Abstract
N-Nonyl-deoxy-galactonojirimycin (N-nonyl-DGJ) has been shown to reduce the amount of hepatitis B virus (HBV) produced by tissue cultures under conditions where cell viability is not affected. We show here that the compound N-nonyl-DGJ was effective against lamivudine-resistant HBV mutants bearing the YMDD motif in the polymerase gene, consistent with the compound's activity being distinct from those of nucleoside inhibitors. To better understand the chemical structures that influence its antiviral activity, a series of imino sugar derivatives were made and tested for their antiviral activity against HBV. This work suggests that the antiviral activity of the alkovirs requires an alkyl chain length of at least eight carbons but that the galactose-based head group can be modified with little or no loss in activity.
Hepatitis B virus (HBV) is the prototypic member of the Hepadnaviridae family of viruses that chronically infects more than 350 million people worldwide (6-9, 17). The major complication is the development of primary hepatocellular carcinoma, estimated to cause more than 500,000 deaths annually (1). Although there is no cure for HBV infection, several therapeutic options now exist (4, 9). However, the poor response rate and the development of resistant mutants highlight the need for alternatives and complements to the conventional therapeutic regimens (10, 21; K.-A. Walters, G. A. Tipples, M. I. Allen, L. D. Condreay, W. R. Addison, and L. Tyrrell, submitted for publication).
In our work developing glucosidase inhibitors as potential mutation-resistant therapeutic agents for HBV and hepatitis C virus, we discovered an imino sugar, N-nonyl-deoxy-galactonojirimycin (N-nonyl-DGJ) (Fig. 1), that possesses potent antiviral activity against HBV in the absence of glucosidase inhibition (2-3, 5, 11-15). In studying this new class of compounds, we have discovered that their mechanism of action is fundamentally different than that of glucosidase inhibitors and may exert an antiviral action at a point before viral envelopment and perhaps prevent the proper encapsidation of the HBV pregenomic RNA (14).
FIG. 1.
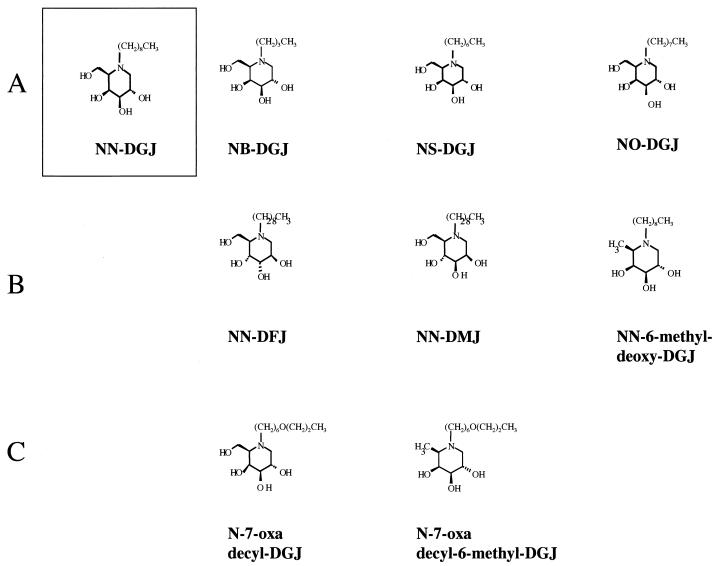
Imino sugars used in this analysis. The imino sugars used in this study are composed of an imino sugar head group and an alkyl chain. (A) Tail modification of N-nonyl-DGJ. N-nonyl-DGJ is the model structure that was modified in this study. Structures shown from left to right are those of N-nonyl-DGJ (NN-DGJ), N-butyl-DGJ (NB-DGJ), N-septyl-DGJ (NS-DGJ), and N-octyl-DGJ (NO-DGJ). (B) Head group modification of N-nonyl-DGJ. Structures shown from left to right are those of N-nonyl-DFJ (NN-DFJ, fucose head group), N-nonyl-DMJ (NN-DMJ, mannose head group), and N-nonyl-6-methyl-DGJ (NN-6-methyl-deoxy-DGJ, made by the replacement of the position 6 hydroxyl with a hydrogen). (C) Further modification of alkyl tail to reduce toxicity. The introduction of an oxygen species is thought to reduce toxicity but maintain efficacy (Walters et al., submitted). Structures shown from left to right are those of N-nonyl-7-oxy-decyl-DGJ (with oxygen in the seventh position in the alkyl chain) and N-nonyl-7-oxy-decyl-6-methyl-DGJ (same as the prior structure but with the 6-methyl modification).
