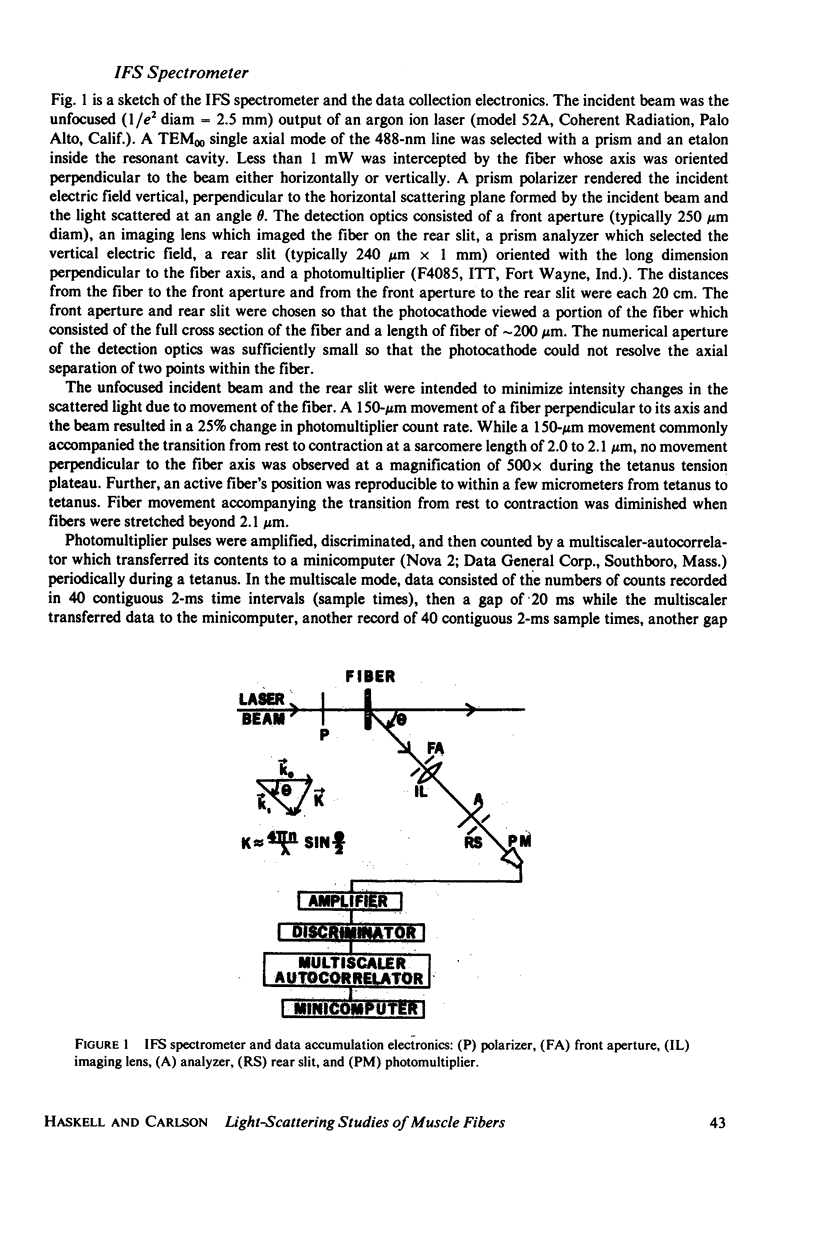
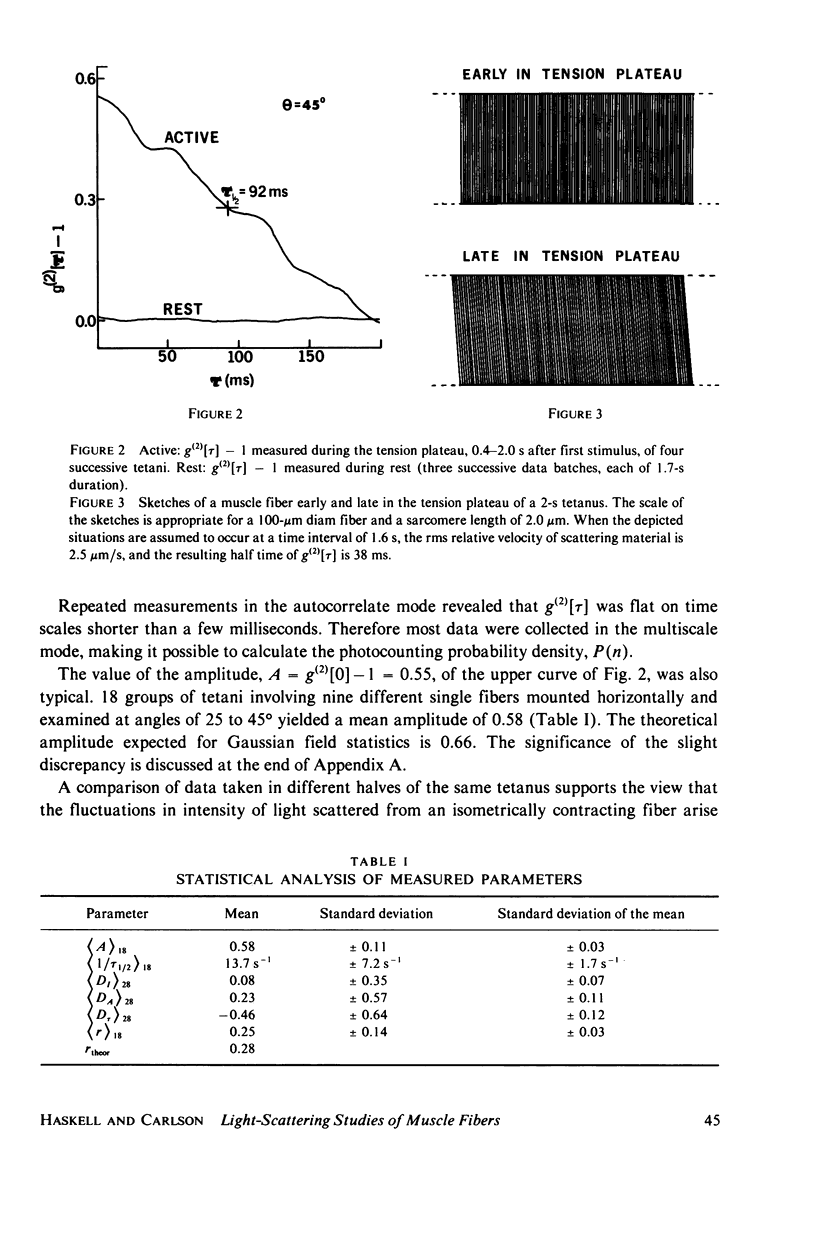
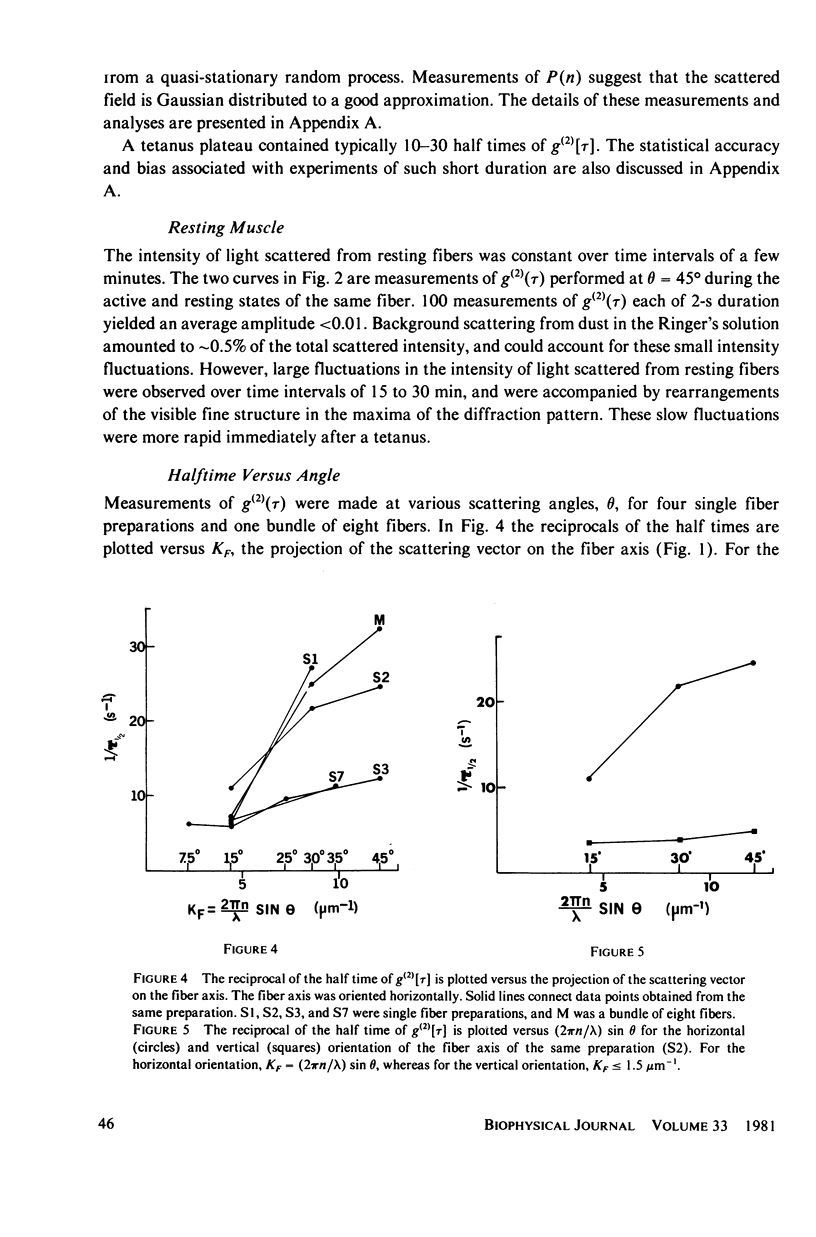
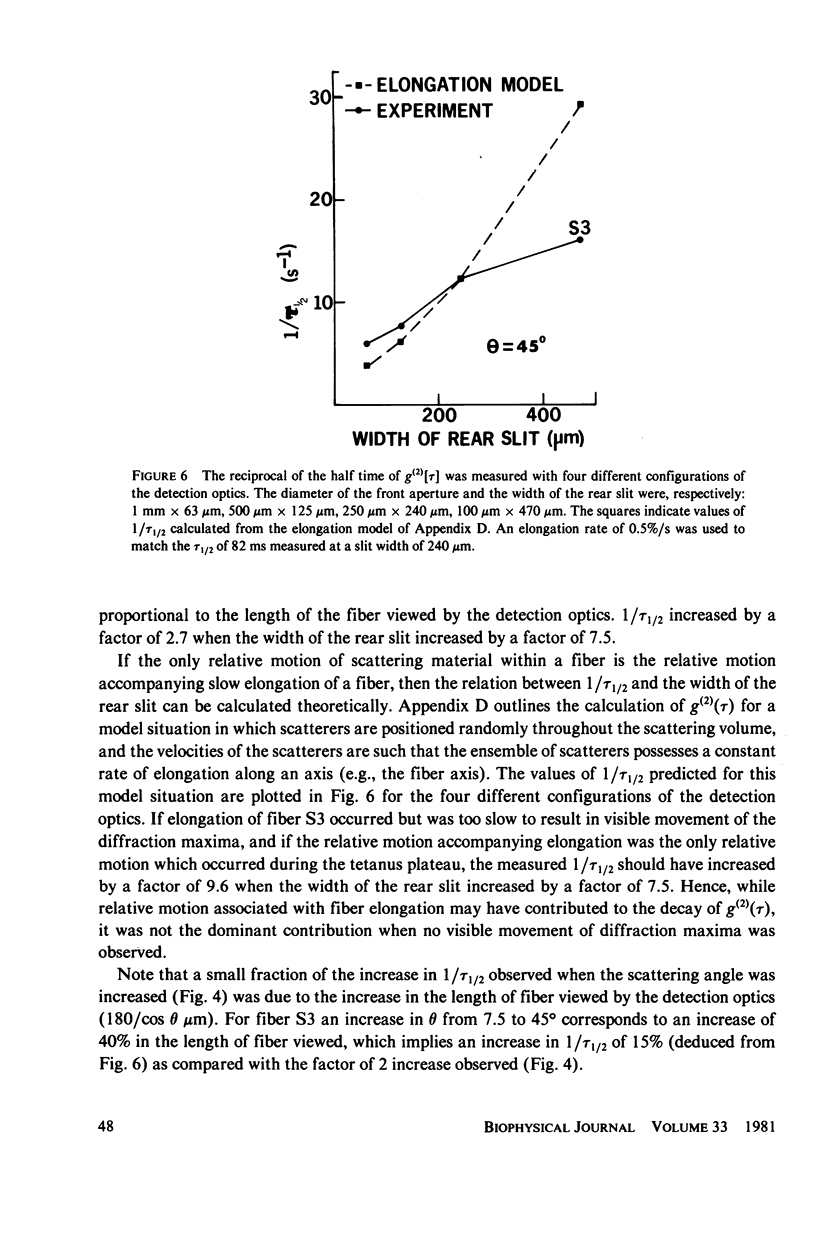
Abstract
Measurements were made of the intensity autocorrelation function, g(2)[tau], of light scattered from intact frog muscle fibers. During the tension plateau of an isometric tenanus, scattered field statistics were approximately Gaussian and intensity fluctuations were quasi-stationary. The half time, tau 1/2, for the decay of g(2)[tau] was typically 70 ms at a scattering angle of 30 degrees. The decay rate, 1/tau 1/2, of g(2)[tau] varied roughly linearly with the projection of the scattering vector on the fiber axis. 1/tau 1/2 was greater during the tension creep phase of tetani of highly stretched fibers, but was roughly independent of sarcomere length during the tension plateau. g(2)[tau] measured during rest or on diffraction pattern maxima during isometric contraction were flat with low amplitudes. These results are consistent with a model of a 200-mu m segment of an isometrically contracting fiber in which scattering material possesses