Abstract
Little is known about the ocular penetration of amphotericin B (AMB) and its lipid formulations, the current drug of choice in fungal endophthalmitis. The ocular distribution of AMB lipid complex (ABLC), liposomal AMB (L-AMB), and AMB deoxycholate (D-AMB) was studied in a rabbit model. D-AMB (1 mg/kg of body weight/day), ABLC (5 mg/kg/day), or L-AMB (5 mg/kg/day) was given intravenously to rabbits as a single dose or as repeated daily doses on 7 consecutive days after induction of unilateral uveitis by intravitreal injection of endotoxin. AMB concentrations in aqueous humor, vitreous humor, and plasma were determined by high-pressure liquid chromatography 16 h after administration of a single dose or 24 h after the last of seven doses. After single-dose administration, L-AMB achieved at least eightfold-higher AMB concentrations in the aqueous of inflamed eyes than ABLC or D-AMB (1.21 ± 0.58 μg/ml versus 0.14 ± 0.04 and 0.11 ± 0.09 μg/ml, respectively). At that time point no drug was detectable in the vitreous. After 7 days of treatment, the concentration of AMB in the vitreous was higher after treatment with L-AMB (0.47 ± 0.21 μg/ml) than after treatment with ABLC (0.27 ± 0.18 μg/ml) and D-AMB (0.16 ± 0.04 μg/ml). Similarly, AMB concentration in the aqueous was higher after repeated doses of L-AMB (0.73 ± 0.43 μg/ml) than after repeated doses of ABLC (0.03 ± 0.02 μg/ml) or D-AMB (0.13 ± 0.06 μg/ml). No AMB was detected in noninflamed eyes. Following systemic administration, AMB distribution to the eye is inflammation dependent and occurs sequentially, first to the aqueous and then to the vitreous. Compared to D-AMB and ABLC, L-AMB reaches higher drug concentrations in both ocular compartments.
Fungal endophthalmitis is a sight-threatening infection that is often difficult to treat. Intravenous amphotericin B (AMB), with or without additional 5-flucytosine, is considered the treatment of choice for severe systemic fungal infections and is often used as adjuvant treatment for intraocular infections caused by a wide range of fungi (24). The usefulness of this polyene, however, is limited by a number of adverse reactions (26), particularly its dose-limiting nephrotoxicity (12, 23). Renal insufficiency in association with azotemia, renal tubular acidosis, and impaired urinary concentrating capacity resulting in electrolyte imbalance often leads to dose reduction or premature discontinuation of the drug (5, 10). The lipid formulations of AMB now available for clinical use may offer therapeutic advantages. Due to their reduced nephrotoxicity and different pharmacodynamic properties, higher doses of the parent compound may be delivered to infected tissues (3, 17, 29).
The exact mechanism responsible for the reduced toxicity of the lipid formulations is not yet known. A recent publication suggests that it may be related to lower concentrations of free AMB found in plasma after administration of lipid-associated AMB. The exposure to free and protein-bound AMB in subjects treated with liposomal AMB (L-AMB) was found to be approximately an order of magnitude lower than in those treated with D-AMB (2).
No data on the ocular distribution of the lipid formulations of AMB are available. Only two clinical case reports on the use of AMB lipid complex (ABLC) (The Liposome Company, Inc., Princeton, N.J.) in the treatment of fungal endophthalmitis have been published (11, 27).
The aim of this study was to determine the ocular distribution of AMB following the systemic administration of the reference AMB deoxycholate (D-AMB) (Bristol-Myers Squibb, Stamford, Conn.) and its lipid formulations, ABLC and L-AMB (NeXstar Pharmaceuticals, Boulder, Colo.), in a rabbit uveitis model.
MATERIALS AND METHODS
Animals.
Male and female adult Burgundy fawn rabbits (2.5 to 3.9 kg) used in this study were provided by an authorized breeding center. The animals were kept in individual cages under well-defined and standardized conditions (humidity- and temperature-controlled room; 13-h light, 11-h dark cycle). They were fed with standard dry food and water ad libitum. All eyes were initially examined with a hand-held slit lamp. Only animals without any sign of ocular inflammation were included in this study. All experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the federal and local ethical and agricultural committees.