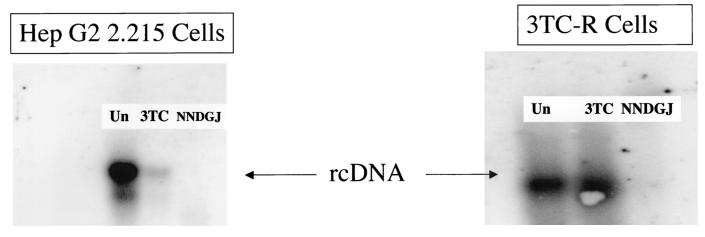
The Food and Drug Administration-approved nucleoside analogue lamivudine (3TC) has shown great promise in the treatment of chronic infection with HBV but is severely hampered by the emergence of resistant virus (4, 9-10, 21). Resistance to 3TC occurs as a result of mutations in the tyrosine-methionine-aspartate-aspartate (YMDD) motif of the HBV RNA-dependent DNA polymerase and may emerge after 9 to 10 months of therapy, with an incidence of >65% after 4 years of 3TC therapy (10, 21). Treatments that will work against 3TC-resistant (3TC-R) virus or inhibit the virus by another mechanism will be essential in the effective treatment of HBV (20). To that end, the ability of N-nonyl-DGJ to inhibit the secretion of 3TC-R virus from a stable 3TC-R-virus-producing cell line was determined by Southern blotting (14, 22). The virus expressed by the 3TC-R-HBV-producing cell line contained both the valine-for-methionine mutation at residue 552 and the methionine-for-leucine mutation at residue 528. N-nonyl-DGJ (alkovir) is a galactose-based imino sugar with a nine-carbon-length alkyl side chain (Fig. 1) and does not inhibit the endoplasmic reticulum (ER) glucosidases (5, 14, 19). Hep G2-derived cells that stably produce either wild-type (Hep G2 2.215) or 3TC-R HBV (Walters et al., submitted) were incubated with either N-nonyl-DGJ or 3TC, and the amount of enveloped HBV in each culture medium was determined by a method that differentiates between enveloped and nonenveloped virus particles (14, 22). Briefly, virus in the culture medium was concentrated by pelleting it through 20% sucrose for 16 h (SW41 rotor, 36,000 rpm). Virus was resuspended in a solution containing 200 μl of 10 mM Tris (pH 7.9), 10 mM EDTA (pH 8.0), and 10 mM MgCl2. Proteinase K was added to a final concentration of 750 μg/ml, and the samples were incubated for 1 h at 37°C. After 1 h, RQ1 DNase (Promega, Madison, Wis.) was added to each tube to a final concentration of 50 U/ml, and the mixtures were incubated at 37°C for 1 h. Sodium dodecyl sulfate was added to a final concentration of 1%, more proteinase K was added to a final concentration of 500 μg/ml, and the reaction was allowed to proceed at 37°C for 4 h. DNA was purified by phenol-chloroform extraction followed by isopropanol precipitation. Viral DNA was separated by electrophoresis on a 1.0% agarose gel, transferred to a nylon membrane, and probed with 32P-labeled HBV probes (14). HBV-specific bands were subsequently identified and quantified by phosphorimage analysis (Bio-Rad, Hercules, Calif.). All bands were within the sensitivity of the machine, and no blots that had saturating pixels were used for analysis. As the left panel of Fig. 2 shows, both 3TC and N-nonyl-DGJ possess the ability to reduce the amount of HBV DNA in the culture medium from Hep G2 2.215 cells compared to untreated controls. Since Hep G2 2.215 cells produce and secrete HBV (18) with a wild-type 3TC-sensitive polymerase gene product, these results are not surprising. The right panel of Fig. 2 shows that treatment of a cell line that produces the 3TC-R virus with 3TC does not result in reduced amounts of HBV DNA detected in the culture medium (compare lanes 1 and 2 of Fig. 2, right panel). In contrast, N-nonyl-DGJ retains the ability to reduce the amount of HBV DNA in the culture medium of cells secreting 3TC-R virus. It should be noted that the 90% inhibitory concentrations of N-nonyl-DGJ against wild-type and 3TC-R virus were similar (data not shown). Thus, N-nonyl-DGJ retains effectiveness against 3TC-R HBV under conditions where 3TC does not.
