Abstract
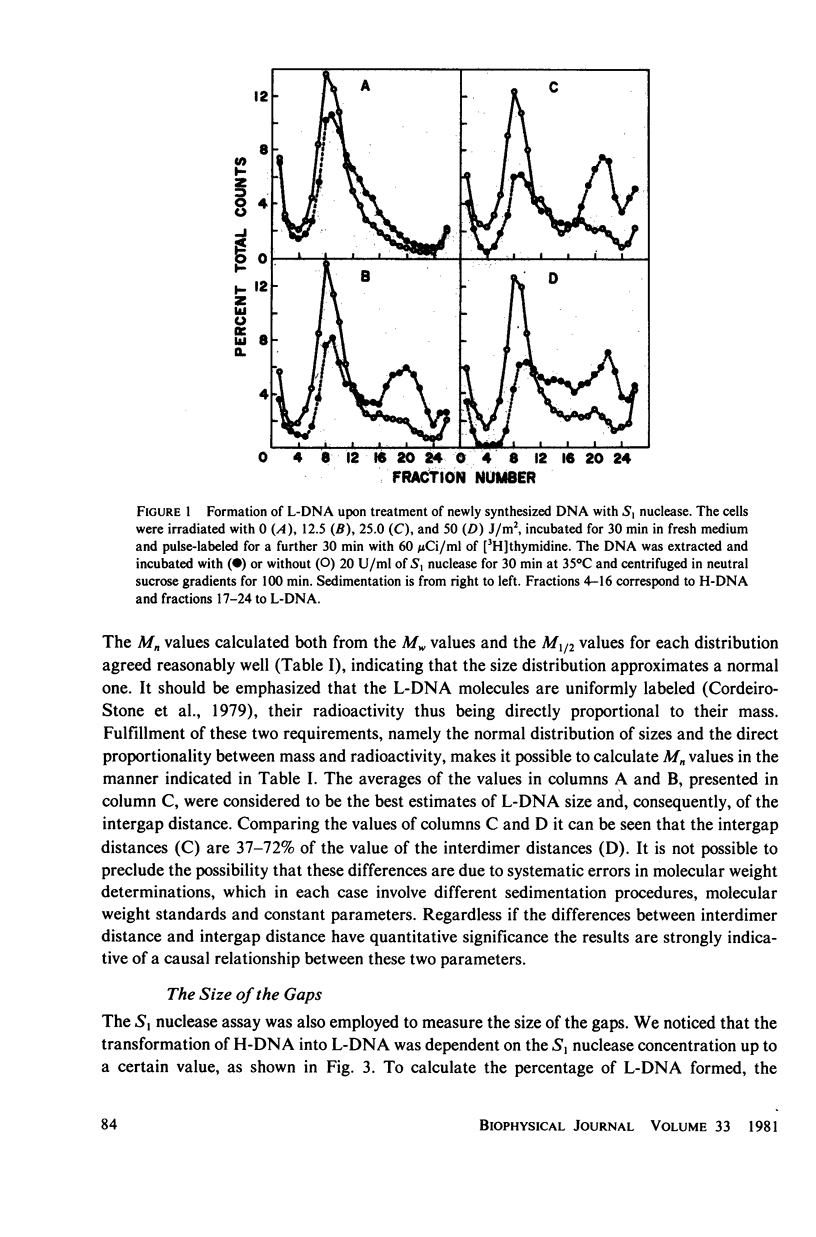
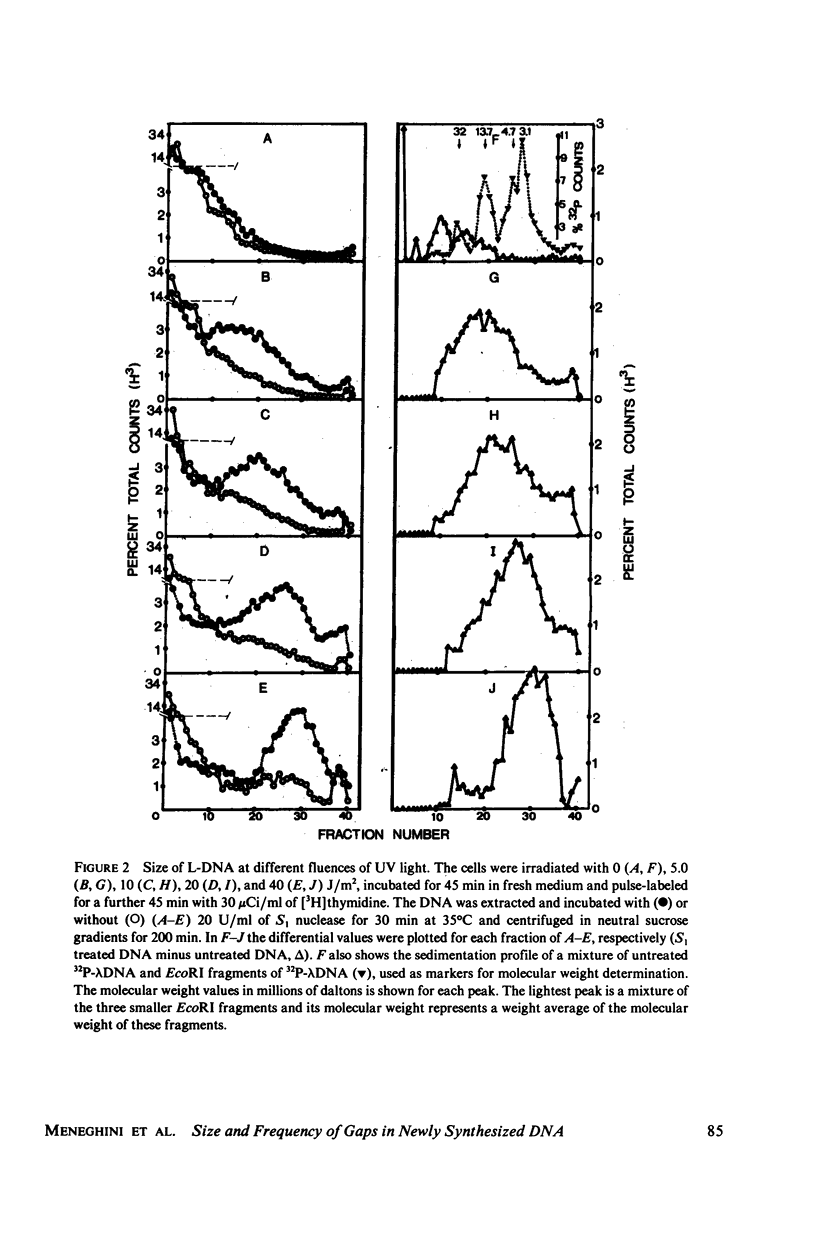
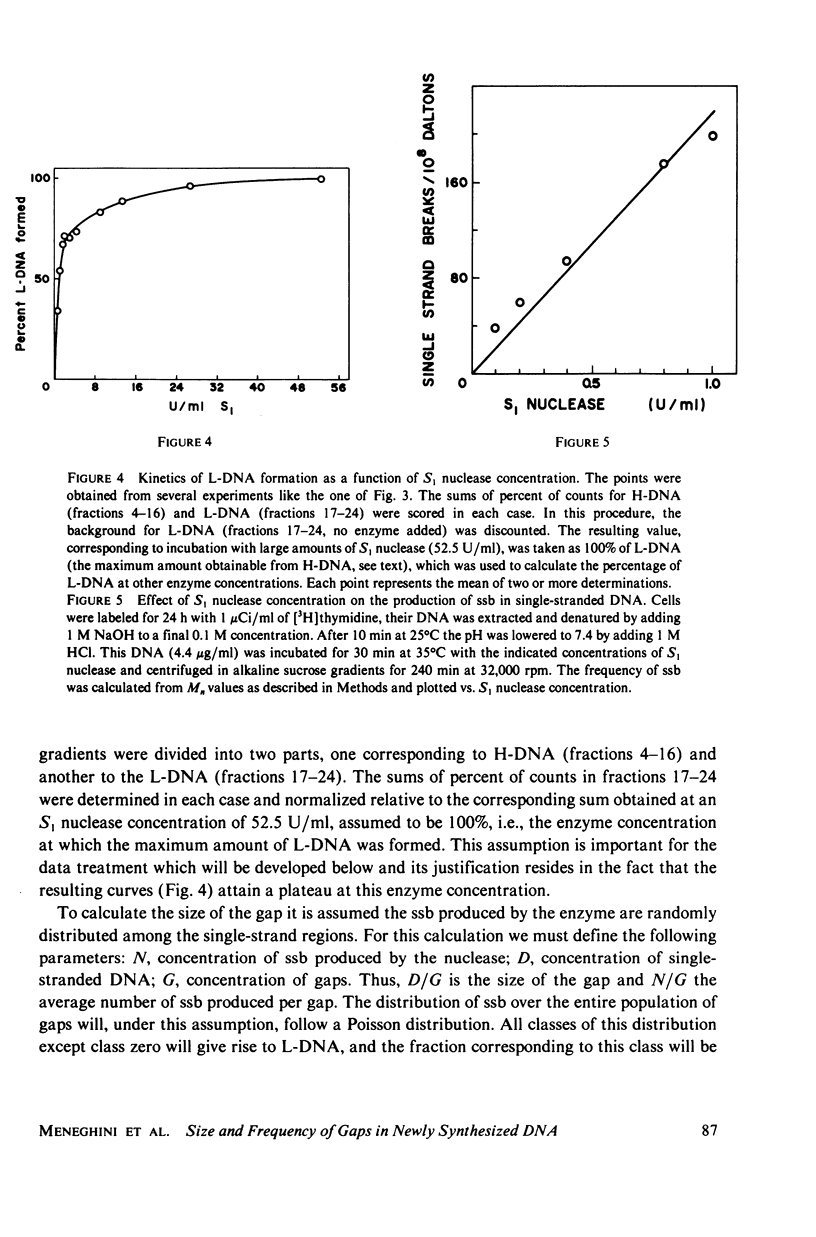
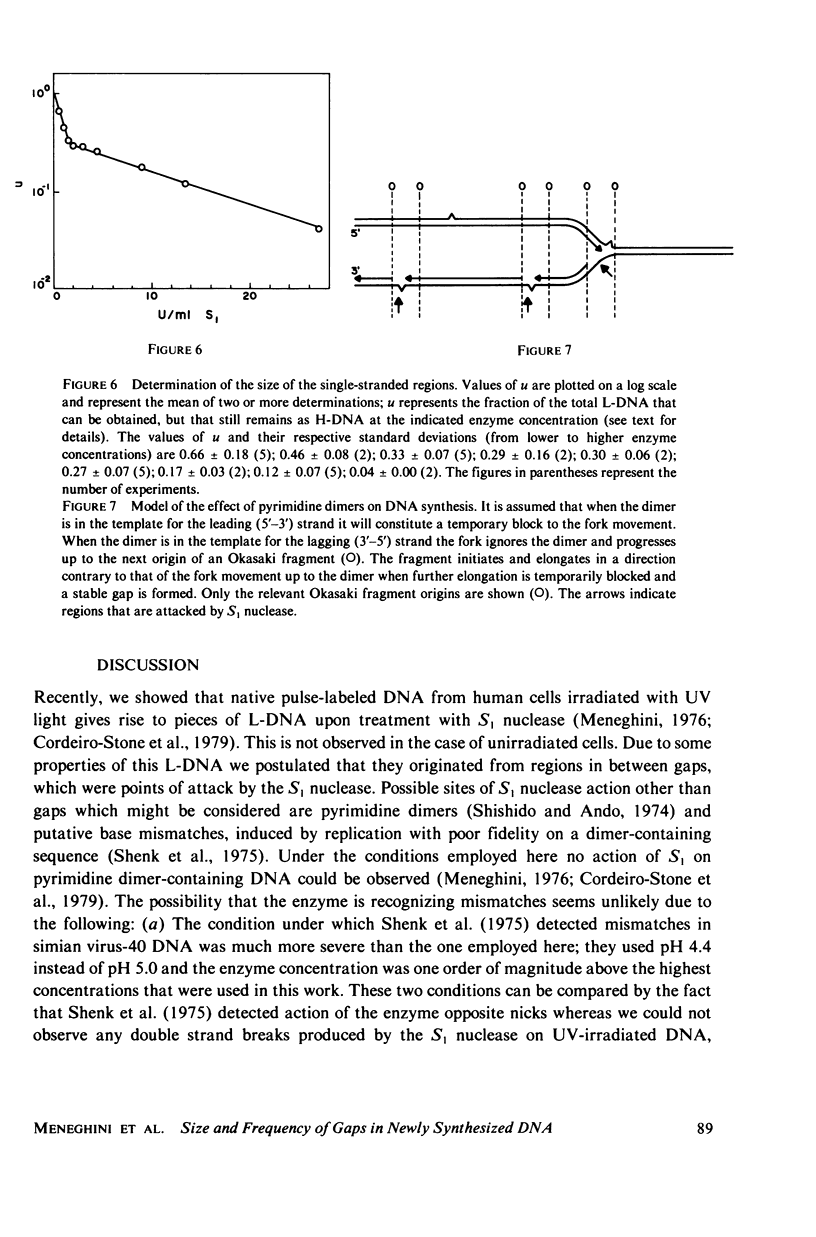
Native newly synthesized DNA from human cells (xeroderma pigmentosum type) irradiated with ultraviolet light releases short pieces of DNA (L-DNA) when incubated with the single-strand specific S1 nuclease. This is not observed in the case of unirradiated cells. Previous experiments had shown that the L-DNA resulted from the action of S1 nuclease upon gaps, i.e., single-stranded DNA discontinuities in larger pieces of double-stranded DNA. We verified that the duplex L-DNA, that arises from the inter-gap regions upon S1 nuclease treatment, has a size which approximates the distance between two pyrimidine dimers on the same strand; this has been observed at different fluences of ultraviolet-light and indicates that the gap is related to or opposite the dimer. A method was devised to measure the size of the gaps. A Poisson distribution analysis of the percentage of the L-DNA produced as a function of S1 nuclease concentration made this possible. 65% of the gaps corresponded to stretches of 1,250 nucleotides and 35% to stretches of 150 nucleotides. These parameters have been considered in the proposition of a model for DNA synthesis on a template containing pyrimidine dimers.