Anesthesia.
Animals were anesthetized with an intramuscular injection of a mixture of ketamine (Ketalar; 35 mg/kg of body weight; Parke-Davis, Ann Arbor, Mich.) and xylazine (Xylapan; 5 mg/kg; Chassot, Bern, Switzerland).
Novocaine (0.2%; Inselspital Pharmacy, Bern, Switzerland) eye drops were used for topical anesthesia. In animals treated with multiple drug doses, vascular access was established by surgical placement of a subcutaneous silastic central venous catheter that enabled repeated drug administrations without further anesthesia (28).
Induction of experimental uveitis.
After washing the ocular surface with sterile novocaine (0.2%), 100 ng of lipopolysaccharide (LPS) from Escherichia coli (Sigma, St. Louis, Mo.) diluted in 10 μl of sterile saline solution was injected exactly 1 h before drug administration in animals treated with a single drug dose (18). Fifty micrograms of LPS diluted in 10 μl of sterile saline solution was injected 24 h before treatment in animals treated with multiple drug doses. The higher LPS dose was administered to maintain intraocular inflammation over the treatment period (8). LPS was injected through the sclera into the vitreous using a 30.5-gauge needle connected to a Hamilton syringe. The injection was performed in the right eye of each animal, taking care to avoid damage to the lens. Inflammation was evaluated by clinical manifestation of iris and conjunctival hyperemia.
Single-dose studies.
Single-dose kinetics of L-AMB (AmBisome; NeXstar Pharmaceuticals) were studied over 24 h. One hour after induction of uveitis, 15 rabbits were given 5-mg/kg L-AMB at 2 mg/ml over 10 min by steady bolus injection into an ear vein. Three to five animals per time point were sacrificed at 4, 8, 16, and 24 h after drug administration.
In a comparative single-dose study, three groups of five rabbits were studied. Animals received either 5-mg/kg ABLC (Abelcet; The Liposome Company) at 2 mg/ml, 5-mg/kg L-AMB at 2 mg/ml, or 1-mg/kg D-AMB (Fungizone; Bristol-Myers Squibb) at 0.5 mg/ml over 10 min by steady intravenous bolus through a peripheral ear vein. AMB colloidal dispersion, another lipid formulation of AMB, was omitted from the study because it is not registered in Switzerland. Rabbits were sacrificed 16 h after injection. The drug dosages used correspond to standard doses recommended for the treatment of human fungal infections, are well tolerated by rabbits, and have been shown to be equivalent in rabbit models of infection (1, 9, 21). All drugs were freshly reconstituted immediately before use, according to the manufacturers' instructions. Fifty mg of lyophilized L-AMB was dissolved in sterile water to a concentration of 4 mg/ml and vigorously shaken for at least 15 s to ensure complete dissolution. This stock solution was used to prepare the final dose at an AMB concentration of 2 mg/ml by adding 5% dextrose. The solution was filtered through a 5-μm-pore-size filter to remove any aggregates. The yellowish suspension of ABLC was shaken until no sediment was visible. The equivalent of the treatment dose of 5 mg/kg of body weight was aspirated from the vial and added to 5% glucose solution through a 5-μm-pore-size filter to a final AMB concentration of 2 mg/ml. A D-AMB stock solution at an AMB concentration of 5 mg/ml was prepared by adding 10 ml of aqua ad iniectabilia to the lyophilized drug and shaken for several minutes. This stock solution was further diluted with 5% glucose solution to a final AMB concentration of 0.5 mg/ml.
Multiple-dose study.
Twenty-four hours after induction of uveitis, three groups (n ≥ 5) of rabbits were given either D-AMB at 1 mg/kg/day, ABLC at 5 mg/kg/day, or L-AMB at 5 mg/kg/day by steady intravenous bolus via a central venous catheter once a day for 7 consecutive days (total of seven doses) (28).
To determine the effect of drug accumulation, animals were sacrificed 24 h after the last dose by cervical dislocation and subsequent exsanguination. Blood samples were collected during bleeding, and plasma was immediately separated by centrifugation. The eyes were promptly enucleated, and aqueous and vitreous humor was collected.
Analytical procedures.
