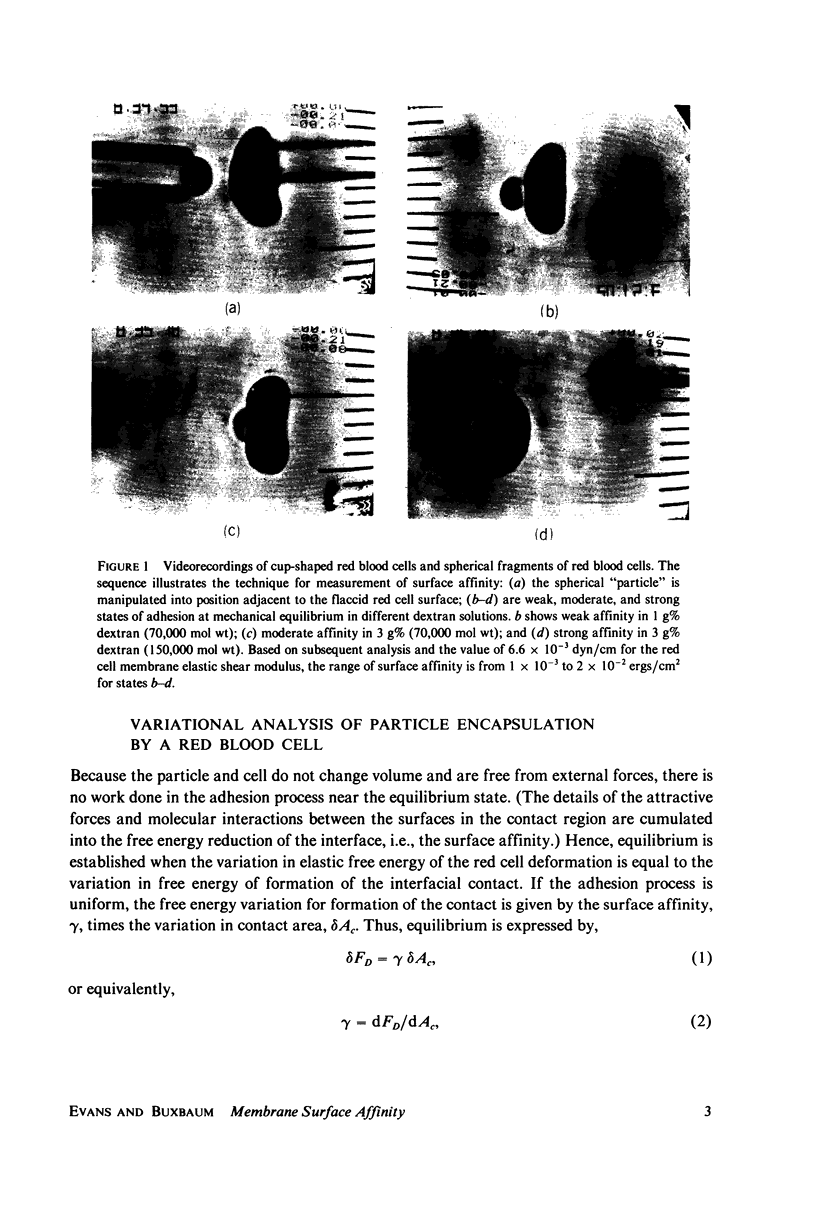
Abstract
An experimental technique and a simple analysis are presented that can be used to quantitate the affinity of red blood cell membrane for surfaces of small beads or microsomal particles up to 3 micrometers Diam. The technique is demonstrated with an example of dextran-mediated adhesion of small spherical red cell fragments to normal red blood cells. Cells and particles are positioned for contact by manipulation with glass micropipets. The mechanical equilibrium of the adhesive contact is represented by the variational expression that the decrease in interfacial free energy due to a virtual increase in contact area is balanced by the increase in elastic energy of the membrane due to virtual deformation. The surface affinity is the reduction in free energy per unit area of the interface associated with the formation of adhesive contact. From numerical computations of equilibrium configurations, the surface affinity is derived as a function of the fractional extent of particle encapsulation. The range of surface affinities for which the results are applicable is increased over previous techniques to several times the value of the elastic shear modulus. It is shown that bending rigidity of the membrane has little effect on the analytical results for particles 1--3 micrometers Diam and that results are essentially the same for both cup- and disk-shaped red cells. A simple analytical model is shown to give a good approximation for surface affinity (normalized by the elastic shear modulus) as a function of the fractional extent of particle encapsulation. The model predicts that a particle would be almost completely vacuolized for surface affinities greater than or equal to 10 times the elastic shear modulus. Based on an elastic shear modulus of 6.6 x 10(-3) dyn/cm, the range for the red cell-particle surface affinity as measured by this technique is from approximately 7 x 10(-4) to 7 x 10(-2) erg/cm2. Also, an approximate relation is derived for the level of surface affinity necessary to produce particle vacuolization by a phospholipid bilayer surface which possesses bending rigidity and a fixed tension.
