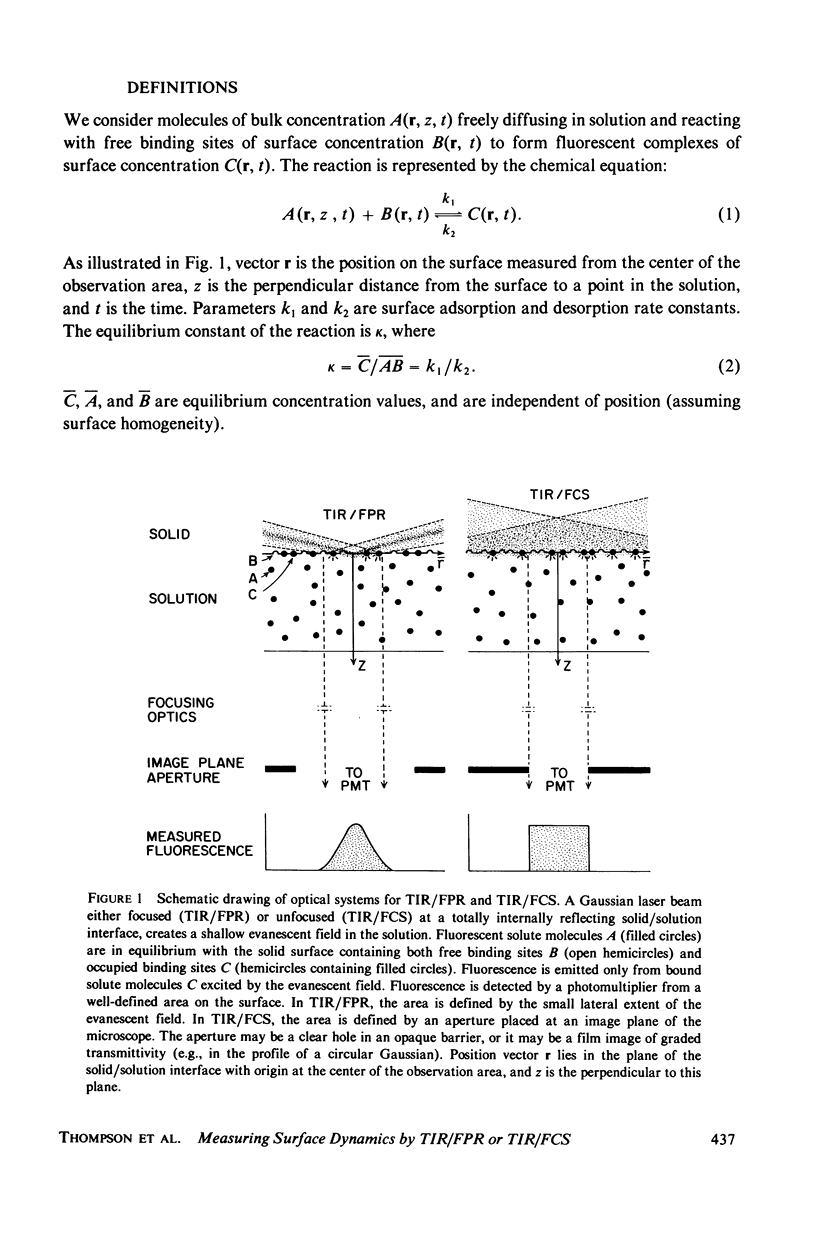
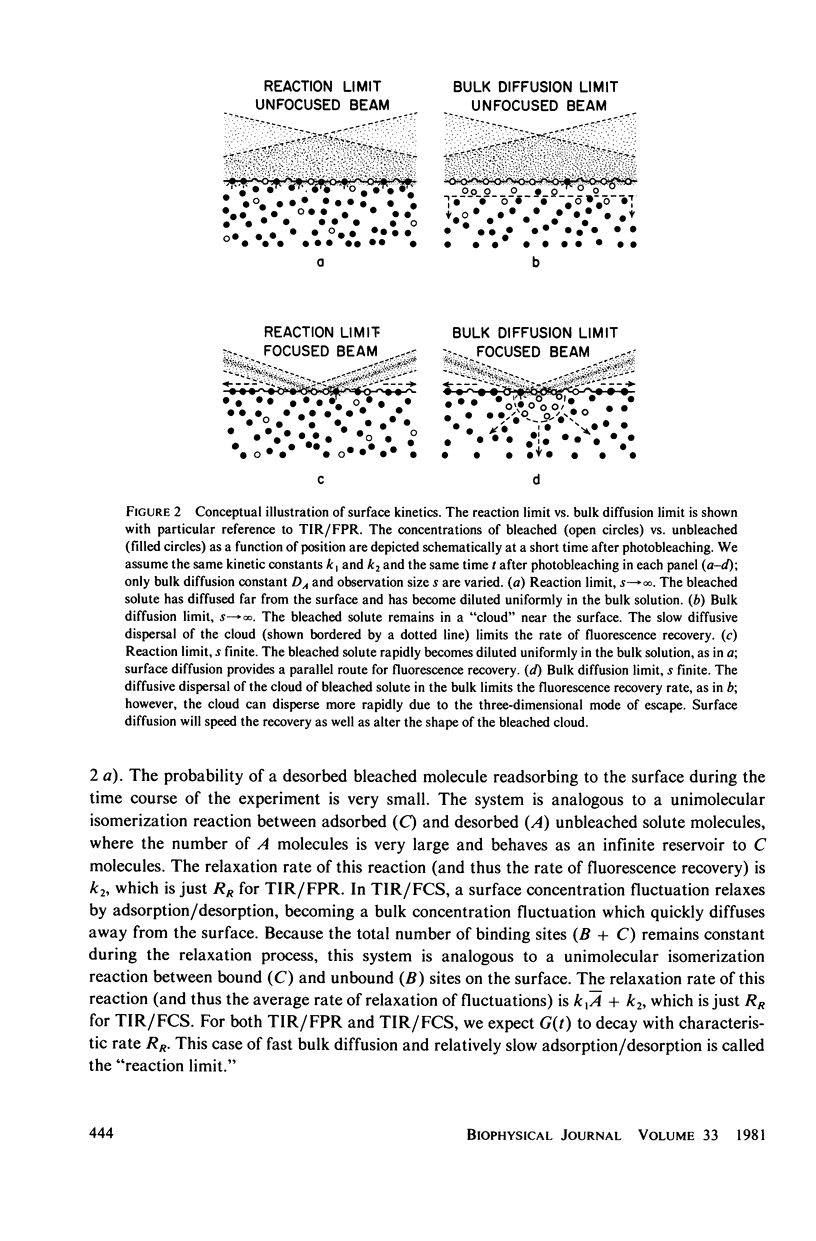
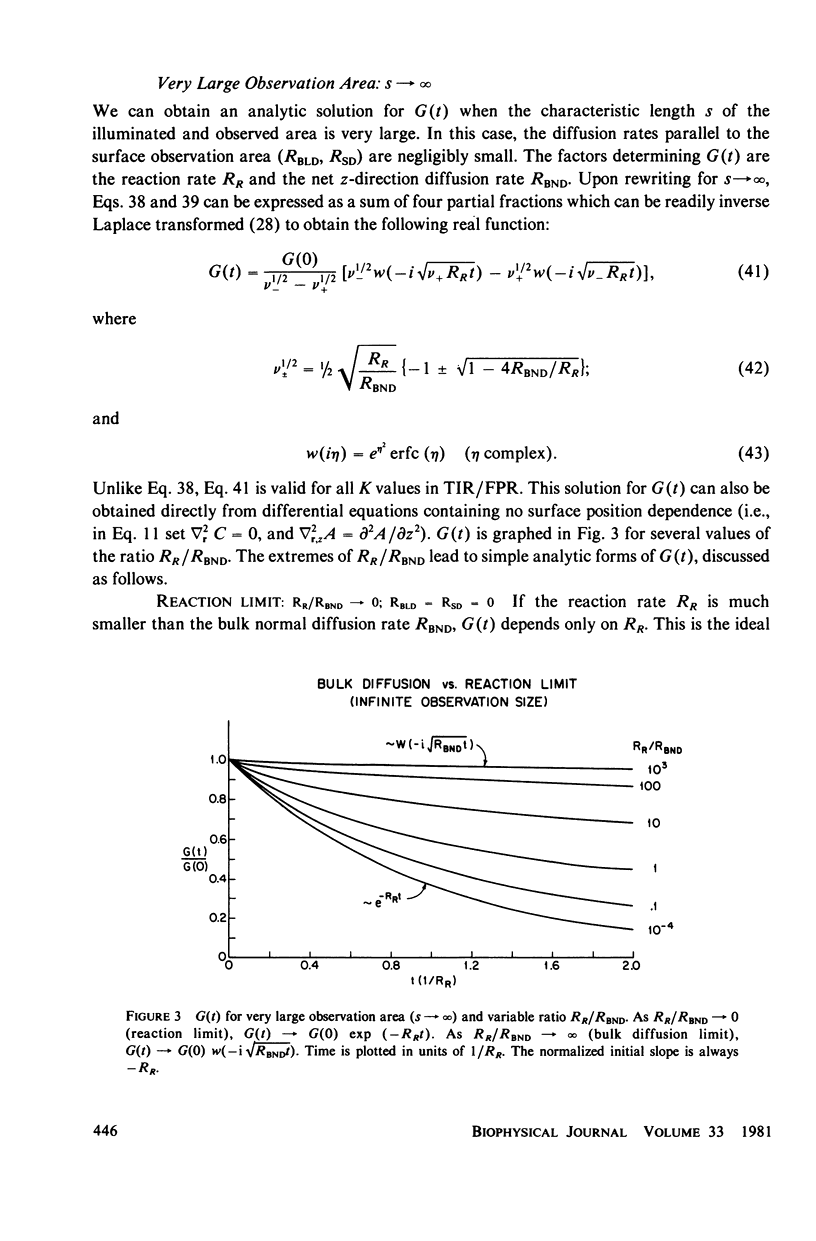
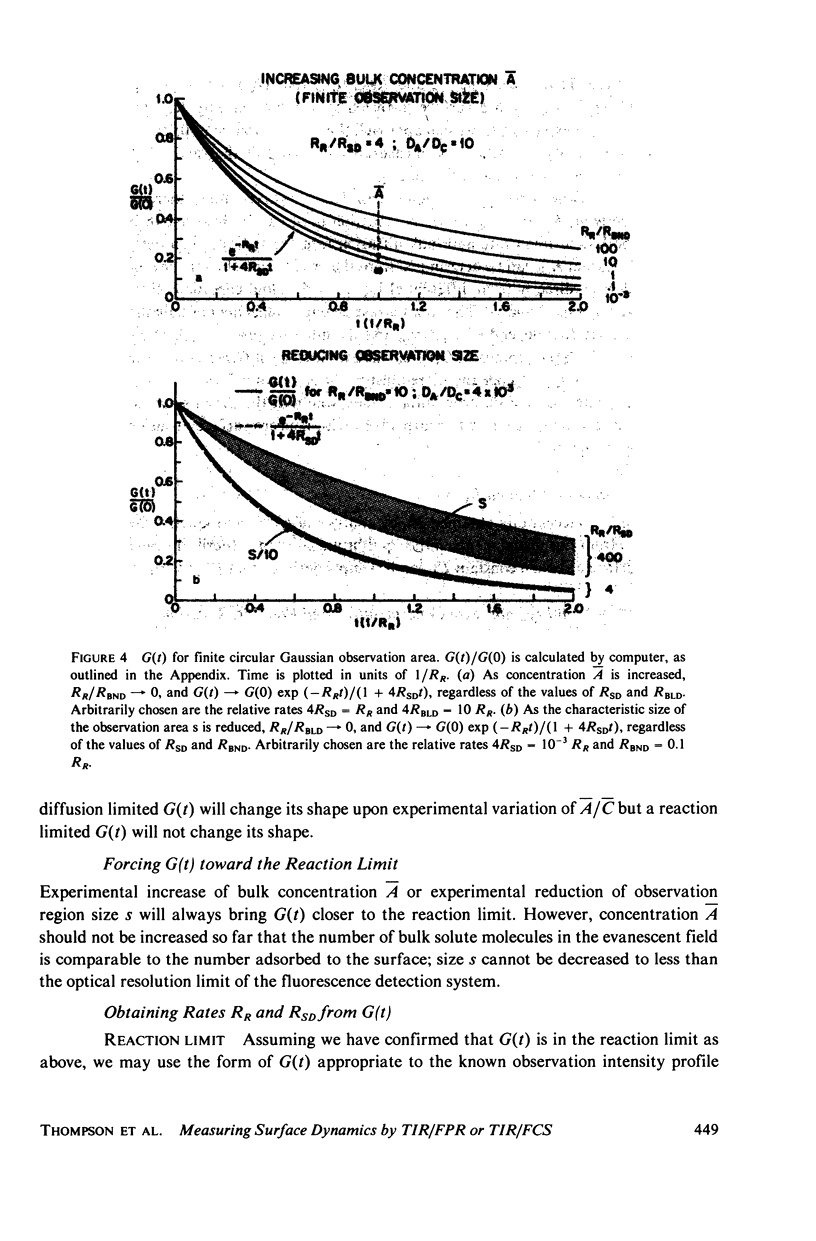
Abstract
The theoretical basis of a new technique for measuring equilibrium adsorption/desorption kinetics and surface diffusion of fluorescent-labeled solute molecules at solid surfaces has been developed. The technique combines total internal reflection fluorescence (TIR) with either fluorescence photobleaching recovery (FPR) or fluorescence correlation spectroscopy (FCS). A laser beam totally internally reflects at a solid/liquid interface; the shallow evanescent field in the liquid excites the fluorescence of surface adsorbed molecules. In TIR/FPR, adsorbed molecules are bleaching by a flash of the focused laser beam; subsequent fluorescence recovery is monitored as bleached molecules exchange with unbleached ones from the solution or surrounding nonilluminated regions of the surface. In TIR/FCS, spontaneous fluorescence fluctuations due to individual molecules entering and leaving a well-defined portion of the evanescent field are autocorrelated. Under appropriate experimental