Abstract
To assess the relationship between antiretroviral drug exposure and lipodystrophy, 69 human immunodeficiency virus type 1-infected patients receiving nelfinavir were investigated cross-sectionally. Lipodystrophy was defined by patients' self-report. Nelfinavir trough concentrations in plasma were significantly related to overall lipodystrophy and peripheral fat wasting scores and appeared to be an independent risk factor for lipodystrophy
In most published reports, lipodystrophy appears to be associated with the use of protease inhibitors (2). Among the mechanisms suggested to have a role in the etiology of lipodystrophy, several could be drug concentration dependent, such as inhibition of adipocyte differentiation (6) or inhibition of steroid metabolism (3). Whatever the mechanism of lipodystrophy, it could be of great importance to identify a relationship between antiretroviral drug concentrations in plasma and lipodystrophy. If this hypothesis were confirmed, therapeutic drug monitoring would help in preventing the occurrence of lipodystrophy in human immunodeficiency virus (HIV) type 1-infected patients with high drug concentrations in plasma. This study was designed to assess the relationship between antiretroviral drug exposure and lipodystrophy in a cohort of patients treated with highly active antiretroviral therapy regimens containing nelfinavir as a protease inhibitor.
The study population consisted of 69 HIV type 1-infected patients receiving highly active antiretroviral therapy including at least two nucleoside analogue reverse transcriptase inhibitors associated with nelfinavir (1,250 mg twice a day). Patients were included in the study after at least 6 months of therapy with nelfinavir. These patients were seen for routine care and were not selected for any adverse events.
Lipodystrophy was defined by patients' self-report (2). Patients rated the severity of peripheral fat wasting and central fat accumulation in six regions (face, arms, legs, buttocks, abdomen, and neck) as none (score 0), mild (score 1), moderate (score 2), or severe (score 3). Scores for peripheral wasting (four sites: face, arms, buttocks, and legs; total score of 0 to 12), central accumulation (two sites: abdomen and neck; total score of 0 to 6), and overall lipodystrophy (six sites, total score of 0 to 18) were assigned (4).
The percentage of body fat was calculated from skin fold thicknesses (1, 5). Venous blood samples were drawn in the morning after 12-h overnight fasting from the day of the medical consultation. Trough concentrations of nelfinavir and its main metabolite M8 in plasma were measured in 100-μl aliquots of plasma in the same blood samples as the biological variables by high-performance liquid chromatography.
Recorded biological data were total and high-density lipoprotein cholesterol, triglyceride, glucose, insulin, C peptide, liver enzymes (alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyltransferase), uricemia, CD4+ lymphocyte counts, and HIV RNA levels (Quantiplex VIH-RNA Chiron [B-DNA] technique; limit of detection, 50 copies per ml).
The Mann-Whitney test was performed to compare the means of two groups, and the Kruskal-Wallis test was performed to compare the means of three or more groups. Proportions were compared by the Fisher exact test. The Spearman rank coefficient was used to study the relationship between quantitative variables. Subsequent backward stepwise logistic regression assessed the independent effects of explanatory variables on the presence of lipodystrophy. Patients receiving lipid-lowering drugs or hypoglycemic oral agents were excluded when cholesterol and triglyceride or insulin and C peptide were the variables studied.
