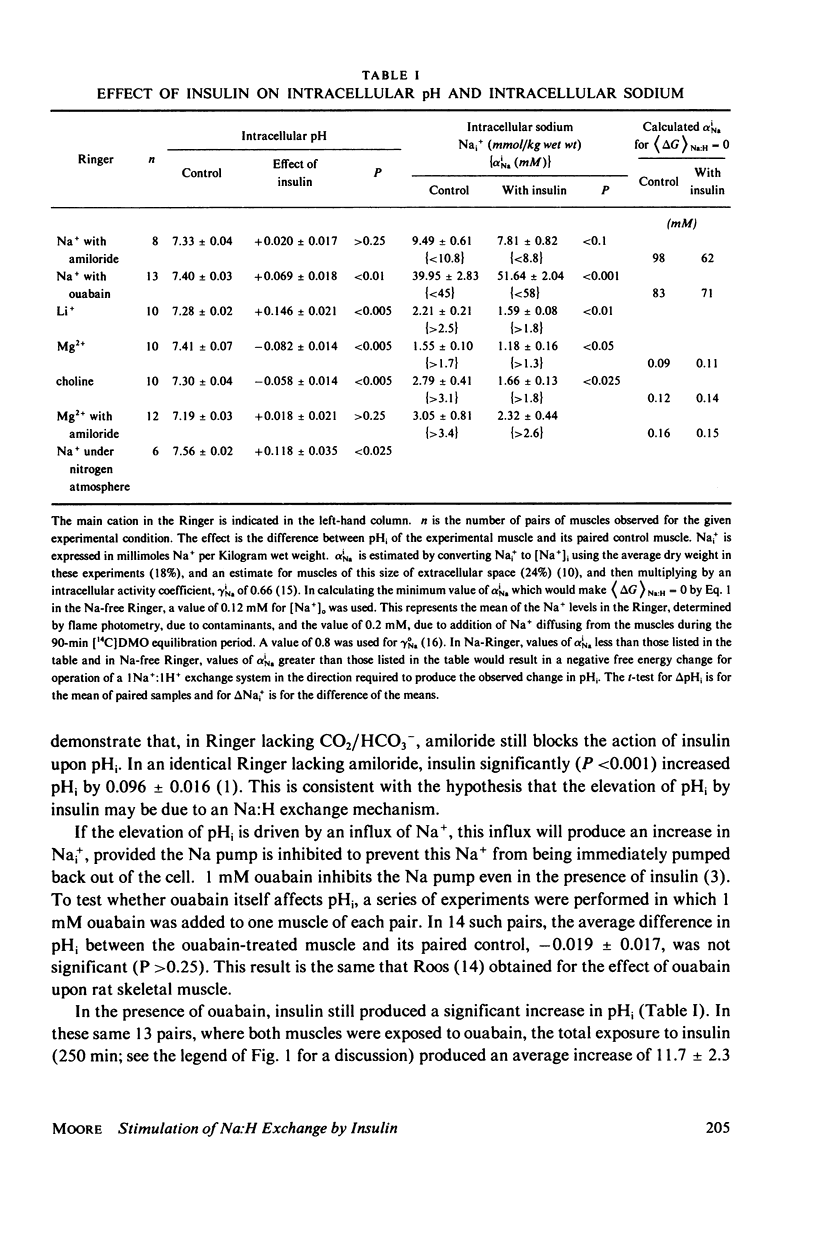
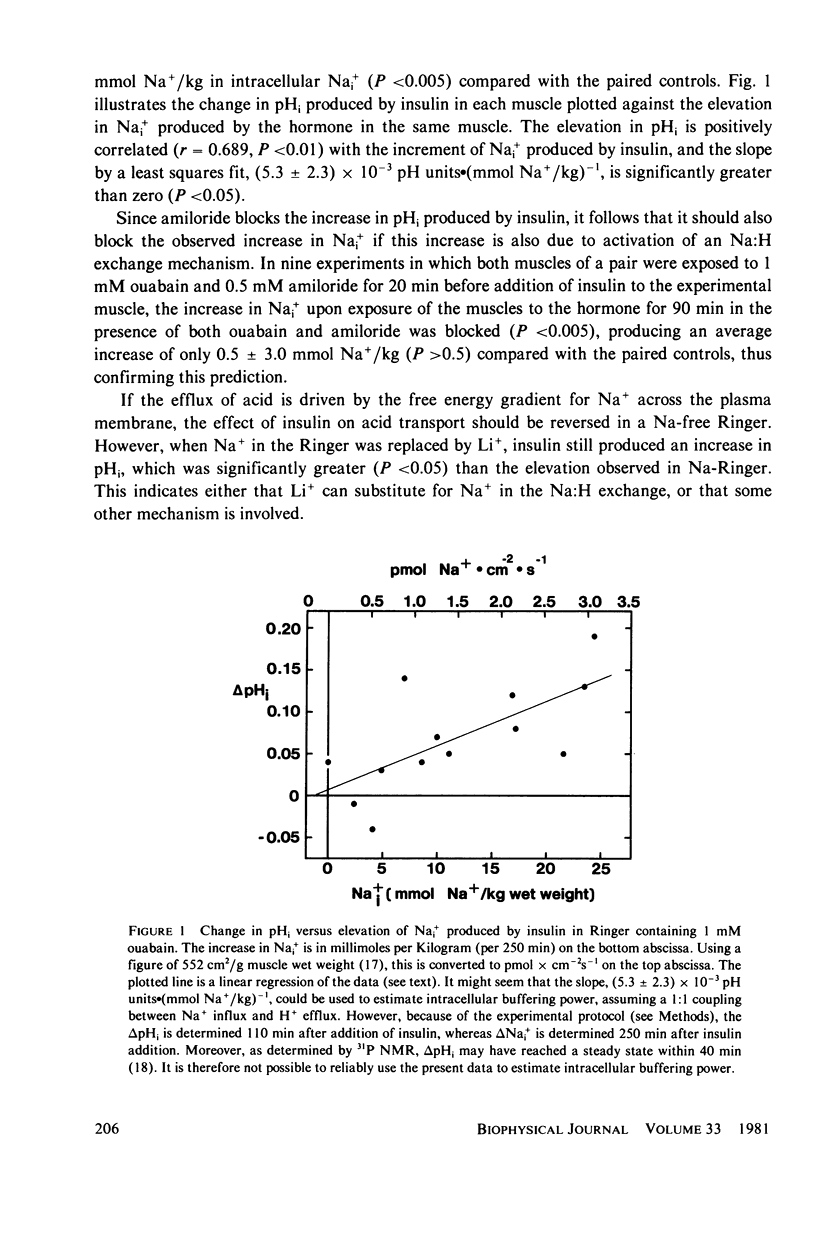
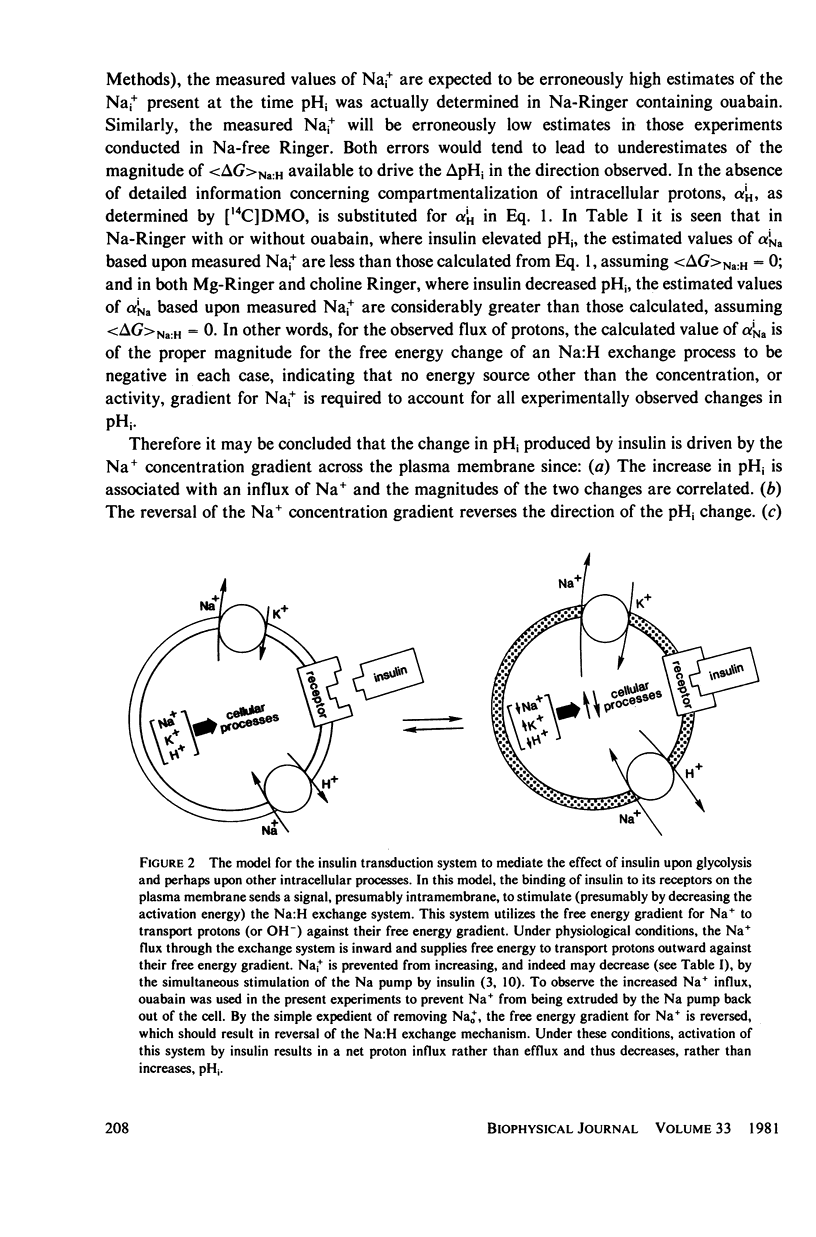
Abstract
In frog skeletal muscle, the increase of intracellular pH (pHi) induced by insulin is correlated with an increase in intracellular Na+ when the sodium pump is inhibited by ouabain. Reversing the Na+ free energy gradient by substituting either Mg2+ or choline for extracellular Na+ converts the effect of insulin to a decrease in pHi, indicating that the action of insulin upon pHi is determined by the Na+ free energy gradient. Moreover, estimates of the Na+ free energy gradient indicate that both the direction and magnitude satisfy the hypothesis that this is the source of energy for the observed changes in pHi. Both the increase in intracellular pH induced by insulin and the associated increase in intracellular Na+ produced by this hormone in the presence of ouabain are blocked by amiloride. This drug also blocks the decrease in pHi by insulin when Mg2+ is substituted for Na+ in the Ringer. In Ringer containing Na+, the increase in pHi by insulin occurs when both metabolic and atmospheric sources of CO2 are eliminated by using a 100% N2 atmosphere. Thus, the mechanism stimulated by insulin is not a Na+-CO3(2-) cotransport system, but is either an Na:H exchange or a Na+-OH- cotransport system which can be inhibited by amiloride. The suggestion is advanced that the Na:H exchange mechanism is part of the membrane transduction system for insulin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Thomas R. C. An investigation of the ionic mechanism of intracellular pH regulation in mouse soleus muscle fibres. J Physiol. 