Plasma, aqueous humor, and vitreous samples were stored at −20°C until analysis. Aqueous humor was drawn from the freshly enucleated eye with a tuberculin syringe using a 30-gauge needle, and the cornea was excised at the limbus. After the eyes were sectioned just behind the lens, vitreous humor was obtained by dissecting it carefully from the retina. Blank aqueous humor and blank vitreous humor were drawn from porcine eyes collected at the abattoir. Blank plasma was prepared from bovine blood. AMB from Streptomyces sp. (Sigma) was used to prepare stock solutions of AMB (40 μg/ml in dimethyl sulfoxide; 2.5 μg/ml in dimethyl sulfoxide and methanol [1:3, vol/vol]). Calibrator and control samples of the various biological specimens were prepared by spiking the blank samples with known amounts of AMB. All vitreous humor samples were kept on ice and homogenized with an Ultra-Turrax T8 microhomogenizer (IKA-Labortechnik, Staufen, Switzerland) at high speed for 10 s. AMB drug levels were determined as total, unassociated AMB concentrations by high-pressure liquid chromatography (HPLC) using a protocol adapted from that of Pleyer et al. (22). Briefly, 100 μl of aqueous humor (100 μl of vitreous humor homogenate) was pretreated by addition of 50 μl (100 μl) of a methanol-acetonitrile mixture (5:95, vol/vol) containing the internal standard (1,1-diphenyl-2-pikryl-hydrazyle; 2.5 μg/ml) followed by vortex mixing for 10 s. One hundred microliters of plasma was pretreated by addition of 100 μl of methanol containing the internal standard at a concentration of 12.5 μg/ml and 300 μl of acetonitrile followed by vortex mixing for 10 s. All mixtures were centrifuged at 16,000 × g for 5 min, and aliquots of the supernatants (50 μl for aqueous and vitreous humor and 20 μl for plasma) were analyzed by HPLC. The HPLC setup comprised a Waters model 501 pump (Waters Corporation, Milford, Mass.), a Waters 717plus autosampler, and a Spectroflow 757 absorbance detector (Kratos Analytical, New York, N.Y.) that was operated at a wavelength of 380 nm. Separation was achieved with a Nova-Pack C18 column (4 μm by 4.6 mm by 150 mm; Waters) using a pH 7.5 mobile phase consisting of a mixture of methanol (75%) and an aqueous buffer that was prepared by addition of 5.2 ml of diethylamine and 2.8 ml of glacial acetic acid to 1,000 ml of water (25%). The flow rate was 1 ml/min. HP ChemStation software (Hewlett-Packard, Palo Alto, Calif.) was used for data recording, storage, and evaluation. Quantitation was based upon the ratio of the peak area of AMB and the internal standard. Calibration curves for aqueous humor (0.1 to 2 μg/ml), for vitreous humor homogenate (0.05 to 1 μg/ml), and for plasma (0.25 to 8 μg/ml) were determined to be linear, with the mean (n = 6) correlation coefficient, r, being ≥0.9981. The lower limit of quantitation was 0.05 μg/ml. The coefficients of variation ranged from 4.4% (for plasma drug level of 3.25 μg/ml, n = 4 [intraday value]) to 10.3% (for aqueous humor drug level of 0.36 μg/ml, n = 4 [interday value]).
Statistical analysis.
Data were first analyzed using the Kruskal-Wallis one-way analysis of variance to compare the three groups. If the Kruskal-Wallis one-way analysis of variance was significant, the Mann-Whitney U test was subsequently used to compare each pair of group means. To account for multiple comparisons, the Bonferroni correction was used to adjust the P values obtained from the Mann-Whitney U tests. Differences with a first-order error of P being <0.05 were considered to be statistically significant. All data analyses were performed with the computer package Statistica for windows 5.1 (StatSoft, Inc. Tulsa, Okla.).
RESULTS
Single-dose kinetics of L-AMB.