Full text
PDF



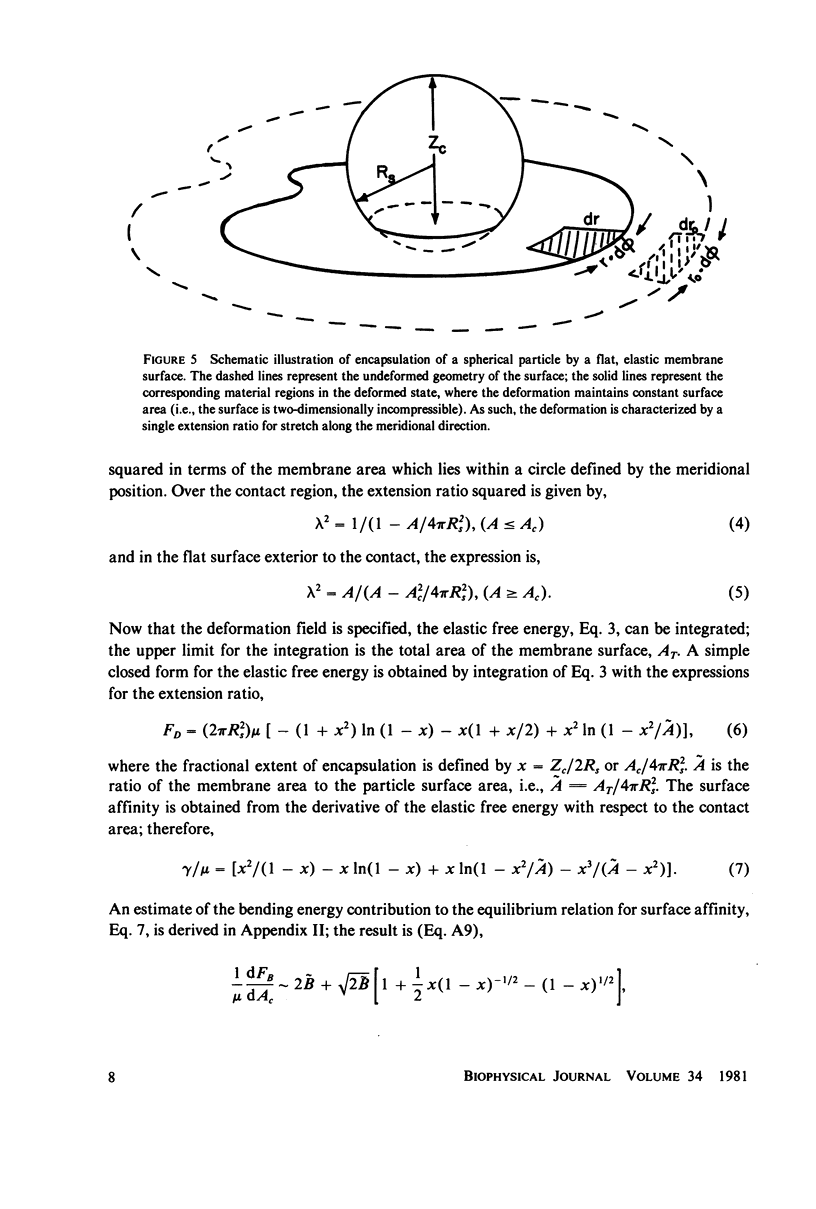

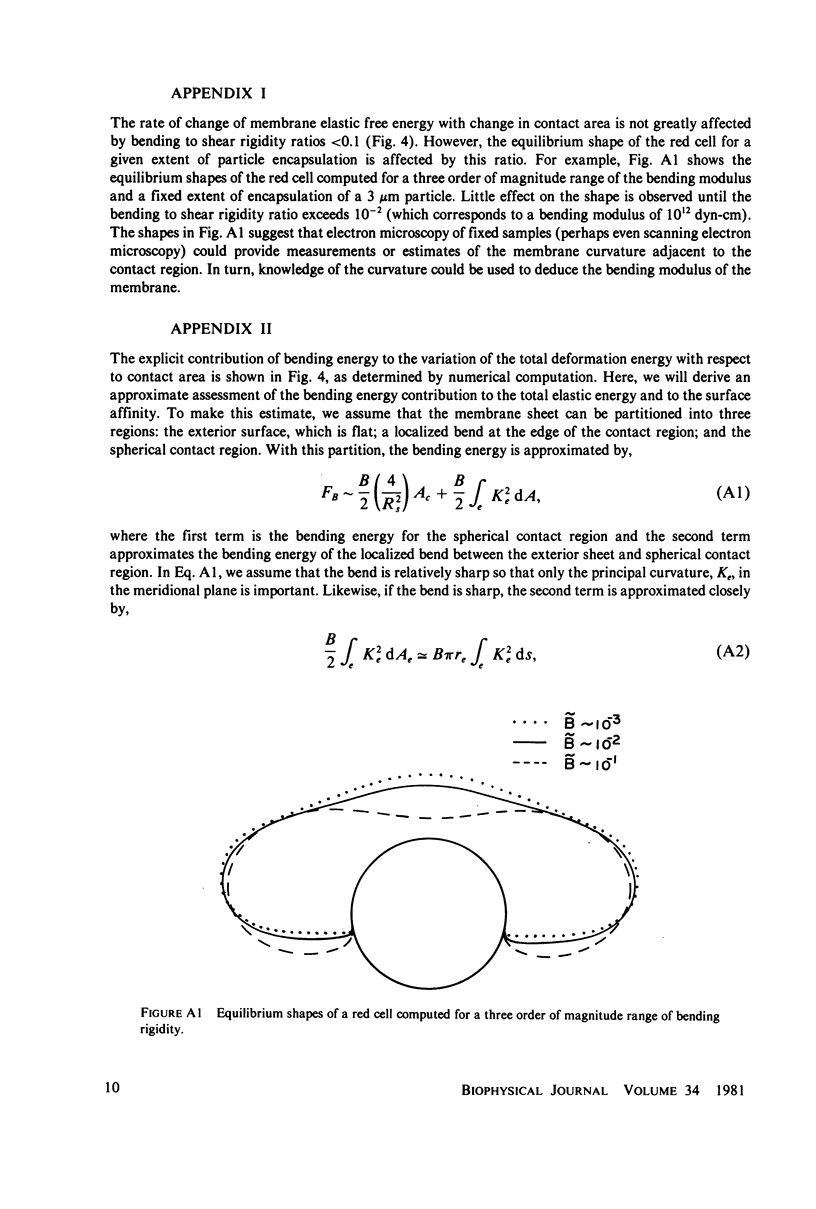


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans E. A. Bending resistance and chemically induced moments in membrane bilayers. Biophys J. 1974 Dec;14(12):923–931. doi: 10.1016/S0006-3495(74)85959-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A. Minimum energy analysis of membrane deformation applied to pipet aspiration and surface adhesion of red blood cells. Biophys J. 1980 May;30(2):265–284. doi: 10.1016/S0006-3495(80)85093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Fung Y. C. Improved measurements of the erythrocyte geometry. Microvasc Res. 1972 Oct;4(4):335–347. doi: 10.1016/0026-2862(72)90069-6. [DOI] [PubMed] [Google Scholar]
- Waugh R., Evans E. A. Thermoelasticity of red blood cell membrane. Biophys J. 1979 Apr;26(1):115–131. doi: 10.1016/S0006-3495(79)85239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]