1977 Dec;273(1):295–316. doi: 10.1113/jphysiol.1977.sp012095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P. J. Amiloride: a potent inhibitor of sodium transport across the toad bladder. J Physiol. 1968 Mar;195(2):317–330. doi: 10.1113/jphysiol.1968.sp008460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder J., Tosteson D. C., Wieth J. O. Effects of bicarbonate on lithium transport in human red cells. J Gen Physiol. 1978 Jun;71(6):721–746. doi: 10.1085/jgp.71.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavryck W. A., Moore R. D., Thompson R. C. Effect of insulin upon membrane-bound (Na+ + K+)-ATPase extracted from frog skeletal muscle. J Physiol. 1975 Oct;252(1):43–58. doi: 10.1113/jphysiol.1975.sp011133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. D., Epel D. Intracellular pH and activation of sea urchin eggs after fertilisation. Nature. 1976 Aug 19;262(5570):661–664. doi: 10.1038/262661a0. [DOI] [PubMed] [Google Scholar]
- MULLINS L. J., NODA K. THE INFLUENCE OF SODIUM-FREE SOLUTIONS ON THE MEMBRANE POTENTIAL OF FROG MUSCLE FIBERS. J Gen Physiol. 1963 Sep;47:117–132. doi: 10.1085/jgp.47.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. D. Elevation of intracellular pH by insulin in frog skeletal muscle. Biochem Biophys Res Commun. 1979 Dec 14;91(3):900–904. doi: 10.1016/0006-291x(79)91964-8. [DOI] [PubMed] [Google Scholar]