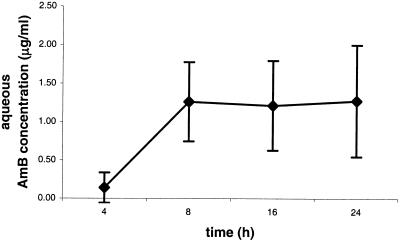
Following the intravenous injection of a single L-AMB dose of 5 mg/kg, the AMB concentration in the aqueous of the inflamed eyes remained stable on a plateau level between 8 and 24 h after treatment (Fig. 1). The mean drug concentration in the aqueous of the inflamed eyes was 0.14 ± 0.19 μg/ml (n = 3) after 4 h, was 1.26 ± 0.51 μg/ml (n = 3) after 8 h, was 1.21 ± 0.58 μg/ml (n = 5) after 16 h, and was 1.28 ± 0.72 μg/ml (n = 4) after 24 h. Inflammation was rated as moderate to severe in all animals. No drug was detectable in the vitreous of the inflamed eyes, nor in the aqueous or vitreous of the contralateral noninflamed eyes.
FIG. 1.
Mean concentration of AMB over time in aqueous humor of eyes with LPS-induced uveitis after a single intravenous dose of L-AMB at 5 mg/kg of body weight. (n = 3 at 4 and 8 h, n = 5 at 16 h, and n = 4 at 24 h). Error bars, standard deviations.
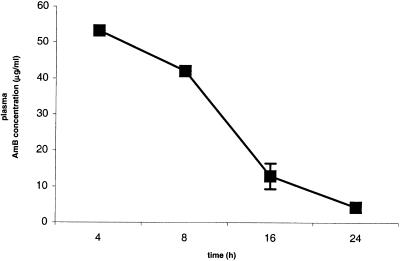
The mean plasma drug concentration was 53.23 μg/ml (n = 2) after 4 h, was 42.08 μg/ml (n = 1) after 8 h, was 12.93 ±3.58 μg/ml (n = 4) after 16 h, and was 4.37 ± 1.39 μg/ml (n = 3) after 24 h (Fig. 2). Concentrations in plasma could not be determined in five samples due to hemolysis induced by accidental freezing of fresh blood.
FIG. 2.
Mean plasma AMB concentration over time (n = 2 at 4 h, n = 1 at 8 h, n = 4 at 16 h, and n = 3 at 24 h). All rabbits were given a single intravenous dose of L-AMB at 5 mg/kg of body weight and were sacrificed at the times indicated. No drug was detectable in the vitreous of the inflamed eyes, nor was any drug in the aqueous or vitreous of the contralateral noninflamed eyes. Error bars, standard deviations.
Comparative single-dose study.
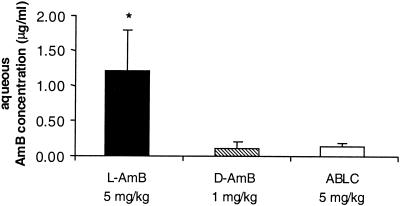
Sixteen hours after intravenous injection of a single dose, mean AMB concentrations in the aqueous of LPS-treated eyes were significantly different (P < 0.01 [Kruskal-Wallis one-way analysis of variance]). AMB concentrations were significantly lower following injection of D-AMB (0.11 ± 0.09 μg/ml [n = 5]) and ABLC (0.14 ± 0.04 μg/ml [n = 5]) than following injection of L-AMB (1.21 ± 0.58 μg/ml [n = 5]) (P = 0.009 [Mann-Whitney U test]) for the comparison of L-AMB with D-AMB or with ABLC (Fig. 3). No significant difference was found between concentrations of D-AMB and ABLC (P = 0.916 [Mann-Whitney U test]). Inflammation was rated as severe in all animals. No drug was detectable neither in the vitreous of the inflamed eyes nor in the aqueous or vitreous of the contralateral noninflamed eyes.
FIG. 3.
Mean AMB concentration in the aqueous humor of LPS-treated rabbit eyes 16 h after the intravenous injection of a single dose of 1-mg/kg D-AMB (n = 5), 5-mg/kg ABLC (n = 5), or 5-mg/kg L-AMB (n = 5). ∗, P = 0.009 (Mann-Whitney U test) for the comparison of L-AMB with D-AMB or ABLC. Error bars, standard deviations.
