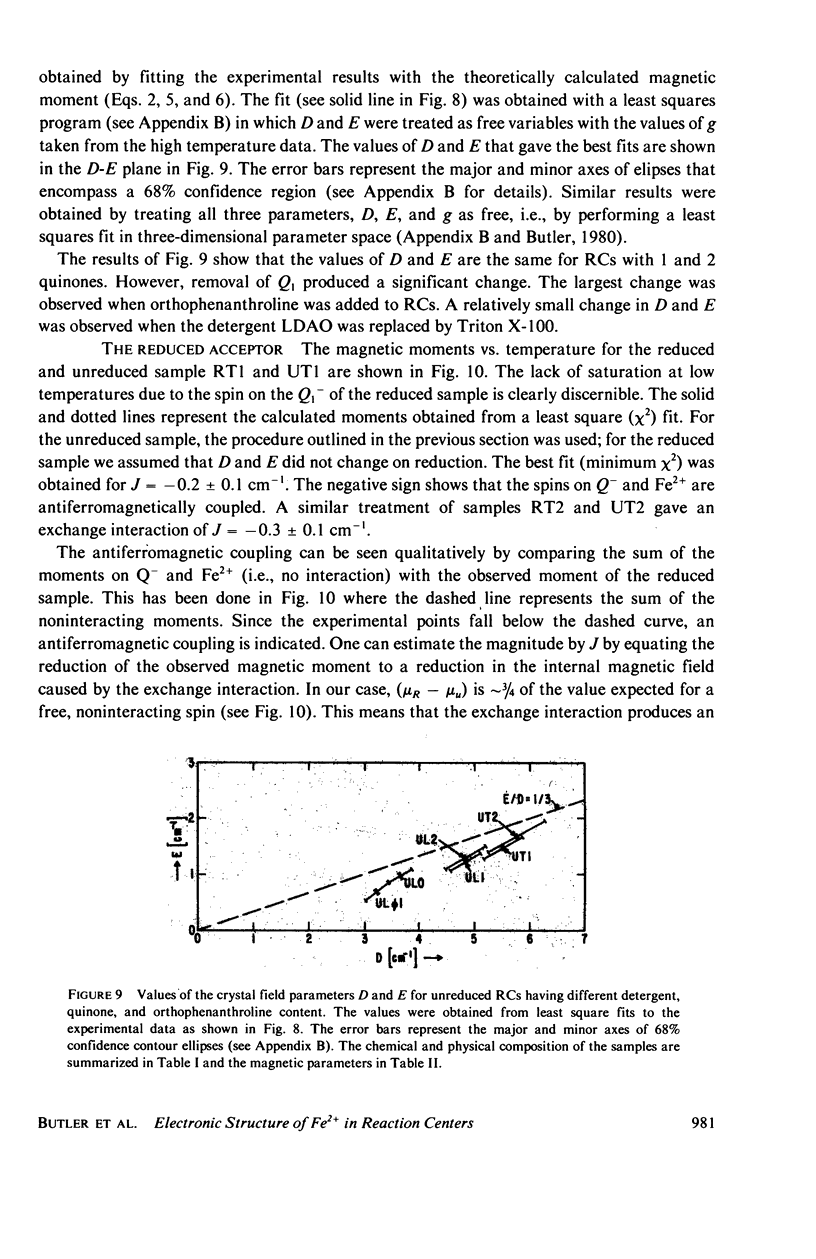
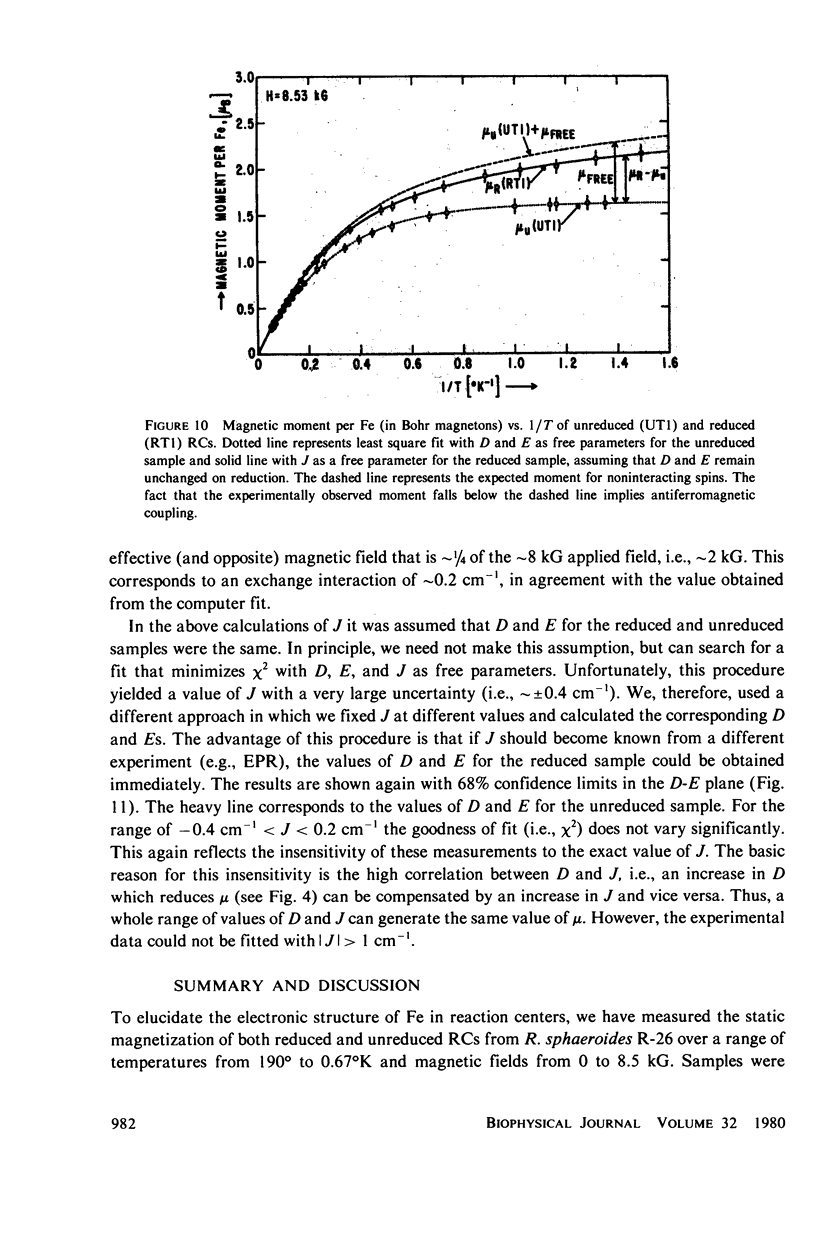
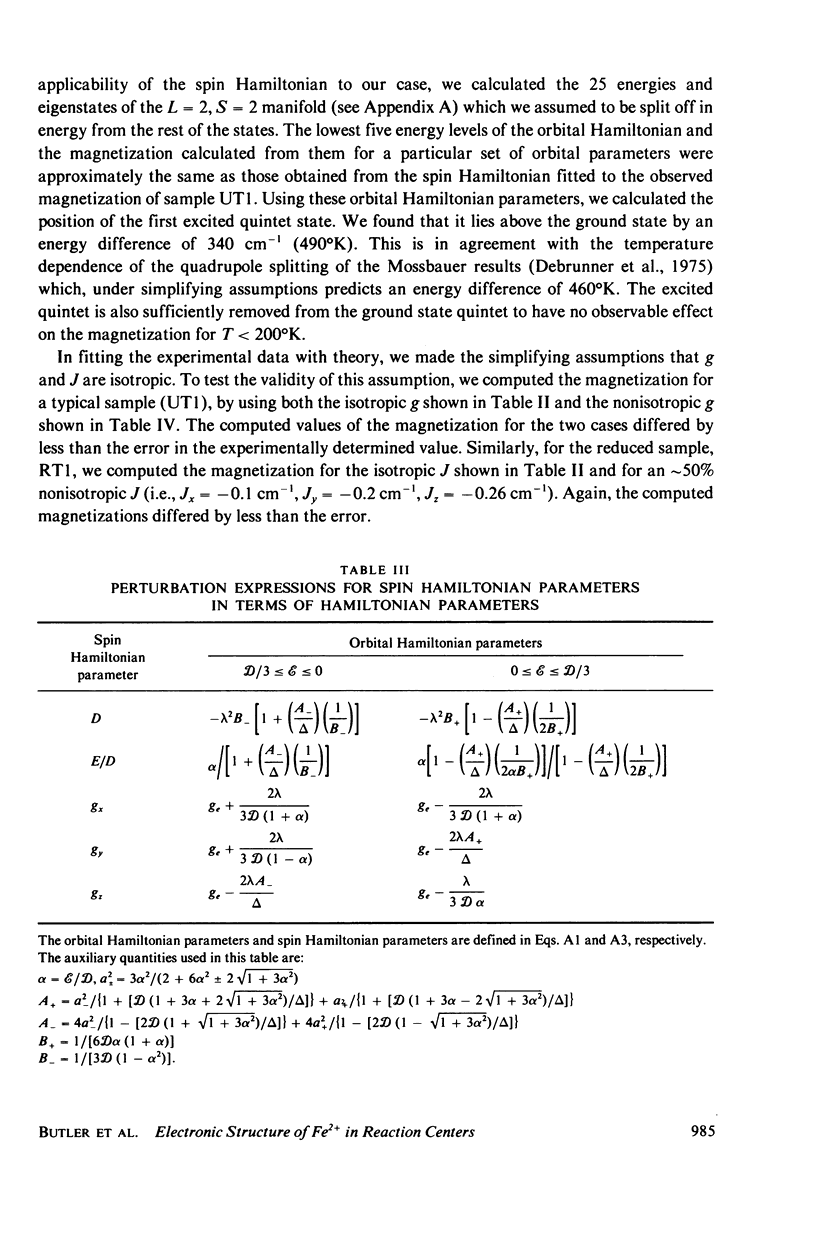
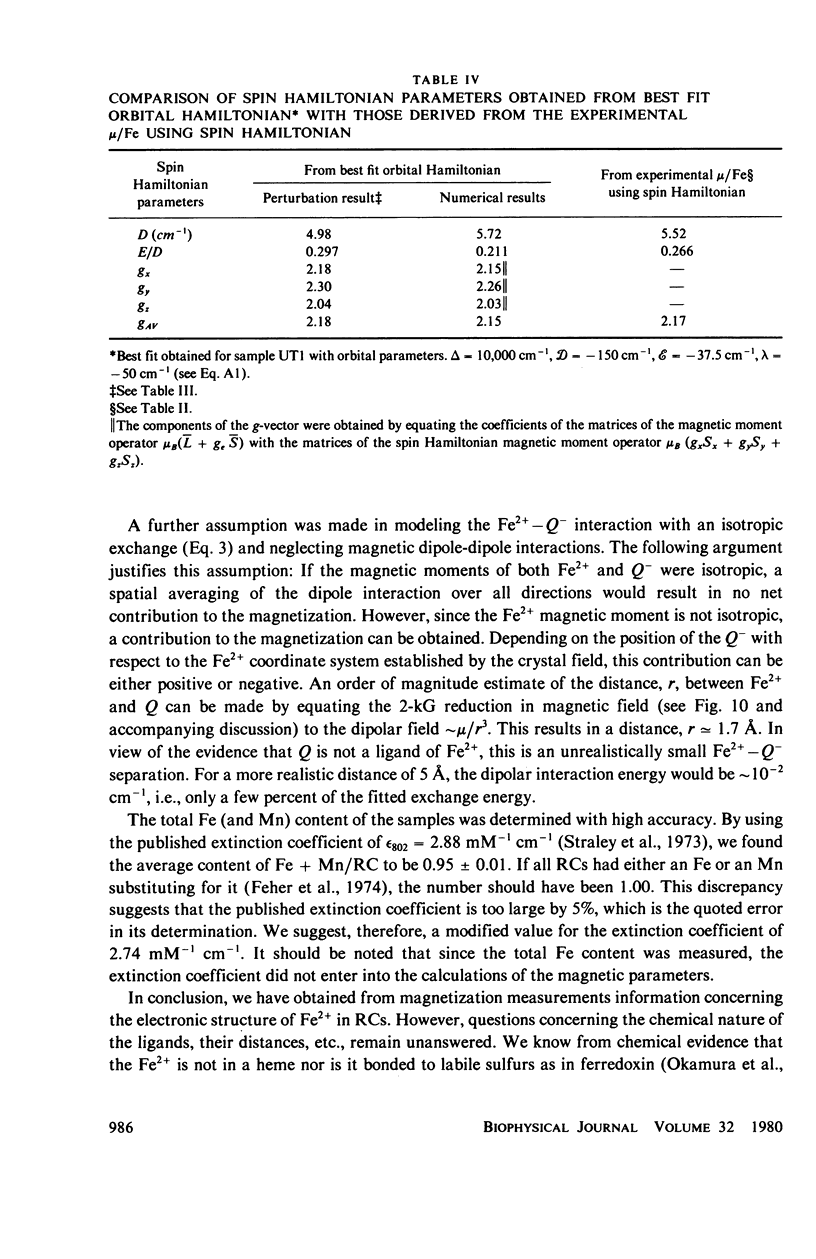
Abstract
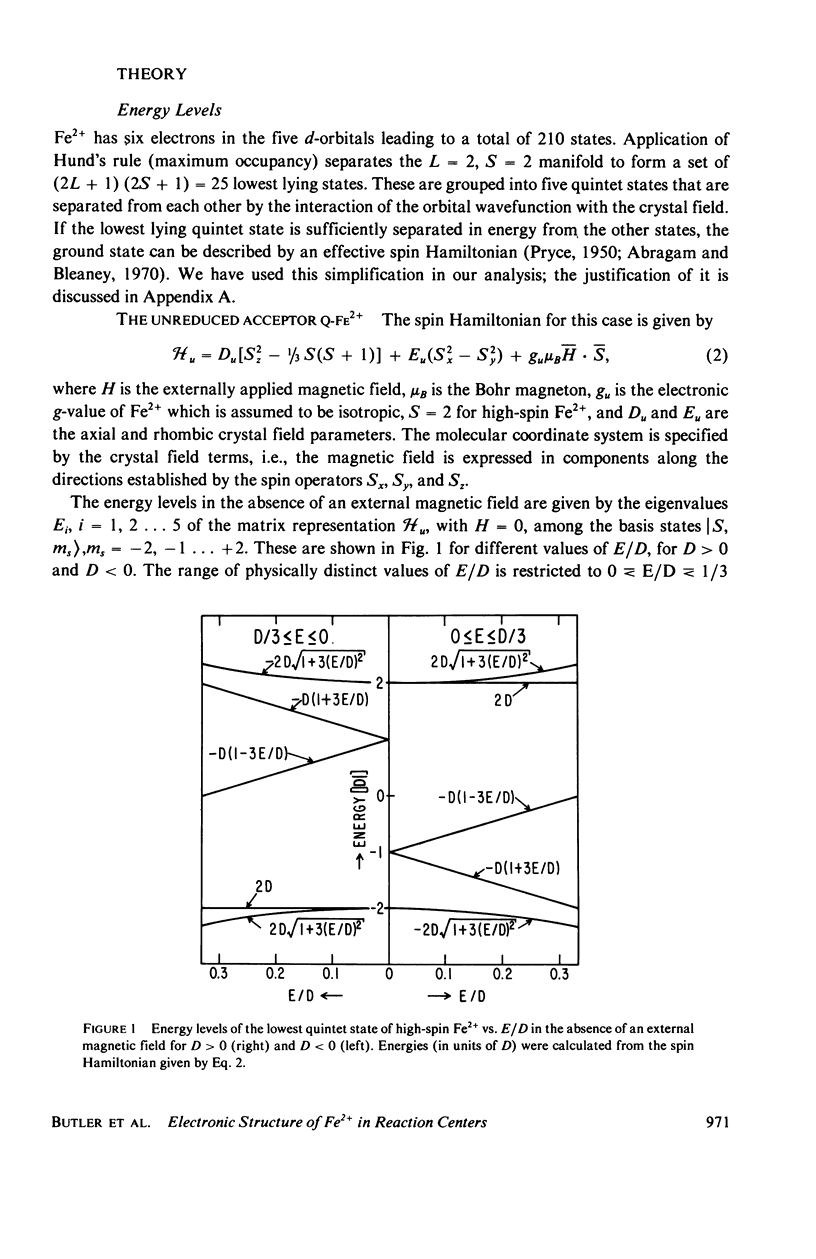
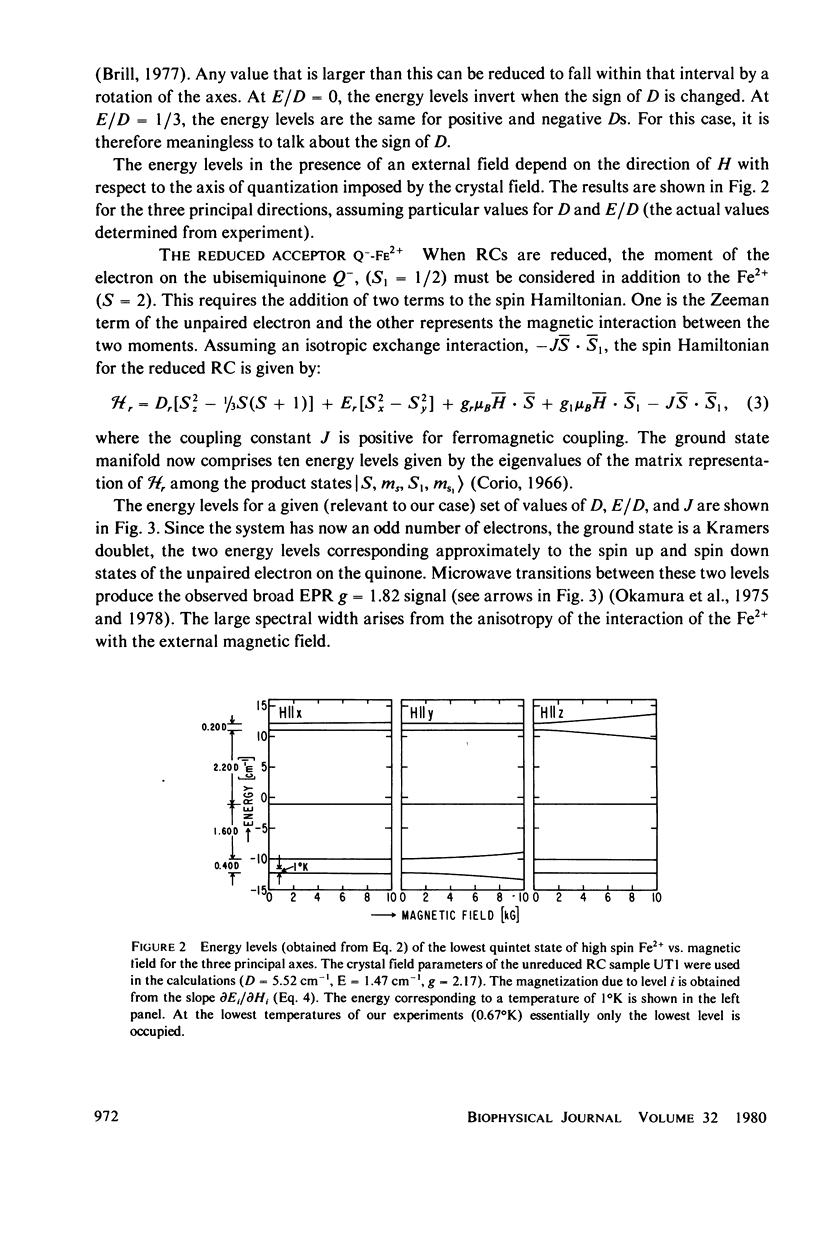
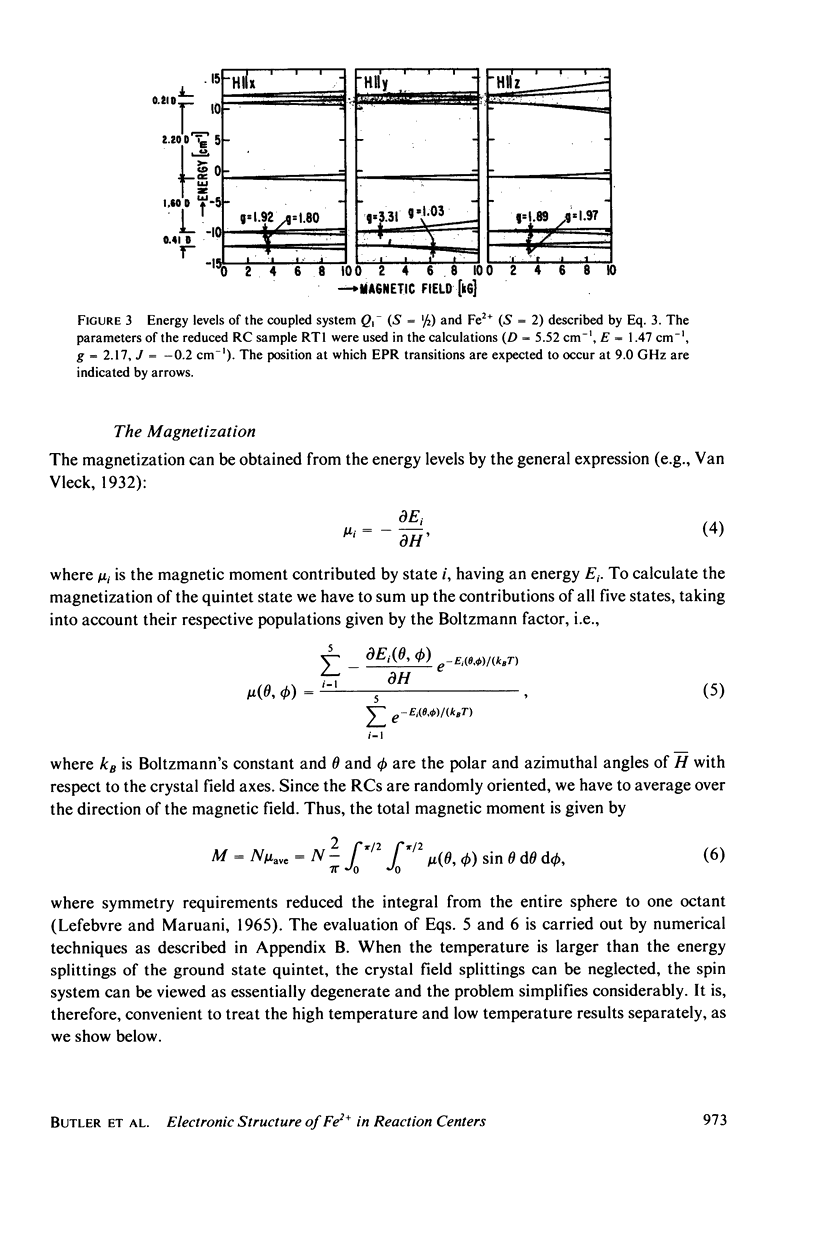
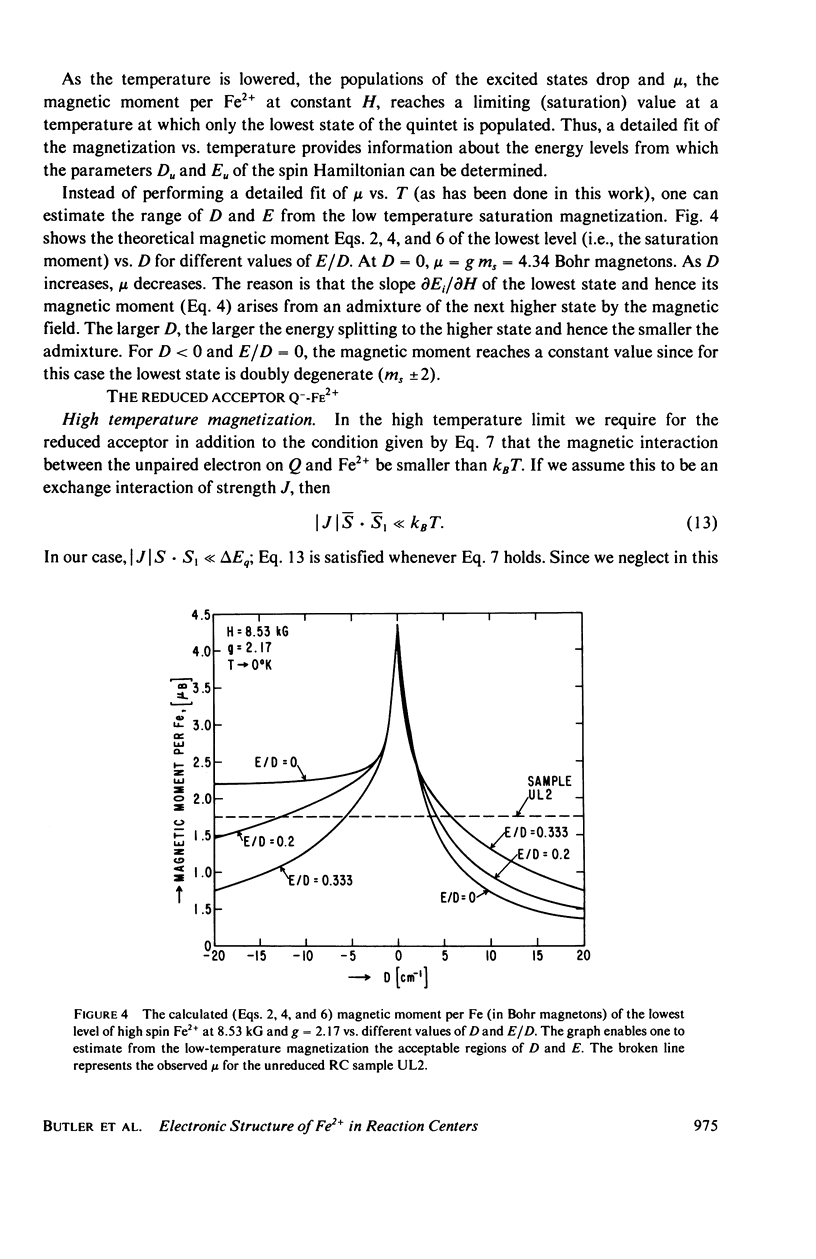
We have measured the static magnetization of unreduced and reduced reaction centers that vary in their quinone content. Measurements were performed in the temperature range 0.7 degrees K less than T less than 200 degrees K and magnetic fields of up to 10 kG. The electronic g-value, crystal