FIG. 2.
N-nonyl-DGJ inhibits the secretion of 3TC-R virus. (A) Hep G2 2.215 cells (which produce wild-type virus) and 3TC-R Hep G2 cells (which produce 3TC-R virus) were treated with either 3TC (15 μM) or N-nonyl-DGJ (NNDGJ; 70 μM) for 7 days, and the amount of virus in each culture medium was detected by Southern blotting as described in Materials and Methods. The left panel shows the effects of 3TC and N-nonyl-DGJ on the secretion of HBV from Hep G2 2.215 cells. As this panel shows, both 3TC and N-nonyl-DGJ inhibit the secretion of HBV into the culture medium. In contrast, the right panel clearly shows that while N-nonyl-DGJ can inhibit the secretion of HBV from cells producing 3TC-R virus, 3TC cannot. Un, untreated; rcDNA, relaxed circular DNA.
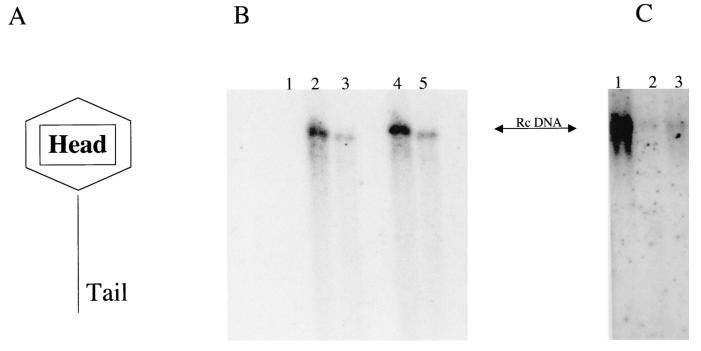
N-nonyl-DGJ can be divided into two functional groups: an imino sugar head group and an alkyl tail (Fig. 1 and 3A). In the case of the imino sugar glucosidase inhibitors, the head group plays a critical role in antiviral activity (5, 14, 19). To test the role of the head group in the antiviral activity of the alkovirs (the imino sugar compounds that do not inhibit the glucosidases [Fig. 1]), we made several compounds in which the head group was changed from a galactose to either a fucose (N-nonyl-deoxyfuconojirimycin [DFJ]), a mannose (N-nonyl-deoxymannojirimycin [DMJ]), or a galactose with a methyl group at position 6 (6-methyl-N-nonyl-DGJ) (5). These compounds were tested in the Hep G2 2.215 cell system as before, and the results are shown in Fig. 3B and C. As Fig. 3B shows, after a 7-day incubation of Hep G2 2.215 cells with either N-nonyl-DGJ or N-nonyl-DFJ at a concentration of 20 μm, reductions (∼12-fold) in HBV secretion (compare lanes 2, 5, and 8) were observed. Analogues with an imino-mannose head group also maintained antiviral activity (data not shown). In addition, as Fig. 3C demonstrates, an imino sugar with a modification of the galactose head group (conversion of the position 6 primary alcohol to a methyl group) also inhibited virus secretion. This highlights the fact that alkyl imino sugars with nonnatural sugar head groups can exert antiviral activity. These data, taken together, suggest that variations of the sugar head group are allowed and to a large extent maintains antiviral activity. It should be mentioned that while imino sugar derivatives with mannose or fucose head groups can inhibit specific steps in the N-linked glycosylation pathway, previous work has determined that compounds that cause glycosylation changes in post-ER compartments (Golgi bodies) have no antiviral activity (11, 23).
FIG. 3.