relative axial velocities of 1-2 mu m/s accompanied by relative axial displacements greater than 0.1 mu m. The slow (1-2 mu m/s) motion of one portion of the fiber relative to another observed under the microscope (500X) during isometric contraction is consistent with the light-scattering results. Structural fluctuations on the scale of the myofibrillar sarcomere which may arise from asynchronous cycling of cross-bridges must involve relative axial velocities less than 3 mu m/s or relative axial displacements less than 0.05 mu m.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner R. F., Carlson F. D. Structural dynamics of frog muscle during isometric contraction. J Gen Physiol. 1975 May;65(5):555–581. doi: 10.1085/jgp.65.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borejdo J., Morales M. F. Fluctuations in tension during contraction of single muscle fibers. Biophys J. 1977 Dec;20(3):315–334. doi: 10.1016/S0006-3495(77)85552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borejdo J., Putnam S., Morales M. F. Fluctuations in polarized fluorescence: evidence that muscle cross bridges rotate repetitively during contraction. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6346–6350. doi: 10.1073/pnas.76.12.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson F. D., Fraser A. B. Dynamics of F-actin and F-actin complexes. J Mol Biol. 1974 Oct 25;89(2):273–281. doi: 10.1016/0022-2836(74)90518-x. [DOI] [PubMed] [Google Scholar]
- Carlson F. D. Letters to the editor: Kinetics. J Mol Biol. 1975 Jun 15;95(1):139–139. doi: 10.1016/0022-2836(75)90341-1. [DOI] [PubMed] [Google Scholar]
- Carlson F. D. Structural fluctuations in the steady state of muscular contraction. Biophys J. 1975 Jul;15(7):633–649. doi: 10.1016/S0006-3495(75)85845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins H. Z., Carlson F. D., Herbert T. J., Woods G. Translational and rotational diffusion constants of tobacco mosaic virus from Rayleigh linewidths. Biophys J. 1969 Apr;9(4):518–546. doi: 10.1016/S0006-3495(69)86402-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott G. F., Lowy J., Millman B. M. Low-angle x-ray diffraction studies of living striated muscle during contraction. J Mol Biol. 1967 Apr 14;25(1):31–45. doi: 10.1016/0022-2836(67)90277-x. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. Tension development in highly stretched vertebrate muscle fibres. J Physiol. 1966 May;184(1):143–169. doi: 10.1113/jphysiol.1966.sp007908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON J., HUXLEY H. E. Quantitative studies on the structure of cross-striated myofibrils. II. Investigations by biochemical techniques. Biochim Biophys Acta. 1957 Feb;23(2):250–260. doi: 10.1016/0006-3002(57)90326-8. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- HUXLEY A. F., NIEDERGERKE R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature. 1954 May 22;173(4412):971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- HUXLEY H. E., HANSON J. Quantitative studies on the structure of cross-striated myofibrils. I. Investigations by interference microscopy. Biochim Biophys Acta. 1957 Feb;23(2):229–249. doi: 10.1016/0006-3002(57)90325-6. [DOI] [PubMed] [Google Scholar]
- HUXLEY H., HANSON J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954 May 22;173(4412):973–976. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]