field parameters D, E, and the exchange interaction, J, between the quinone spin and Fe2+ were determined using the spin Hamiltonian formalism. The effective moment mu eff/Fe2+ of both reduced and unreduced samples were determined to be 5.35 +/- 0.15 Bohr magnetons. This shows, in agreement with previous findings, that Fe2+ does not change its valence state when the reaction centers are reduced. Typical values of D congruent to +5 cm-1 and E/D congruent to 0.27 are consistent with Fe being in an octahedral environment with rhombic distortion. The values of D and E were approximately the same for reaction centers having one and two quinones. These findings imply that quinone is most likely not a ligand of Fe. The Fe2+ and the spin on the quinone in reduced reaction centers were found to be coupled with an exchange interaction 0 less than /J/ less than 1 cm-1. The validity of the spin Hamiltonian was checked by using an orbital Hamiltonian to calculate energy levels of the 25 states of the S = 2, L = 2 manifold and comparing the magnetization of the lowest five states with those obtained from the spin Hamiltonian. Using the orbital Hamiltonian, we calculated the position of the first excited quintet state to be 340 cm-1 above the ground state quintet. This is in good agreement with the temperature dependence of the quadrupole splitting as determined by Mossbauer spectroscopy.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blankenship R. E., Parson W. W. The involvement of iron and ubiquinone in electron transfer reactions mediated by reaction centers from photosynthetic bacteria. Biochim Biophys Acta. 1979 Mar 15;545(3):429–444. doi: 10.1016/0005-2728(79)90152-x. [DOI] [PubMed] [Google Scholar]
- Feher G., Isaacson R. A., McElroy J. D., Ackerson L. C., Okamura M. Y. On the question of the primary acceptor in bacterial photosynthesis:manganese