Full text
PDF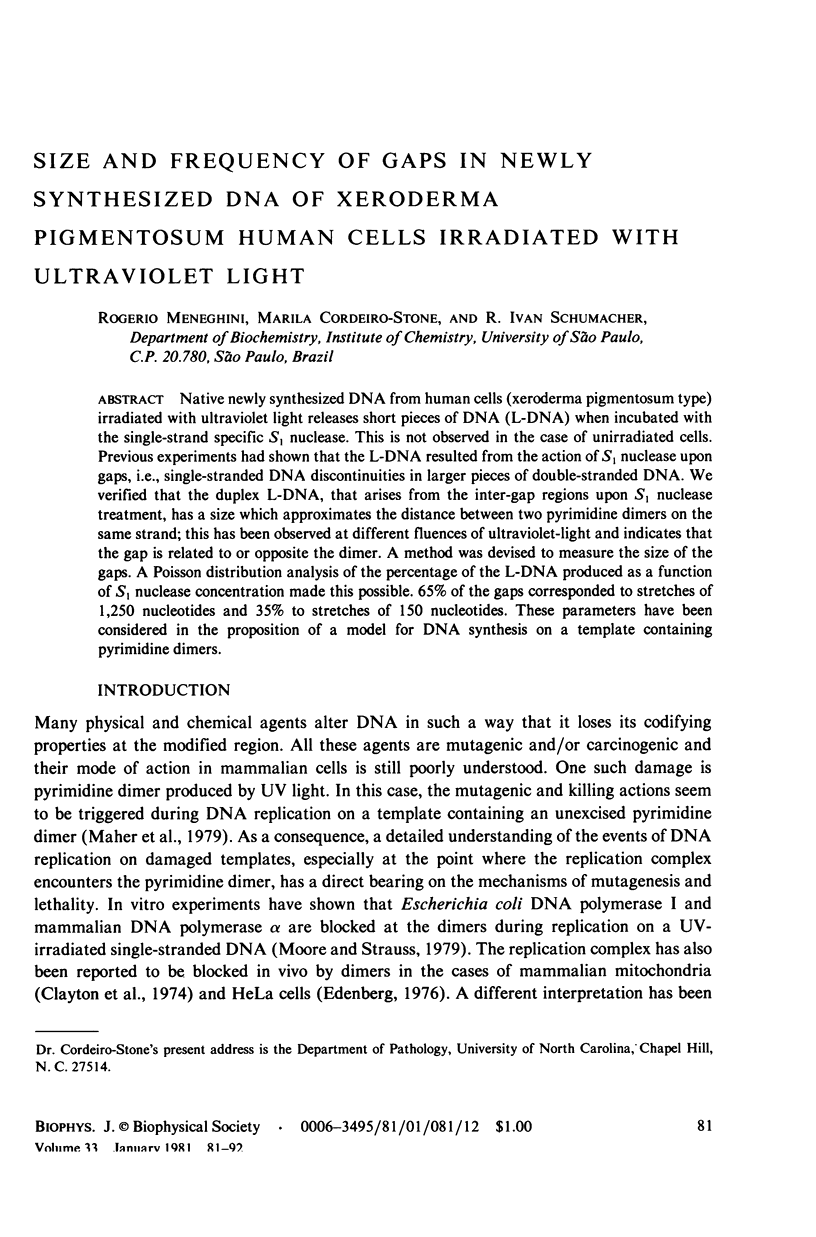







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buhl S. N., Setlow R. B., Regan J. D. Steps in DNA chain elongation and joining after ultra-violet irradiation of human cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1972 Nov;22(5):417–424. doi: 10.1080/09553007214551301. [DOI] [PubMed] [Google Scholar]
- Clarkson J. M., Hewitt R. R. Significance of dimers to the size of newly synthesized DNA in UV-irradiated Chinese hamster ovary cells. Biophys J. 1976 Oct;16(10):1155–1164. doi: 10.1016/S0006-3495(76)85764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A., Doda J. N., Friedberg E. C. The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2777–2781. doi: 10.1073/pnas.71.7.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro-Stone M., Schumacher R. I., Meneghini R. Structure of the replication fork in ultraviolet light-irradiated human cells. Biophys J. 1979 Aug;27(2):287–300. doi: 10.1016/S0006-3495(79)85218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J., Huberman J. A. Eukaryotic chromosome replication. Annu Rev Genet. 1975;9:245–284. doi: 10.1146/annurev.ge.09.120175.001333. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J. Inhibition of DNA replication by ultraviolet light. Biophys J. 1976 Aug;16(8):849–860. doi: 10.1016/S0006-3495(76)85735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmann U. K., Lett J. T. Review and evaluation of molecular weight calculations from the sedimentation profiles of irradiated DNA. Radiat Res. 1973 Apr;54(1):152–162. [PubMed] [Google Scholar]
- Fujiwara Y., Tatsumi M. Replicative bypass repair of ultraviolet damage to DNA of mammalian cells: caffeine sensitive and caffeine resistant mechanisms. Mutat Res. 1976 Oct;37(1):91–110. doi: 10.1016/0027-5107(76)90058-0. [DOI] [PubMed] [Google Scholar]
- Higgins N. P., Kato K., Strauss B. A model for replication repair in mammalian cells. J Mol Biol. 1976 Mar 5;101(3):417–425. doi: 10.1016/0022-2836(76)90156-x. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J Mol Biol. 1972 May 28;66(3):319–337. doi: 10.1016/0022-2836(72)90418-4. [DOI] [PubMed] [Google Scholar]