(A to C) Alterations of the head group do not affect drug efficacy. (A) The imino sugars used in this study are composed of a head group and a tail group. DGJ is a galactose analogue in which the ring oxygen has been replaced with a nitrogen atom and the anomeric hydroxyl group of galactose is absent. Head groups that mimic glucose (DNJ) inhibit the ER glucosidase; other head groups do not but may inhibit other non-ER-based N-linked glycosylation steps (13). (B) Conversion of the imino-galactose head group to imino fucose [DFJ] does not alter efficacy. As described in Materials and Methods, Hep G2 2.215 cells were left untreated (lane 2) or treated with either N-nonyl-DGJ at 20 μM (lane 3) or N-nonyl-DFJ at 2 μM (lane 4) or 20 μM (lane 5). As the figure shows, both N-nonyl-DGJ and N-nonyl-DFJ retained the ability to inhibit the secretion of HBV. Lane 1 contains HBV secreted from Hep G2 cells (negative control). (C) Modification of the galactose ring structure also does not alter the efficacy of N-nonyl-DGJ. The position 6 hydroxyl group in the imino-galactose ring was replaced with a hydrogen. This replacement alters the ability of the molecule to inhibit glycolipid biosynthesis (5, 19). Lane 1, HBV DNA secreted from untreated Hep G2 2.215 cells; lane 2, HBV DNA secreted from N-nonyl-DGJ (20 μM)-treated cells; lane 3, HBV DNA secreted from N-nonyl-6-methyl-DGJ (20 μM)-treated cells. Rc DNA, relaxed circular DNA.
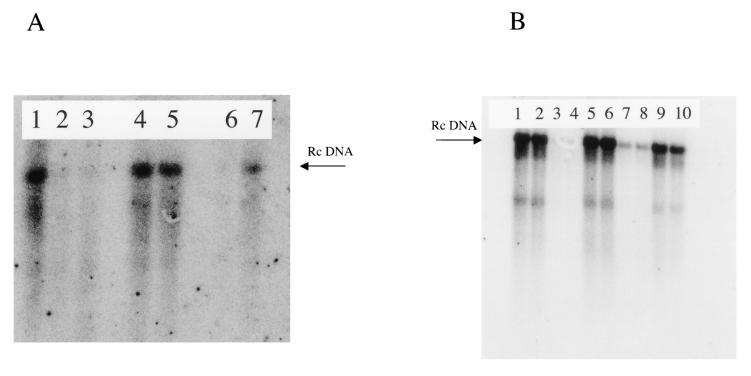
Initial experiments to test the role of the tail group in the activity of N-nonyl-DGJ involved shorter tail variants to determine if a critical length was required for antiviral efficacy. The structures of these compounds are shown in Fig. 1, and the results are shown in Fig. 4. As before, Hep G2 2.215 cells were either left untreated or treated with the concentrations of 3TC, N-nonyl-DGJ, N-septyl-DGJ, or N-octyl-DGJ indicated in the legend to Fig. 4. As Fig. 4A shows, decreasing the chain length to seven (N-septyl-DGJ) (lanes 4 and 5 of Fig. 4A) correlated with a loss of antiviral activity, while N-octyl-DGJ maintained a degree of efficacy (Fig. 4B, lanes 6 and 7). N-alkylated derivatives of DGJ (or deoxynojirimycin [DNJ]) that have an N-alkyl chain length of greater than three carbon atoms act as inhibitors of glycolipid biosynthesis (16). Further evidence that glycolipid inhibition has no effect on the replication and secretion of HBV was tested by using N-butyl-DGJ, a shorter alkylated derivative of N-nonyl-DGJ. Figure 4B shows the effect of shorter alkyl chains on HBV secretion and the effect of modification of the alkyl chain on antiviral efficacy. As before, Hep G2 2.215 cells were either left untreated (Fig. 4B, lanes 1 and 2) or treated with the concentrations of 3TC (Fig. 4B, lanes 3 and 4), N-butyl-DGJ (Fig. 4B, lanes 5 and 6), N-nonyl-DGJ (Fig. 4B, lanes 7 and 8), or N-7-oxy-decyl-DGJ (Fig. 4B, lanes 9 and 10) indicated in the legend to Fig. 4. N-butyl-DGJ is an inhibitor of the ceramide-specific glucosyltransferase that is involved in glycolipid biosynthesis (16). Clearly, as Fig. 4A shows, N-butyl-DGJ, and hence glycolipid inhibition, has no effect upon HBV secretion. Modification of the alkyl chain by the introduction of an oxygen species into the N-alkyl carbon chain at position 7 has been shown to improve toxicity while maintaining efficacy against α-glucosidase for DNJ-based imino sugars (5, 19). However, for the alkovirs, the addition of an oxygen species into a decyl tail destroys the antiviral activity. This finding suggests that the nonyl-alkyl chain is important for antiviral activity (Fig. 4B, group 5).
FIG. 4.