substituting for iron in reaction centers of Rhodopseudomonas spheroides R-26. Biochim Biophys Acta. 1974 Oct 18;368(1):135–139. doi: 10.1016/0005-2728(74)90104-2. [DOI] [PubMed] [Google Scholar]
- Feher G., Okamura M. Y., McElroy J. D. Identification of an electron acceptor in reaction centers of Rhodopseudomonas spheroides by EPR spectroscopy. Biochim Biophys Acta. 1972 Apr 20;267(1):222–226. doi: 10.1016/0005-2728(72)90155-7. [DOI] [PubMed] [Google Scholar]
- Feher G. Some chemical and physical properties of a bacterial reaction center particle and its primary photochemical reactants. Photochem Photobiol. 1971 Sep;14(3):373–387. doi: 10.1111/j.1751-1097.1971.tb06180.x. [DOI] [PubMed] [Google Scholar]
- Loach P. A., Hall R. L. The question of the primary electron acceptor in bacterial photosynthesis. Proc Natl Acad Sci U S A. 1972 Apr;69(4):786–790. doi: 10.1073/pnas.69.4.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura M. Y., Isaacson R. A., Feher G. Primary acceptor in bacterial photosynthesis: obligatory role of ubiquinone in photoactive reaction centers of Rhodopseudomonas spheroides. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3491–3495. doi: 10.1073/pnas.72.9.