Sixty-nine patients (59 males and 10 females) were included in the study (Table 1). The mean duration of protease inhibitor and nelfinavir therapies was 28.4 ± 11.7 and 17.4 ± 5.8 months, respectively. Five patients received a lipid-lowering drug, and two received a hypoglycemic oral agent. The most frequent combinations of antiretroviral drugs were stavudine-lamivudine (3TC)-nelfinavir (n = 28) and zidovudine-3TC-nelfinavir (n = 26). Forty-seven patients (69%) reported at least one sign of overall lipodystrophy. Twenty-nine (42%) and 33 (48%) reported at least one sign of central accumulation or peripheral wasting, respectively. Total fat mass calculated from skin fold thicknesses was significantly decreased for patients reporting overall lipodystrophy ([21.4 ± 3.4]% versus [25.8 ± 8.1]% [P = 0.009]). Mean trough concentrations of nelfinavir, M8, and the sum of nelfinavir and M8 in plasma were 2.60 ± 1.54, 0.57 ± 0.54, and 3.11 ± 1.85 mg/liter, respectively. Coefficients of variation were 60, 96, and 60%, respectively. Trough concentrations of nelfinavir were significantly higher in women than in men (3.5 ± 2.0 versus 2.4 ± 1.4 mg/liter, respectively; P = 0.04). As a high correlation was shown between nelfinavir and M8 (r = 0.46, P = 0.0001), M8 values were not further included in the analysis. Of the total of 69 patients included in this analysis, 47 (70.5%) had a viral load below 50 copies/ml. No relationship was shown between plasma nelfinavir concentrations and viral load by a Spearman rank correlation test (r = −0.12, P = 0.32). Nelfinavir trough concentrations in plasma were significantly related to overall lipodystrophy score (r = 0.38, P = 0.001) and peripheral fat wasting score (r = 0.38, P = 0.001) but not central fat accumulation score. The population of patients was divided into three groups according to nelfinavir trough concentrations in plasma (below the 25th percentile [i.e., 1.4 mg/liter], between the 25th and the 75th percentile, and above the 75th percentile [i.e., 3.3 mg/liter]). The scores of overall lipodystrophy and peripheral fat wasting but not central fat accumulation were significantly different among the three groups (Fig. 1). Nelfinavir trough concentration in plasma appeared to be an independent risk factor for overall lipodystrophy and peripheral fat wasting but not central fat accumulation in a multivariate analysis (Table 2). The other risk factors of lipodystrophy (score of lipodystrophy above the median) shown by a logistic regression analysis were duration of nucleoside reverse transcriptase inhibitor (NRTI) treatment and patient's gender (female gender was associated with increased risk). These risk factors were significantly associated with overall lipodystrophy and peripheral wasting but not central accumulation (Table 2). Although patients taking the stavudine-3TC-nelfinavir combination tended to have higher scores of lipodystrophy than patients taking the zidovudine-3TC-nelfinavir combination, differences did not reach statistical significance (score of lipodystrophy for overall lipodystrophy, 1.4 ± 2.2 in patients receiving zidovudine-3TC-nelfinavir compared to 3.3 ± 4.4 in patients receiving stavudine-3TC-nelfinavir [P = 0.06]; central fat accumulation, 0.5 ± 0.9 compared to 1.0 ± 1.3 [P = 0.16]; peripheral fat wasting, 0.8 ± 1.8 compared to 2.3 ± 3.4 [P = 0.06]). Duration of exposure to NRTIs was increased in the stavudine group (37 ± 24.0 months in the zidovudine group versus 47.4 ± 35.3 months in the stavudine group). These results are consistent with the results of the multivariate analysis, including duration of treatment with NRTI, which did not show stavudine as a risk factor for lipodystrophy. Serum alanine transaminase, gamma-glutamyltransferase, triglyceride, total cholesterol, and insulinemia were significantly higher in patients classified in the overall lipodystrophy group. For each of these variables, differences were also shown when only peripheral fat wasting and not central fat accumulation was considered (Table 3). Using a logistic regression analysis, we showed an association between the risk of hypercholesterolemia and duration of protease inhibitor treatment. No significant influence of the other factors studied was detected (exposure to stavudine or zidovudine, duration of treatment with NRTI, and trough levels of nelfinavir). On the basis of this result, hypercholesterolemia is not a relevant indication for dose adjustment of nelfinavir. High concentrations of nelfinavir in plasma were associated with an increased risk of lipodystrophy. Scores of lipodystrophy for overall lipodystrophy and peripheral wasting were significantly related to trough concentrations of nelfinavir. Results from this investigation suggest that a study should be conducted to determine whether decreasing the nelfinavir dose for patients with high trough concentrations in plasma can prevent or reverse lipodystrophy (while maintaining virologic suppression). Attention should be paid, however, to the risk of viral rebound after a dose decrease. In our cohort, viral load values were not related to trough concentrations of nelfinavir in plasma. However, Burger et al. showed a relationship between plasma nelfinavir concentrations and virologic efficacy (D. M. Burger, P. W. Hugen, J. Droste, A. D. Huitema, et al., presented at the 2nd International Workshop on Clinical Pharmacology of HIV Therapy, Noordwijk, The Netherlands, 2 to 4 April 2001). The lack of correlation of viral response to nelfinavir drug levels could be due to prior nelfinavir resistance. Only patients receiving nelfinavir were included in our study. As a consequence, the results that we obtained may not be applicable to other protease inhibitors. Similar studies should be performed with other protease inhibitors especially in combination with ritonavir used as a booster since concentrations obtained in plasma are dramatically increased. Despite these limitations, our study provides evidence that peripheral fat wasting is possibly accounted for by modifiable risk factors. Other potential variables such as NRTI exposure, diet, exercise, and baseline body mass index should be also considered. Our results warrant further evaluation of therapeutic drug monitoring to increase the tolerance of nelfinavir in HIV-infected patients and to decrease the risk of lipodystrophy without resulting in unacceptable virologic failure.