conditions, the rate constants and surface diffusion coefficient can be readily obtained from the TIR/FPR and TIR/FCS curves. In general, the shape of the theoretical TIR/FPR and TIR/FCS curves depends in a complex manner upon the bulk and surface diffusion coefficients, the size of the iluminated or observed region, and the adsorption/desorption/kinetic rate constants. The theory can be applied both to specific binding between immobilized receptors and soluble ligands, and to nonspecific adsorption processes. A discussion of experimental considerations and the application of this technique to the adsorption of serum proteins on quartz may be found in the accompanying paper (Burghardt and Axelrod. 1981. Biophys. J. 33:455).
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg O. G., Blomberg C. Association kinetics with coupled diffusional flows. Special application to the lac repressor--operator system. Biophys Chem. 1976 Jul;4(4):367–381. doi: 10.1016/0301-4622(76)80017-8. [DOI] [PubMed] [Google Scholar]
- Borejdo J., Putnam S., Morales M. F. Fluctuations in polarized fluorescence: evidence that muscle cross bridges rotate repetitively during contraction. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6346–6350. doi: 10.1073/pnas.76.12.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt T. P., Axelrod D. Total internal reflection/fluorescence photobleaching recovery study of serum albumin adsorption dynamics. Biophys J. 1981 Mar;33(3):455–467. doi: 10.1016/S0006-3495(81)84906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Feher G., Weissman M. Fluctuation spectroscopy: determination of chemical reaction kinetics from the frequency spectrum of fluctuations. Proc Natl Acad Sci U S A. 1973 Mar;70(3):870–875. doi: 10.1073/pnas.70.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever I. A simple visual surface immunology test. J Immunol Methods. 1978;24(1-2):57–61. doi: 10.1016/0022-1759(78)90086-8. [DOI] [PubMed] [Google Scholar]
- Magde D., Elson E. L., Webb W. W. Fluorescence correlation spectroscopy. II. An experimental realization. Biopolymers. 1974 Jan;13(1):29–61. doi: 10.1002/bip.1974.360130103. [DOI] [PubMed] [Google Scholar]