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura M. Y., Steiner L. A., Feher G. Characterization of reaction centers from photosynthetic bacteria. I. Subunit structure of the protein mediating the primary photochemistry in Rhodopseudomonas spheroides R-26. Biochemistry. 1974 Mar 26;13(7):1394–1403. doi: 10.1021/bi00704a013. [DOI] [PubMed] [Google Scholar]
- PUMPHREY A. M., REDFEARN E. R. A method for determining the concentration of ubiquinone in mitochondrial preparations. Biochem J. 1960 Jul;76:61–64. doi: 10.1042/bj0760061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson W. W., Case G. D. In Chromatium, a single photochemical reaction center oxidizes both cytochrome C552 and cytochrome C555. Biochim Biophys Acta. 1970;205(2):232–245. doi: 10.1016/0005-2728(70)90253-7. [DOI] [PubMed] [Google Scholar]
- Parson W. W. The role of P870 in bacterial photosynthesis. Biochim Biophys Acta. 1968 Jan 15;153(1):248–259. doi: 10.1016/0005-2728(68)90167-9. [DOI] [PubMed] [Google Scholar]
- Straley S. C., Parson W. W., Mauzerall D. C., Clayton R. K. Pigment content and molar extinction coefficients of photochemical reaction centers from Rhodopseudomonas spheroides. Biochim Biophys Acta. 1973 Jun 28;305(3):597–609. doi: 10.1016/0005-2728(73)90079-0. [DOI] [PubMed] [Google Scholar]