Sixteen hours after injection of a single dose of D-AMB or ABLC, no AMB was detectable in the plasma. In contrast, following L-AMB injection, the mean AMB concentration in plasma was 12.93 ± 3.58 μg/ml (n = 4) at the same time point.
Multiple-dose study.
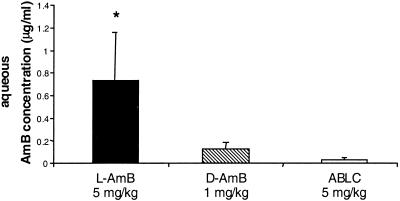
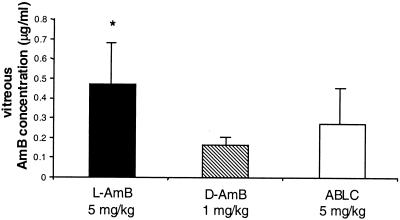
Twenty-four hours after the last of seven consecutive daily doses, the mean AMB concentration in the aqueous of LPS-treated eyes was significantly higher (Kruskal-Wallis test [P < 0.005]) following L-AMB treatment (0.73 ± 0.43 μg/ml [n = 6]) than following ABLC (0.03 ± 0.02 μg/ml [n = 5]) or D-AMB (0.13 ± 0.06 μg/ml [n = 6]) treatment (for L-AMB versus D-AMB, P = 0.013 [Mann-Whitney U test]; for L-AMB versus ABLC, P = 0.008 [Mann-Whitney U test]) (Fig. 4). Cumulative doses of L-AMB also led to a significantly higher mean intravitreal AMB concentration in inflamed eyes (0.47 ± 0.21 μg/ml [n = 6]) than repeat doses of D-AMB (0.16 ± 0.04 μg/ml [n = 6]) (P = 0.0039) (Fig. 5). Following repeat doses of ABLC, the mean AMB concentration in the vitreous of LPS-treated eyes was 0.27 ± 0.18 μg/ml (n = 5) (P = 0.12 [comparison of ABLC versus L-AMB]). Inflammation was rated as severe in the beginning and mild to moderate at the end of treatment in all animals. At 24 h following the last of seven daily doses of L-AMB, the plasma AMB concentration was 16.02 ± 7.68 μg/ml (n = 6), approximately fourfold higher than at the same interval after a single dose. Similar to the findings of the single-dose study, no AMB was detectable in plasma 24 h after the last of seven doses of D-AMB or ABLC.
FIG. 4.
Mean AMB concentration in the aqueous humor of LPS-treated rabbit eyes 24 h after the last of 7 daily doses of 1-mg/kg D-AMB (n = 6), 5-mg/kg ABLC (n = 5), or 5-mg/kg L-AMB (n = 6) via central venous catheter. For L-AMB versus D-AMB, P is 0.013 (Mann-Whitney U test) (*); for L-AMB versus ABLC, P is 0.008 (Mann-Whitney U test). Error bars, standard deviations.
FIG. 5.
Mean AMB concentration in the vitreous humor of LPS-treated rabbit eyes 24 h after the last of 7 daily doses of 1-mg/kg D-AMB (n = 6), 5-mg/kg ABLC (n = 5), or 5-mg/kg L-AMB (n = 6) via central venous catheter. ∗, P = 0.0039 (Mann-Whitney U test) for the comparison of L-AMB with D-AMB (P = 0.08 for L-AMB versus ABLC). Error bars, standard deviations.
DISCUSSION
AMB and its various lipid formulations exhibit different pharmacokinetic properties that determine their distinct toxicities, tissue distributions, and organ-specific relative fungicidal efficacies. While recent reports indicate that these differences may be used to optimize AMB therapy by delivering higher drug doses to infected target organs, little is known how this insight might impact the treatment of fungal eye infections (14). Systemic treatment with AMB, often combined with pars plana vitrectomy, is currently the treatment of choice for severe fungal endophthalmitis (6). However, information on the penetration of AMB into the eye after systemic D-AMB treatment is limited, and the ocular distribution of ABLC or L-AMB has never been studied.