- Maher V. M., Dorney D. J., Mendrala A. L., Konze-Thomas B., McCormick J. J. DNA excision-repair processes in human cells can eliminate the cytotoxic and mutagenic consequences of ultraviolet irradiation. Mutat Res. 1979 Sep;62(2):311–323. doi: 10.1016/0027-5107(79)90087-3. [DOI] [PubMed] [Google Scholar]
- Meneghini R. Gaps in DNA synthesized by ultraviolet light-irradiated WI38 human cells. Biochim Biophys Acta. 1976 Apr 2;425(4):419–427. doi: 10.1016/0005-2787(76)90006-x. [DOI] [PubMed] [Google Scholar]
- Meneghini R., Hanawalt P. T4-endonuclease V-sensitive sites in DNA from ultraviolet-irradiated human cells. Biochim Biophys Acta. 1976 Apr 2;425(4):428–437. doi: 10.1016/0005-2787(76)90007-1. [DOI] [PubMed] [Google Scholar]
- Moore P., Strauss B. S. Sites of inhibition of in vitro DNA synthesis in carcinogen- and UV-treated phi X174 DNA. Nature. 1979 Apr 12;278(5705):664–666. doi: 10.1038/278664a0. [DOI] [PubMed] [Google Scholar]
- Painter R. B. DNA damage and repair in eukaryotic cells. Genetics. 1974 Sep;78(1):139–148. doi: 10.1093/genetics/78.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. D., Cleaver J. E. Postreplication repair: questions of its definition and possible alteration in xeroderma pigmentosum cell strains. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3927–3931. doi: 10.1073/pnas.76.8.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Rhodes C., Rigby P. W., Berg P. Biochemical method for mapping mutational alterations in DNA with S1 nuclease: the location of deletions and temperature-sensitive mutations in simian virus 40. Proc Natl Acad Sci U S A. 1975 Mar;72(3):989–993. doi: 10.1073/pnas.72.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishido K., Ando T. Cleavage of ultraviolet light-irradiated DNA by single strand-specific S1 endonuclease. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1380–1388. doi: 10.1016/0006-291x(74)90466-5. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]