- Roberts H., Hess B. Kinetics of cytochrome c oxidase from yeast. Membrane-facilitated electrostatic binding of cytochrone c showing a specific interaction with cytochrome c oxidase and inhibition by ATP. Biochim Biophys Acta. 1977 Oct 12;462(1):215–234. doi: 10.1016/0005-2728(77)90204-3. [DOI] [PubMed] [Google Scholar]
- Schranner R., Richter P. H. Rate enhancement by guided diffusion. Chain length dependence of repressor-operator association rates. Biophys Chem. 1978 May;8(2):135–150. doi: 10.1016/0301-4622(78)80005-2. [DOI] [PubMed] [Google Scholar]
- Watkins R. W., Robertson C. R. A total internal-reflection technique for the examination of protein adsorption. J Biomed Mater Res. 1977 Nov;11(6):915–938. doi: 10.1002/jbm.820110611. [DOI] [PubMed] [Google Scholar]
- Weaver D. L. Diffusion-controlled mean reaction times in biological systems with elliptical symmetry. Biophys Chem. 1979 Nov;10(3-4):245–251. doi: 10.1016/0301-4622(79)85013-9. [DOI] [PubMed] [Google Scholar]
- Wong M., Bayer M. E., Litwin S. Virus--cell interaction: prediction of the time course of observable effects from virus interaction at cell injection sites, and mechanisms leading to attachment. FEBS Lett. 1978 Nov 1;95(1):26–30. doi: 10.1016/0014-5793(78)80044-1. [DOI] [PubMed] [Google Scholar]