Our findings indicate that AMB distributes to ocular structures sequentially, to the aqueous before the vitreous humor, in an inflammation-dependent manner, and that important differences exist in the ocular penetration among the various formulations of AMB. Using standard doses, AMB concentrations in the aqueous of inflamed eyes were significantly higher (and potentially fungicidal) after single-dose administration of L-AMB than after single-dose administration of either D-AMB or ABLC. After repeated doses, L-AMB also achieved higher AMB concentrations in both aqueous and vitreous than did D-AMB or ABLC.
Within 8 h after systemic administration of L-AMB, drug concentration in the aqueous reached a plateau that remained essentially unchanged during the following 16 h. Similarly stable AMB concentrations in the aqueous have been reported by Green et al. (13) in their study of single-dose kinetics of D-AMB in rabbits. Sixteen hours after single-dose administration, drug concentrations in the aqueous after both D-AMB and ABLC treatment were significantly lower than those after L-AMB treatment. Similar drug levels have been reported in patients treated with D-AMB for fungal endophthalmitis (7, 20).
Remarkably, no AMB was detectable in the vitreous after single-dose administration of the drugs studied. This is in agreement with results published by Green et al. (13), who only found trace amounts of AMB in the vitreous after single-dose administration of D-AMB to rabbits. Successful treatment of endophthalmitis, however, may critically depend on the AMB concentration reached in the vitreous.
In severe cases of endogenous fungal endophthalmitis, pars plana vitrectomy is commonly combined with intravitreal injection of 5 to 10 μg of D-AMB diluted in 0.1 to 0.2 ml of 5% glucose solution to rapidly reach fungicidal concentrations. Due to the invasive nature of the procedure, its repeated use is limited, and systemic treatment with AMB is usually initiated at the same time. In this setting the highest drug concentration that can eventually be reached in the vitreous by systemic administration may be more important than the speed of drug penetration.
Compared to the endogenous form, exogenous fungal endophthalmitis is often detected at an earlier stage, when mainly anterior ocular structures including the cornea and anterior chamber are involved. A drug that rapidly reaches therapeutically active antifungal concentrations in all affected ocular tissues would be highly desirable.
Only after repeated doses was AMB detected in the vitreous. Concentrations were higher after treatment with L-AMB than after treatment with either other drug. However, none of the drugs achieved concentrations above 0.5 μg/ml, indicating that the fungicidal activity might be marginal except for the most sensitive fungal strains (16).
Compared to single-dose administrations, repeated dosing did not lead to higher drug levels in the aqueous humor. This finding might be due to the different intervals after the last dose at which drug levels were determined. However, this seems unlikely considering the stable drug concentration in the aqueous observed for both D-AMB (13) and L-AMB (this study) between 8 and 24 h after administration. Alternatively, the intensity of the LPS-induced uveitis might decrease over time and thus reduce drug penetration. Indeed, the absence of drug in noninflamed eyes indicates that inflammation of ocular structures is crucial for drug penetration, a notion that is supported by the findings of Fisher et al. (7) and Green et al. (13) for human and rabbit eyes, respectively.
L-AMB has been shown to achieve the highest plasma drug levels of all AMB formulations, a finding that we repeated with remarkably good agreement with published results (19). In a rabbit model of Candida albicans meningoencephalitis, the higher concentrations of L-AMB and D-AMB in plasma, compared to those of ABLC, correlated with higher concentrations in brain tissue and greater antifungal efficacy. Hence, when the blood-brain barrier is disrupted by inflammation, concentration gradients between plasma and tissue appear to be the principle determinants for drug delivery to the central nervous system (14). The correlation between drug levels in plasma and ocular tissues observed with L-AMB indicate that, similar to the brain, concentration gradients may be the major determinant for the penetration of AMB into the inflamed eye.
Compared to those obtained with single-dose administration, plasma levels achieved by L-AMB increase approximately fourfold after repeated doses. The apparently discrepant lack of a comparable increase in intraocular concentrations after chronic dosing may be further evidence that the LPS-induced uveitis is abating over the course of the experiment.
The various formulations of AMB have similar pharmacokinetics in rabbits and humans (15, 17), and the rabbit model is of established relevance for human eye diseases. Our findings may provide a potential rationale for the use of L-AMB in selected cases of human fungal endophthalmitis. When only the choroid is involved, parenteral treatment seems to be sufficient. For retinal or vitreal infection, however, additional intraocular injection of AMB with or without vitrectomy is indicated (4, 25). Vitrectomy also provides confirmation of the diagnosis and allows simultaneous instillation of intravitreal AMB.