Structure-activity relationship analysis identifies N-alkylated tails as important for function. (A) The importance of the tail group in the activity of the alkovirs was tested by modification of the tail length in N-nonyl-DGJ. Briefly, Hep G2 2.215 cells were either left untreated (lane 1) or treated with 3TC at 1.0 μM (lane 2), N-nonyl-DGJ at 20 μM (lane 3), N-septyl-DGJ at 20 μM (lane 4), N-septyl-DGJ at 2 μM (lane 4), N-octyl-DGJ at 20 μM (lane 6), or N-octyl-DGJ at 2 μM (lane 7) for 7 days, and the amount of virus was determined in each culture medium by Southern blot hybridization. (B) Modification of the tail with an oxygen species can eliminate activity. Blots show the amount of HBV DNA secreted from untreated Hep G2 2.215 cells (lanes 1 and 2) and from those treated with 3 μM 3TC (lanes 3 and 4), 500 μM N-butyl-DGJ (lanes 5 and 6), 20 μM N-nonyl-DGJ (lanes 7 and 8), or 1,000 μM N-7-oxy-decyl-DGJ (lanes 9 and 10). Rc DNA, relaxed circular DNA.
The present study has elucidated some critical structural features of N-nonyl-DGJ that influence its antiviral activity. The data reported here are consistent with the following structural conclusions. From the structural perspective, we have learned that the sugar head group need not be galactonojirimycin for retention of antiviral activity. Either fuconojirimycin or mannojirimycin can be substituted for galactonojirimycin, with little reduction in antiviral activity (Fig. 3). The alkyl side chain length appears to be critical, with antiviral activity decreasing sharply with side chains of fewer than eight carbons. Moreover, interruption of the side chain with oxygenation also reduced activity. Taken together, these data suggest that a minimum alkyl side chain length of eight carbons and a sugar head group are core elements of the alkovir antivirals.
We have also shown that N-nonyl-DGJ is effective against the 3TC-R HBV mutant. This suggests that the mechanism of antiviral action of N-nonyl-DGJ does not involve the polymerase catalytic domain and is consistent with a nonpolymerase target. In addition, this finding highlights the potential of this compound as a therapeutic agent (22).
Acknowledgments
This work was supported by the Hepatitis B Foundation of America, an appropriation from the Commonwealth of Pennsylvania, NIH grant number 1R41AI/DK49924-01, and Synergy Pharmaceuticals, Inc. Anand Mehta is the Bruce Witte Research Scholar of the Hepatitis B Foundation.
Nicole Zitzmann (Oxford University) is thanked for careful reading of the manuscript.
REFERENCES
- 1.Beasley, R. P., and L. Y. Hwang. 1991. Overview on the epidemiology of hepatocellular carcinoma, p. 532-535. In F. B. Hollinger, S. M. Lemon, and M. Margolis (ed.), Viral hepatitis and liver disease. The Williams & Willkins Co., Baltimore, Md.
- 2.Block, T., F. Platt, L. Xuanyong, W. Gerlich, G. Foster, B. Blumberg, and R. Dwek. 1994. Secretion of human hepatitis B virus is inhibited by the imino sugar N-butyldeoxynojirimycin. Proc. Natl. Acad. Sci. USA 91:2235-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Block, T. M., X. Lu, A. Mehta, B. Blumberg, B. Tennant, M. Ebling, B. Korba, D. M. Lansky, G. S. Jacob, and R. A. Dwek. 1998. Treatment of chronic hepadnavirus infection in a woodchuck animal model with an inhibitor of protein folding and trafficking. Nat. Med. 4:610-614. [DOI] [PubMed]
- 4.Doong, S. L., C. H. Tsai, R. F. Schinazi, D. C. Liotta, and Y. C. Cheng. 1991. Inhibition of the replication of hepatitis B virus in vitro by 2′,3′-dideoxy-3′-thiacytidine and related analogues. Proc. Natl. Acad. Sci. USA 88:8495-8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durantel, D., N. Branza-Nichita, S. Carrouée-Durantel, T. D. Butters, R. A. Dwek, and N. Zitzmann. 2001. Study of the mechanism of antiviral action of iminosugar derivatives against bovine viral diarrhea virus. J. Virol. 75:8987-8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganem, D. 1991. Assembly of hepadnaviral virions and subviral particles. Curr. Top. Microbiol. Immunol. 168:61-83. [DOI] [PubMed] [Google Scholar]
- 7.Heermann, K. H., and W. H. Gerlich. 1992. Surface proteins of hepatitis B virus, p. 104-144. In A. Maclachlan (ed.), Molecular biology of HBV. CRC Press, Boca Raton Fla.