TABLE 1.
Demographic characteristics of the population (n = 69)
| Type of value | Age (yr) | Ht (cm) | Wt (kg) | Viral load (copies/ml) | CD4 (cells/mm3) |
|---|---|---|---|---|---|
| Mean | 46.2 | 172.4 | 68.8 | 2,642.2 | 474.7 |
| SD | 10.6 | 8.6 | 10.6 | 12,341.9 | 238.9 |
| Minimum | 26.4 | 150 | 41 | 25 | 7 |
| Maximum | 76.1 | 192 | 92 | 94,000 | 1,218 |
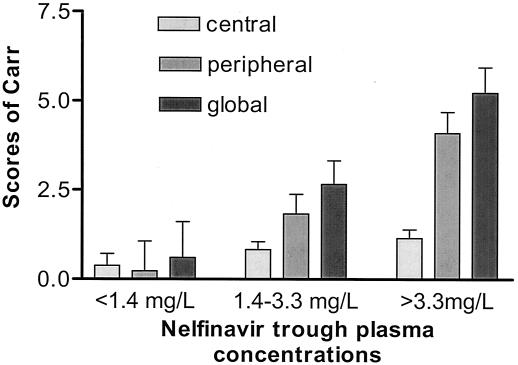
FIG. 1.
Scores of lipodystrophy for overall lipodystrophy, central fat accumulation, and peripheral wasting in patients with nelfinavir trough concentrations below the 25th percentile (1.4 mg/liter) (group 1), between the 25th percentile (1.4 mg/liter) and the 75th percentile (3.3 mg/liter) (group 2), and above the 75th percentile (3.3 mg/liter) (group 3). By the Kruskal-Wallis test, a significant difference was shown for overall lipodystrophy and peripheral fat wasting: overall lipodystrophy, F = 3.94, P = 0.02; central fat accumulation, F = 1.32, P = 0.27; peripheral wasting, F = 3.75, P = 0.03. A Kruskal-Wallis multiple-comparison test found significant differences between groups 1 and 3 (P < 0.05) but not between groups 2 and 3 or between groups 2 and 1 for overall lipodystrophy and peripheral wasting.
TABLE 2.
Risk factors for clinical lipodystrophy (logistic regression)a
| Variable | Central accumulation
|
Peripheral wasting
|
Overall lipodystrophy
|
|||
|---|---|---|---|---|---|---|
| Odds ratio | P | Odds ratio | P | Odds ratio | P | |
| Age | Removed | Removed | Removed | |||
| Sex | Removed | 10.56 | 0.03 | 10.17 | 0.02 | |
| Nelfinavir trough level | Removed | 1.90 | 0.01 | 1.94 | 0.005 | |
| Stavudine | 2.09 | 0.16 | Removed | Removed | ||
| 3TC | Removed | 0.27 | 0.14 | Removed | ||
| Treatment duration | ||||||
| NRTI | Removed | 1.03 | 0.008 | 1.02 | 0.07 | |
| Protease inhibitor | 1.03 | 0.16 | Removed | Removed | ||
A backward (step-down) variable selection was performed. In this procedure, all variables were included in the model. The variable that was the least significant was removed. This deletion of variables continued until all of the variables in the model were significant at a level below 0.20. Levels of significance below 0.05 are in boldface.
TABLE 3.