In summary, the results of this study show that, compared to those obtained for D-AMB and ABLC, higher drug concentrations can be achieved with L-AMB both in the aqueous and vitreous humor of inflamed eyes, suggesting a potential usefulness of this AMB formulation in the adjuvant treatment of fungal endophthalmitis.
Acknowledgments
This work was supported in part by independent grants from NeXstar-Gilead and Fresenius-Kabi, Stans, Switzerland.
We thank Gyula Vucic and Daniel Mettler from the Department of Clinical Research, University of Bern, who performed the catheter implantations, and Franziska Flueckiger, Silvia Kurth, and Hans Liechti, members of the laboratory, for expert technical assistance.
REFERENCES
- 1.Allende, M. C., J. W. Lee, P. Francis, K. Garrett, H. Dollenberg, J. Berenguer, C. A. Lyman, P. A. Pizzo, and T. J. Walsh. 1994. Dose-dependent antifungal activity and nephrotoxicity of amphotericin B colloidal dispersion in experimental pulmonary aspergillosis. Antimicrob. Agents Chemother. 38:518-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bekersky, I., R. M. Fielding, D. E. Dressler, J. W. Lee, D. N. Buell, and T. J. Walsh. 2002. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob. Agents Chemother. 46:834-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brajtburg, J., and J. Bolard. 1996. Carrier effects on biological activity of amphotericin B. Clin. Microbiol. Rev. 9:51231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brod, R. D., H. W. Flynn, Jr., J. G. Clarkson, S. C. Pflugfelder, W. W. Culbertson, and D. Miller. 1990. Endogenous Candida endophthalmitis. Management without intravenous amphotericin B. Ophthalmology 97:666-672. [DOI] [PubMed] [Google Scholar]
- 5.Carlson, M. A., and R. E. Condon. 1994. Nephrotoxicity of amphotericin B. J. Am. Coll. Surg. 179:361-381. [PubMed] [Google Scholar]
- 6.Essman, T. F., H. W. Flynn, Jr., W. E. Smiddy, R. D. Brod, T. G. Murray, J. L. Davis, P. E. Rubsamen. 1997. Treatment outcomes in a 10-year study of endogenous fungal endophthalmitis. Ophthalmic Surg. Lasers 28:185-194. [PubMed] [Google Scholar]
- 7.Fisher, J. F., A. T. Taylor, J. Clark, R. Rao, and A. Espinel-Ingroff. 1983. Penetration of amphotericin B into the human eye. J. Infect. Dis. 147:164. [DOI] [PubMed] [Google Scholar]
- 8.Forrester, J. V., B. V. Worgul, and G. R. Meriam. 1980. Endotoxin-induced uveitis in the rat. Albrecht von Graefes Arch. Klein. Ophthalmol. 213:221-233. [DOI] [PubMed] [Google Scholar]
- 9.Francis, P., J. W. Lee, A. Hoffman, J. Peter, A. Francesconi, J. Bacher, J. Shelhamer, P. A. Pizzo, and T. J. Walsh. 1994. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar D-mannitol and serum galactomannan as markers of infection. J. Infect. Dis. 169:356-368. [DOI] [PubMed] [Google Scholar]
- 10.Gallis, H. A., R. H. Drew, and W. W. Pickard. 1990. Amphotericin B: 30 years of clinical experience. Rev. Infect. Dis. 12:308-329. [DOI] [PubMed] [Google Scholar]
- 11.Goldblum, D., B. E. Frueh, S. Zimmerli, and M. Böhnke. 2000. Treatment of post-keratitis Fusarium endophthalmitis with amphotericin B lipid complex. Cornea 19:853-857. [DOI] [PubMed] [Google Scholar]
- 12.Graybill, J. R. 1996. The future of antifungal therapy. Clin. Infect. Dis. 22:S166-178. [DOI] [PubMed] [Google Scholar]
- 13.Green, W. R., J. E. Bennett, and R. D. Goos. 1965. Ocular penetration of amphotericin B. Arch. Ophthalmol. 73:769-775. [DOI] [PubMed] [Google Scholar]