- Moore R. D., Fidelman M. L., Seeholzer S. H. Correlation between insulin action upon glycolysis and change in intracellular pH. Biochem Biophys Res Commun. 1979 Dec 14;91(3):905–910. doi: 10.1016/0006-291x(79)91965-x. [DOI] [PubMed] [Google Scholar]
- Moore R. D., Rabovsky J. L. Mechanism of insulin action on resting membrane potential of frog skeletal muscle. Am J Physiol. 1979 May;236(5):C249–C254. doi: 10.1152/ajpcell.1979.236.5.C249. [DOI] [PubMed] [Google Scholar]
- Neville M. C., White S. Extracellular space of frog skeletal muscle in vivo and in vitro: relation to proton magnetic resonance relaxation times. J Physiol. 1979 Mar;288:71–83. [PMC free article] [PubMed] [Google Scholar]
- Russell J. M., Boron W. F. Role of choloride transport in regulation of intracellular pH. Nature. 1976 Nov 4;264(5581):73–74. doi: 10.1038/264073a0. [DOI] [PubMed] [Google Scholar]
- Russell J. M. Effects of ammonium and bicarbonate-CO2 on intracellular chloride levels in Aplysia neurons. Biophys J. 1978 Apr;22(1):131–137. doi: 10.1016/S0006-3495(78)85478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SJODIN R. A., HENDERSON E. G. TRACER AND NON-TRACER POTASSIUM FLUXES IN FROG SARTORIUS MUSCLE AND THE KINETICS OF NET POTASSIUM MOVEMENT. J Gen Physiol. 1964 Mar;47:605–638. doi: 10.1085/jgp.47.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venosa R. A. Inward movement of sodium ions in resting and stimulated frog's sartorius muscle. J Physiol. 1974 Aug;241(1):155–173. doi: 10.1113/jphysiol.1974.sp010646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]


