Abstract
Frog rod outer segments were labeled with the sulfhydryl-reactive label iodoacetamido tetramethylrhodamine. The bulk of the label reacted with the major disk membrane protein, rhodopsin. Fluorescence photobleaching and recovery (FPR) experiments on labeled rods showed that the labeled proteins diffused rapidly in the disk membranes. In these FPR experiments we observed both the recovery of fluorescence in the bleached spot and the loss of fluorescence from nearby, unbleached regions of the photoreceptor. These and previous experiments show that the redistribution of the fluorescent labeled proteins after bleaching was due to diffusion. The diffusion constant, D, was (3.0 +/- 10(-9) cm2 s-1 if estimated from the rate of recovery of fluorescence in the bleached spot, and (5.3 +/- 2.4) x 10(-9) cm2 s-1 if estimated from the rate of depletion of fluorescence from nearby regions. The temperature coefficient, Q10, for diffusion was 1.7 +/- 0.5 over the range 10 degrees--29 degrees C. These values obtained by FPR are in good agreement with those previously obtained by photobleaching rhodopsin in fresh, unlabeled rods. This agreement indicates that the labeling and bleaching procedures required by the FPR method did not significantly alter the diffusion rate of rhodopsin. Moreover, the magnitude of the diffusion constant for rhodopsin is that to be expected for an object of its diameter diffusing in a bilayer with the viscosity of the disk membrane. In contrast to the case of rhodopsin, FPR methods applied to other membrane proteins have yielded much smaller diffusion constants. The present results help indicate that these smaller diffusion constants are not artifacts of the method but may instead be due to interactions the diffusing proteins have with other components of the membrane in addition to the viscous drag imposed by the lipid bilayer.
Full text
PDF




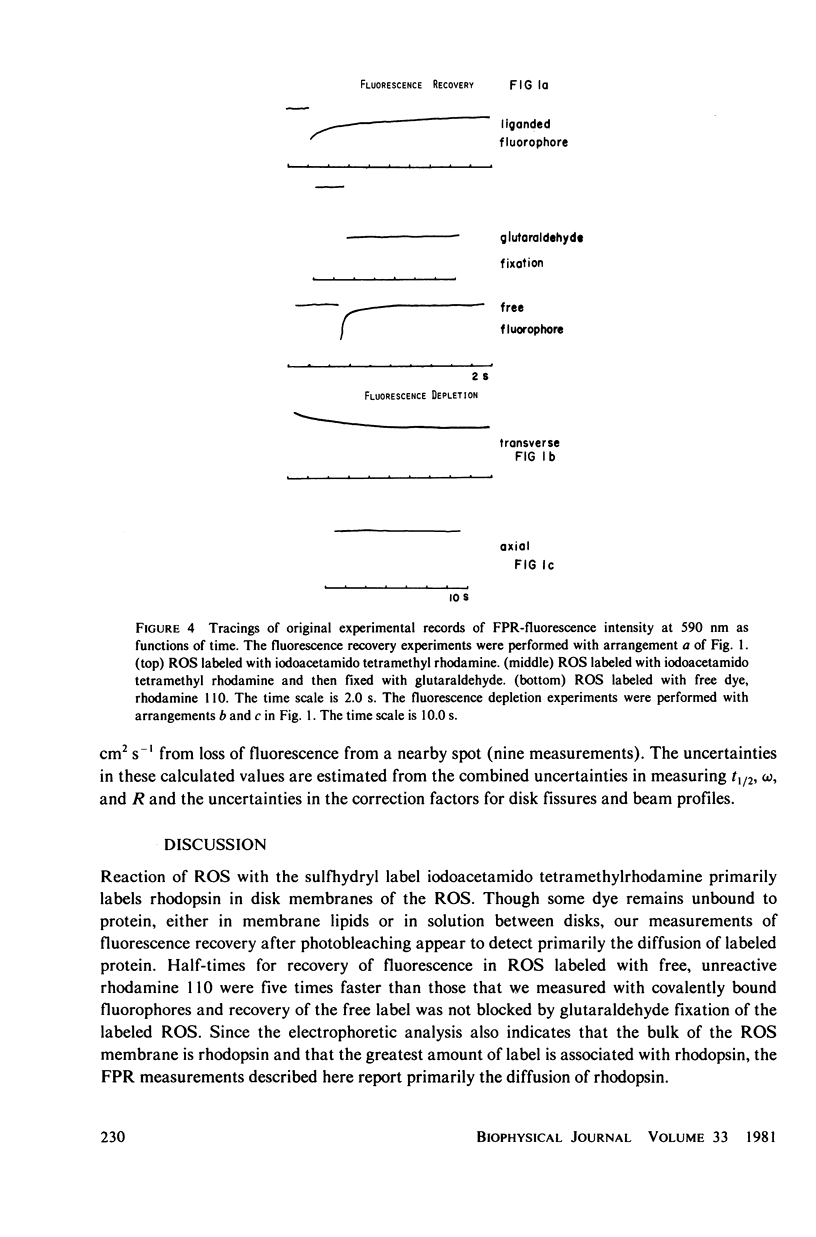


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Edidin M., Wei T. Y. Diffusion rates of cell surface antigens of mouse-human heterokaryons. I. Analysis of the population. J Cell Biol. 1977 Nov;75(2 Pt 1):475–482. doi: 10.1083/jcb.75.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hanski E., Rimon G., Levitzki A. Adenylate cyclase activation by the beta-adrenergic receptors as a diffusion-controlled process. Biochemistry. 1979 Mar 6;18(5):846–853. doi: 10.1021/bi00572a017. [DOI] [PubMed] [Google Scholar]
- Kaplan M. W., Deffebach M. E. Birefringence measurements of structural inhomogeneities in Rana pipiens rod outer segments. Biophys J. 1978 Jul;23(1):59–70. doi: 10.1016/S0006-3495(78)85432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Koppel D. E., Axelrod D., Jacobson K., Webb W. W., Elson E. L. Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2409–2413. doi: 10.1073/pnas.73.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow V. P., Roth S., Edidin M. Effects of temperature on glycosyltransferase activity in the plasma membrane of L cells. Exp Cell Res. 1979 Jun;121(1):55–61. doi: 10.1016/0014-4827(79)90443-9. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Schindler M., Koppel D. E. Lateral mobility of integral membrane proteins is increased in spherocytic erythrocytes. Nature. 1980 Jun 12;285(5765):510–511. doi: 10.1038/285510a0. [DOI] [PubMed] [Google Scholar]
- Strittmatter P., Rogers M. J. Apparent dependence of interactions between cytochrome b5 and cytochrome b5 reductase upon translational diffusion in dimyristoyl lecithin liposomes. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2658–2661. doi: 10.1073/pnas.72.7.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D. E., Edidin M., Dragsten P. R. Effect of bleaching light on measurements of lateral diffusion in cell membranes by the fluorescence photobleaching recovery method. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2043–2045. doi: 10.1073/pnas.77.4.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. W., Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969 Aug;42(2):392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]