Abstract
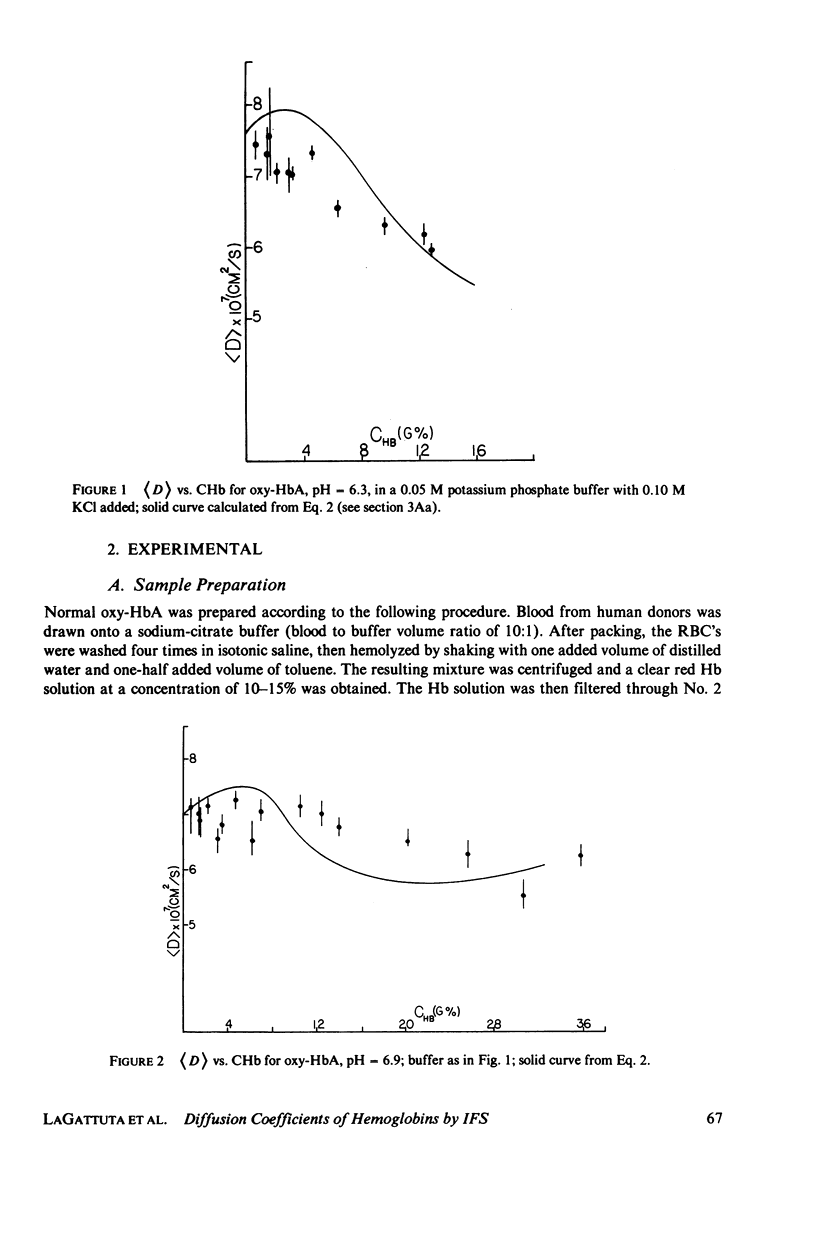
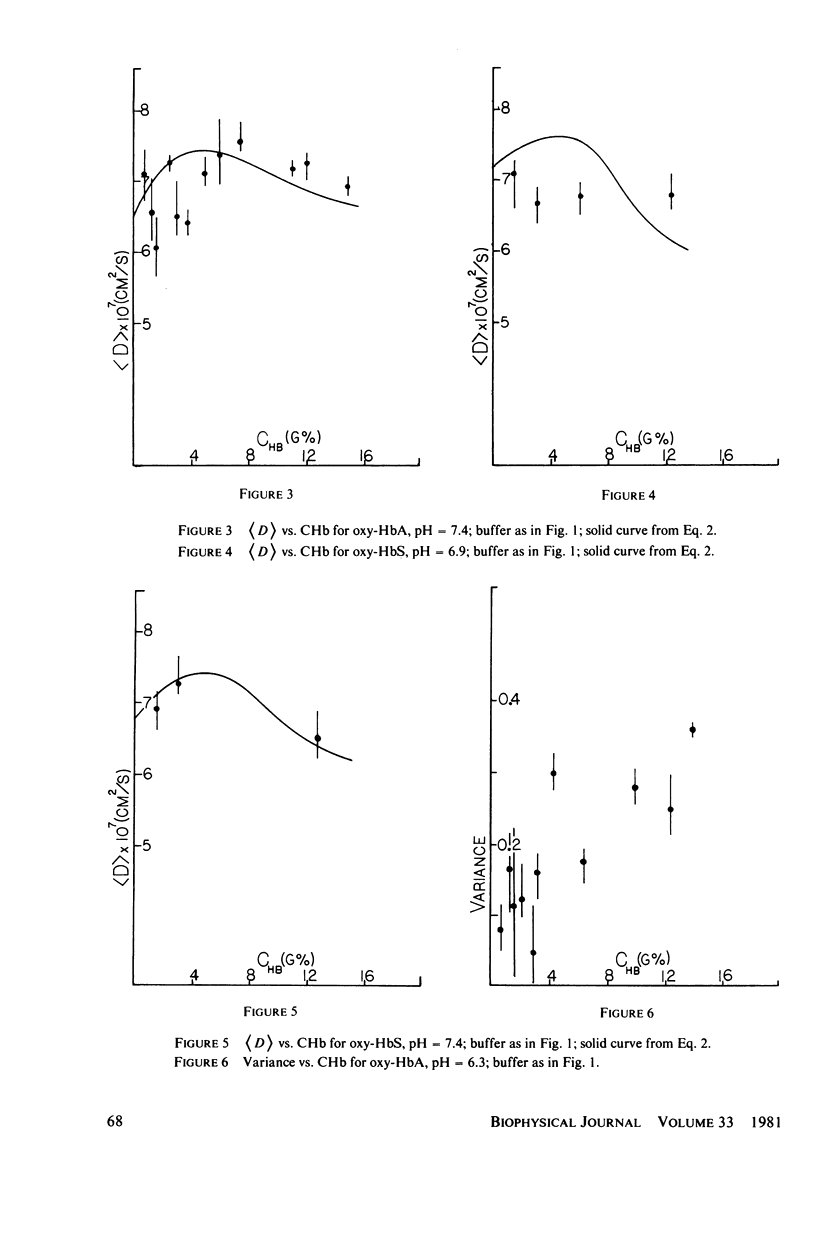
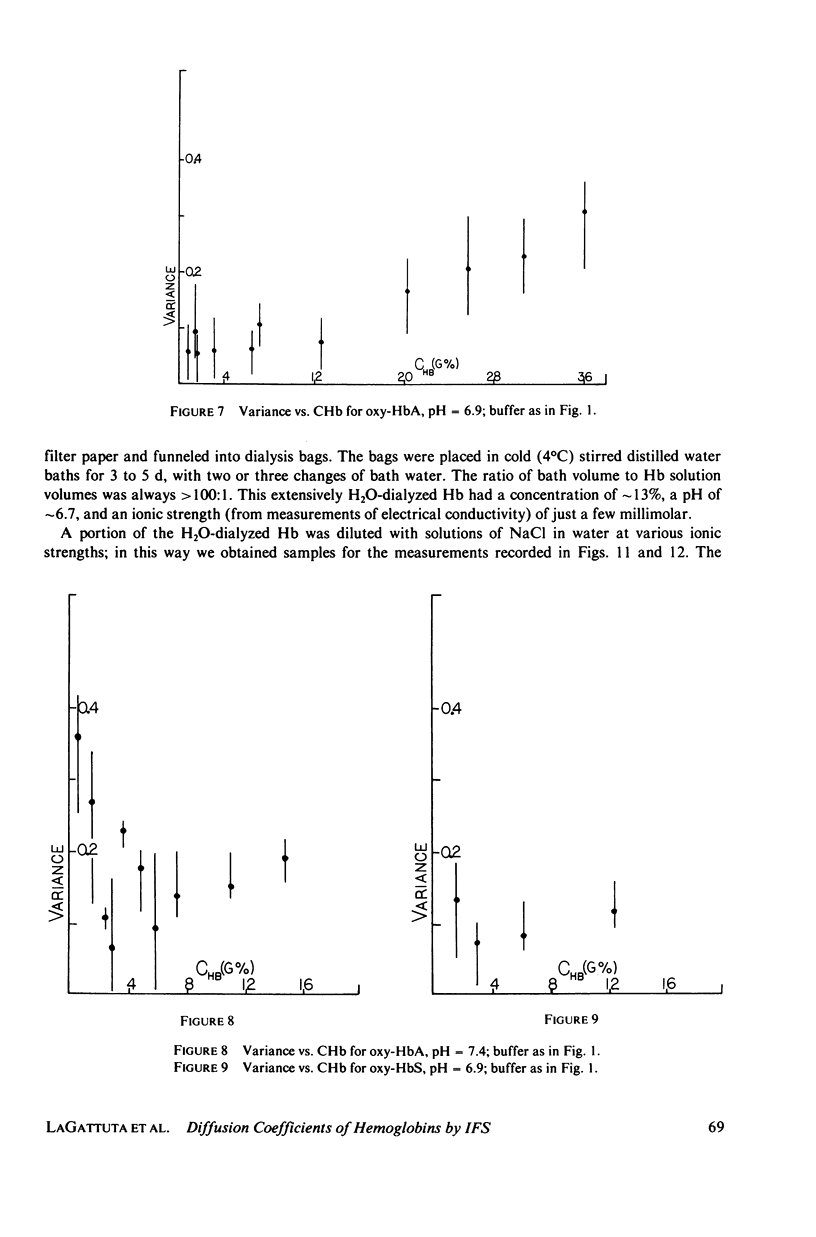
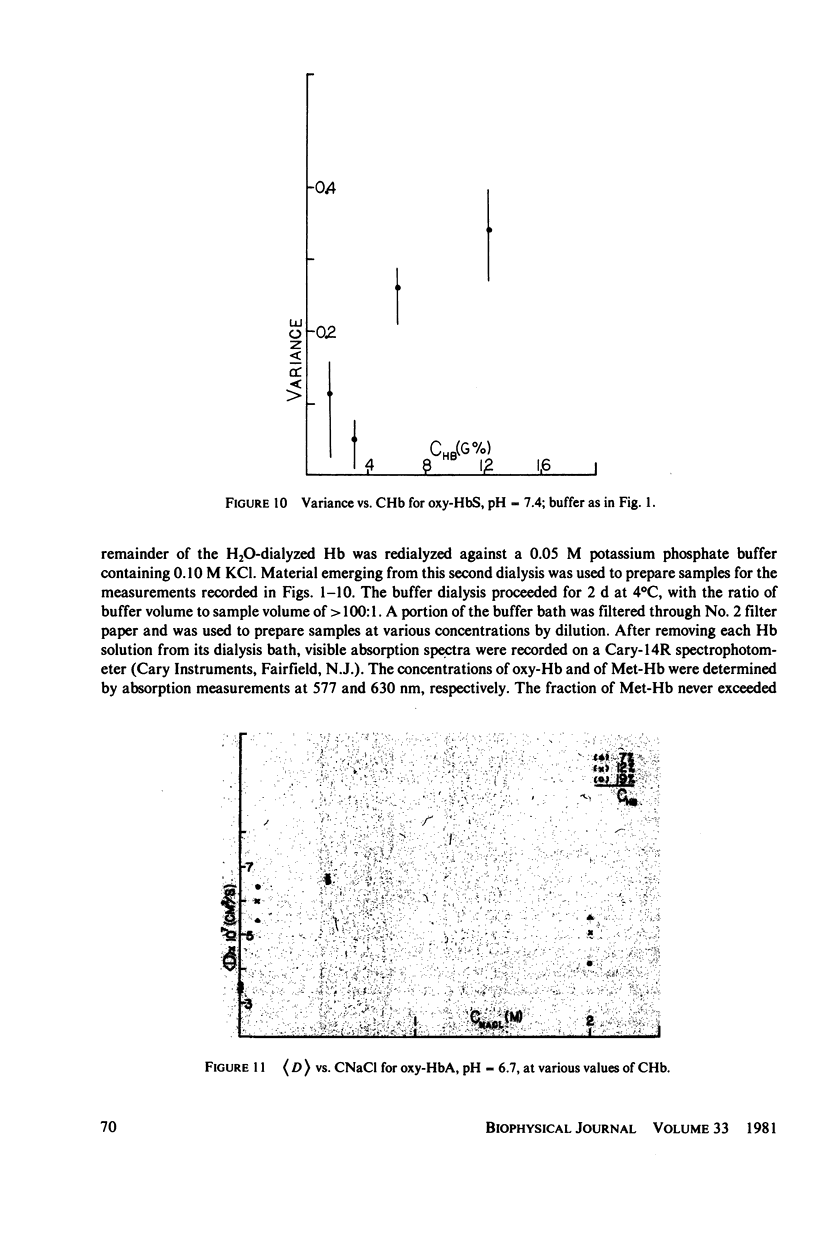
Measurements of the mutual diffusion coefficients (D) of the liganded human hemoglobins (Hb) oxy-HbA and oxy-HbS were performed as a function of Hb concentration (CHb), pH, and ionic strength (tau) by intensity fluctuation spectroscopy (IFS). Average diffusion coefficients, (D), and normalized variances, ((D/(D) - 1)2), were recorded. Results are reported and select features are discussed quantitatively. (a) for tau = 0.15 M, the shape of the (d) vs. CHb curve is found to vary with pH. We developed a precise description of this effect in the form of an algebraic relationship between (D), CHb, and Z, the titration charge. (b) only slight differences between the (D) values of oxy-HbS and oxy-HbA are observed, at tau = 0.15 M, for CHb Less Than or Equal To 10 g%. These differences are explained by the theory of part a. (c) No evidence of aggregation is found in solutions of oxy-HbA or oxy-HbS, at tau = 0.15 