- 8.Hollinger, F. B. 1990. Hepatitis B virus, p. 2171-2236. In B. N. Fields, D. M. Knipe, et al. (ed.), Fields virology, vol. 2. Raven Press, Ltd., New York, N.Y.
- 9.Hoofnagle, J. H., and A. M. Di Bisceglie. 1997. Drug therapy: the treatment of chronic viral hepatitis. N. Engl. J. Med. 336:347-356. [DOI] [PubMed] [Google Scholar]
- 10.Liaw, Y. F. 2001. Impact of YMDD mutations during lamivudine therapy in patients with chronic hepatitis B. Antivir. Chem. Chemother. 12:67-71. [PubMed] [Google Scholar]
- 11.Lu, X., A. Mehta, T. Butters, R. A. Dwek, and T. M. Block. 1995. Evidence that N-linked glycosylation is necessary for hepatitis B virus secretion. Virology 213:660-665. [DOI] [PubMed] [Google Scholar]
- 12.Lu, X., A. Mehta, M. Dadmarz, R. A. Dwek, B. S. Blumberg, and T. M. Block. 1997. Aberrant trafficking and behavior of hepatitis B virus glycoproteins in cells in which glycosylation processing is inhibited. Proc. Natl. Acad. Sci. USA 94:2380-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta, A., X. Lu, R. A. Dwek, B. Blumberg, and T. M. Block. 1997. HBV envelope glycoproteins vary drastically in their sensitivity to glycan processing—evidence that alteration of a single N-linked glycosylation site can regulate HBV secretion. Proc. Natl. Acad. Sci. USA 94:1822-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta, A., S. Carrouee, B. Conyers, R. Jordan, T. Butters, R. A. Dwek, and T. M. Block. 2001. Inhibition of hepatitis B virus DNA replication by imino sugars without the inhibition of the DNA polymerase: therapeutic implications. Hepatology 33:1488-1495. [DOI] [PubMed] [Google Scholar]
- 15.Ou, W. J., P. H. Cameron, D. Y. Thomas, and J. M. Bergeron. 1993. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature 364:771-776. [DOI] [PubMed] [Google Scholar]
- 16.Platt, F. M., and T. D. Butters. 2000. New therapeutic prospects for the glycosphingolipid lysosomal storage diseases. Biochem. Pharmacol. 56:421-430. [DOI] [PubMed] [Google Scholar]
- 17.Robinson, W. S. 1990. Hepadnaviridae and their replication, p. 2137-2169. In B. N. Fields, D. M. Knipe, et al. (ed.), Fields virology. Raven Press, Ltd., New York, N.Y.
- 18.Sells, M. A., M. L. Chen, and G. Acs. 1987. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc. Natl. Acad. Sci. USA 84:1005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan, A., L. van den Broek, S. van Boeckel, H. Ploegh, and J. Bolscher. 1991. Chemical modification of the glucosidase inhibitor 1-deoxynojirimycin. Structure-activity relationships. J. Biol. Chem. 266:14504-14510. [PubMed] [Google Scholar]
- 20.Terrault, N., and M. Ma. 2001. Adding to the hepatitis B virus treatment arsenal: alpha-glucosidase inhibitor derivatives. Hepatology 33:1544-1546. [DOI] [PubMed] [Google Scholar]
- 21.Tipples, G. A., M. Ma, K. P. Fischer, V. G. Bain, N. M. Kneteman, and D. L. J. Tyrrell. 1996. Mutation in the HBV RNA-dependent DNA polymerase confers resistance to lamivudine in vivo. Hepatology 24:714-717. [DOI] [PubMed] [Google Scholar]
- 22.Wei, Y., J. E. Tavis, and D. Ganem. 1996. Relationship between viral DNA synthesis and virion envelopment in hepatitis B viruses. J. Virol. 70:6455-6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zitzmann, N., A. S. Mehta, S. Carrouée, T. D. Butters, F. M. Platt, J. McCauley, B. S. Blumberg, R. A. Dwek, and T. M. Block. 1999. Imino sugars inhibit the formation and secretion of bovine viral diarrhea virus, a pestivirus model of hepatitis C virus: implications for the development of broad spectrum anti-hepatitis agents. Proc. Natl. Acad. Sci. USA 96:11878-11882. [DOI] [PMC free article] [PubMed] [Google Scholar]