- 14.Groll, A. H., N. Giri, V. Petraitis, R. Petraitiene, M. Candelario, J. S. Bacher, J. S. Piscitelli, T. J. Walsh. 2000. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J. Infect. Dis. 182:274-282. [DOI] [PubMed] [Google Scholar]
- 15.Groll, A. H., S. C. Piscitelli, and T. J. Walsh. 1998. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv. Pharmacol. 44:343-500. [DOI] [PubMed] [Google Scholar]
- 16.Hanson, L. H., and D. A. Stevens. 1992. Comparison of antifungal activity of amphotericin B deoxycholate suspension with that of amphotericin B cholesteryl sulfate colloidal dispersion. Antimicrob. Agents Chemother. 36:486-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiemenz, J. W., and T. J. Walsh. 1996. Lipid formulations of amphotericin B: recent progress and future directions. Clin. Infect. Dis. 22:S133-144. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni, P. S., and M. Mancino. 1993. Studies on intraocular inflammation produced by intravitreal human interleukins in rabbits. Exp. Eye Res. 56:275-279. [DOI] [PubMed] [Google Scholar]
- 19.Lee, J. W., M. A. Amantea, P. A. Francis, E. E. Navarro, J. Bacher, P. A. Pizzo, T. J. Walsh. 1994. Pharmacokinetics and safety of a unilamellar liposomal formulation of amphotericin B (AmBisome) in rabbits. Antimicrob. Agents Chemother. 38:713-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Day, D. M., W. S. Head, R. D. Robinson, W. H. Stern, and J. M. Freeman. 1985. Intraocular penetration of systemically administered antifungal agents. Curr. Eye Res. 4:131-134. [DOI] [PubMed] [Google Scholar]
- 21.Perfect, J. R., and K. A. Wright. 1994. Amphotericin B lipid complex in the treatment of experimental cryptococcal meningitis and disseminated candidosis. J. Antimicrob. Chemother. 33:73-81. [DOI] [PubMed] [Google Scholar]
- 22.Pleyer, U., J. Grammer, J. H. Pleyer, P. Kosmidis, D. Friess, K. H. Schmidt, H. J. Thiel. 1995. Amphotericin B Wirkspiegel in der Cornea. Ophthalmologie 92:469-475. [PubMed] [Google Scholar]
- 23.Sabra, R., and R. A. Branch. 1990. Amphotericin B nephrotoxicity. Drug Safety 5:94-108. [DOI] [PubMed] [Google Scholar]
- 24.Samiy, N., and D. J. D'Amico. 1996. Endogenous fungal endophthalmitis. Int. Ophthalmol. Clin. 36:147-162. [DOI] [PubMed] [Google Scholar]
- 25.Smiddy, W. E. 1998. Treatment outcomes of endogenous fungal endophthalmitis. Curr. Opin. Ophthalmol. 9:66-70. [DOI] [PubMed] [Google Scholar]
- 26.Tester-Dalderup, C. B. M. 1996. Antifungal drugs, p. 774-779. In M. N. G. Dukes (ed.), Meyler's side effects of drugs, 13th ed. Elsevier Science BV, Amsterdam, The Netherlands.
- 27.Virata, S. R., J. A. Kylstra, J. C. Brown, D. A. Wohl, and M. S. Cohen. 1999. Worsening of endogenous Candida albicans endophthalmitis during therapy with intravenous lipid complex amphotericin B. Clin. Infect. Dis. 28:1177-1178. [DOI] [PubMed] [Google Scholar]
- 28.Walsh, T. J., J. Bacher, and P. A. Pizzo. 1988. Chronic silastic central venous catheterization for induction, maintenance, and support of persistent granulocytopenia in rabbits. Lab. Anim. Sci. 38:467-471. [PubMed] [Google Scholar]
- 29.Wong-Beringer, A., R. A. Jacobs, and B. J. Guglielmo. 1998. Lipid formulations of amphotericin B: clinical efficacy and toxicities. Clin. Infect. Dis. 27:603-618. [DOI] [PubMed] [Google Scholar]