M, for CHb Less Than or Equal To 10 g%. (d) Indications of aggregation appear in oxy-HbA solutions at very low concentrations of salt. An estimate is made of the extent of aggregation, and the average radius of a cluster is determined.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert S. S., Banks G. The concentration dependence of the hemoglobin mutual diffusion coefficient. Biophys Chem. 1976 May;4(3):287–296. doi: 10.1016/0301-4622(76)80077-4. [DOI] [PubMed] [Google Scholar]
- Elbaum D., Nagel R. L., Herskovits T. T. Aggregation of deoxyhemoglobin S at low concentrations. J Biol Chem. 1976 Dec 10;251(23):7657–7660. [PubMed] [Google Scholar]
- Girling R. L., Schmidt W. C., Jr, Houston T. E., Amma E. L., Huisman T. H. Molecular packing and intermolecular contacts of sickling deer type III hemoglobin. J Mol Biol. 1979 Jul 5;131(3):417–433. doi: 10.1016/0022-2836(79)90001-9. [DOI] [PubMed] [Google Scholar]
- Jones C. R., Johnson C. S., Jr Photon correlation spectroscopy of hemoglobin: diffusion of oxy-HbA and oxy-HbS. Biopolymers. 1978 Jun;17(6):1581–1593. doi: 10.1002/bip.1978.360170615. [DOI] [PubMed] [Google Scholar]