Metabolic data and body composition for patients according to lipodystrophy classificationa
| Component (unit) | Central accumulation
|
Peripheral wasting
|
Overall lipodystrophy
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | P | Yes | No | P | Yes | No | P | |
| Uricemia (mmol/liter) | 343 ± 90 | 399 ± 112 | 0.08 | 352 ± 91 | 377 ± 113 | 0.51 | 364 ± 106 | 367 ± 95 | 0.82 |
| ALAT (IU/liter) | 32.4 ± 25.1 | 24.3 ± 18.1 | 0.05 | 32.8 ± 25.4 | 23.2 ± 16.6 | 0.06 | 32.3 ± 24.7 | 21.5 ± 14.6 | 0.03 |
| ASAT (IU/liter) | 24.2 ± 18.3 | 19.8 ± 10.7 | 0.32 | 23.8 ± 18.2 | 19.7 ± 10.0 | 0.75 | 23.6 ± 17.6 | 19.0 ± 8.3 | 0.65 |
| γGT (IU/liter) | 67.9 ± 98.5 | 36.6 ± 46.3 | 0.06 | 51.7 ± 46.0 | 48.7 ± 92.8 | 0.01 | 62.4 ± 87.1 | 33.8 ± 50.3 | 0.004 |
| Triglyceride (mmol/liter) | 2.2 ± 1.1 | 1.9 ± 0.9 | 0.46 | 2.4 ± 10 | 1.8 ± 1.0 | <0.006 | 2.2 ± 1.0 | 1.8 ± 1.0 | 0.04 |
| Cholesterol (mmol/liter) | 6.2 ± 1.4 | 5.8 ± 1.1 | 0.15 | 6.4 ± 1.3 | 5.6 ± 1.1 | 0.009 | 6.3 ± 1.3 | 5.6 ± 1.0 | 0.02 |
| HDL cholesterol (mmol/liter) | 1.3 ± 0.3 | 1.3 ± 0.5 | 0.39 | 1.2 ± 0.4 | 1.4 ± 0.5 | 0.67 | 1.2 ± 0.3 | 1.4 ± 0.6 | 0.26 |
| Glycemia (mmol/liter) | 5.3 ± 1.8 | 5.2 ± 0.8 | 0.30 | 5.5 ± 1.7 | 5.1 ± 0.6 | 0.67 | 5.4 ± 1.6 | 5.1 ± 0.7 | 0.87 |
| Insulinemia (mU/liter) | 17.2 ± 10.2 | 14.3 ± 7.4 | 0.26 | 18.8 ± 9.6 | 12.6 ± 7.0 | 0.004 | 17.6 ± 9.2 | 12.9 ± 7.6 | 0.03 |
| C peptide (mmol/liter) | 3.0 ± 1.8 | 2.3 ± 0.9 | 0.18 | 3.0 ± 1.4 | 2.3 ± 1.4 | 0.02 | 2.8 ± 14 | 2.3 ± 1.3 | 0.08 |
Levels of significance of 0.05 or less are in boldface. Abbreviations: ALAT, alanine aminotransferase; IU, international units; ASAT, aspartate aminotransferase; γGT, gamma-glutamyltransferase; HDL, high-density lipoprotein.
Acknowledgments
Acknowledgments go to Philippe Gerhardt, Salim Hilab, Christina Pantoja, and Randa Jdid for their help.
REFERENCES
- 1.Aboul-Seoud, M. A., and A. Aboul-Seoud. 2001. Estimation of body fat from skinfold thickness. Comput. Methods Programs Biomed. 65:201-206. [DOI] [PubMed] [Google Scholar]
- 2.Carr, A., K. Samaras, A. Thorisdottir, G. R. Kaufmann, D. J. Chisholm, and D. A. Cooper. 1999. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet 353:2093-2099. [DOI] [PubMed] [Google Scholar]
- 3.Christeff, N., J. C. Melchior, P. deTruchis, C. Perronne, E. A. Nunez, and M. L. Gougeon. 1999. Lipodystrophy defined by a clinical score in HIV-infected men on highly active antiretroviral therapy: correlation between dyslipidaemia and steroid hormone alterations. AIDS 13:2251-2260. [DOI] [PubMed] [Google Scholar]
- 4.Durnin, J. V., and J. Womersley. 1974. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br. J. Nutr. 32:77-97. [DOI] [PubMed] [Google Scholar]
- 5.Durnin, J. V., and J. Womersley. 1973. The metabolic effects, and the composition of the tissue lost, in weight reduction by obese patients on treatment with fenfluramine. Br. J. Pharmacol. 49:115-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang, B., K. MacNaul, D. Szalkowski, Z. H. Li, J. Berger, and D. E. Moller. 1999. Inhibition of adipocyte differentiation by HIV protease inhibitors. J. Clin. Endocrinol. Metab. 84:4274-4277. [DOI] [PubMed] [Google Scholar]