- Keller K. H., Canales E. R., Yum S. I. Tracer and mutual diffusion coefficients of proteins. J Phys Chem. 1971 Feb 4;75(3):379–387. doi: 10.1021/j100673a015. [DOI] [PubMed] [Google Scholar]
- Lindstrom T. R., Koenig S. H., Boussios T., Bertles J. F. Intermolecular interactions of oxygenated sickle hemoglobin molecules in cells and cell-free solutions. Biophys J. 1976 Jun;16(6):679–689. doi: 10.1016/S0006-3495(76)85721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdoff-Fairchild B., Chiu C. C. X-ray diffraction studies of fibers and crystals of deoxygenated sickle cell hemoglobin. Proc Natl Acad Sci U S A. 1979 Jan;76(1):223–226. doi: 10.1073/pnas.76.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel R. L., Bookchin R. M., Johnson J., Labie D., Wajcman H., Isaac-Sodeye W. A., Honig G. R., Schilirò G., Crookston J. H., Matsutomo K. Structural bases of the inhibitory effects of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S. Proc Natl Acad Sci U S A. 1979 Feb;76(2):670–672. doi: 10.1073/pnas.76.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAULING L., ITANO H. A. Sickle cell anemia a molecular disease. Science. 1949 Nov 25;110(2865):543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- Raj T., Flygare W. H. Diffusion studies of bovine serum albumin by quasielastic light scattering. Biochemistry. 1974 Jul 30;13(16):3336–3340. doi: 10.1021/bi00713a024. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr Concerted formation of the gel of hemoglobin S. Proc Natl Acad Sci U S A. 1973 May;70(5):1506–1508. doi: 10.1073/pnas.70.5.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W. W., Luzzana M. R., Penniston J. T., Johnson C. S., Jr Pregelation aggregation of sickle cell hemoglobin. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1260–1263. doi: 10.1073/pnas.71.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Casimir W., Kaiser N., Keilmann F., Mayer A., Vogel H. Dielectric properties of oxyhemoglobin and deoxyhemoglobin in aqueous solution at microwave frequencies. Biopolymers. 1968;6(12):1705–1715. doi: 10.1002/bip.1968.360061205. [DOI] [PubMed] [Google Scholar]


