Abstract
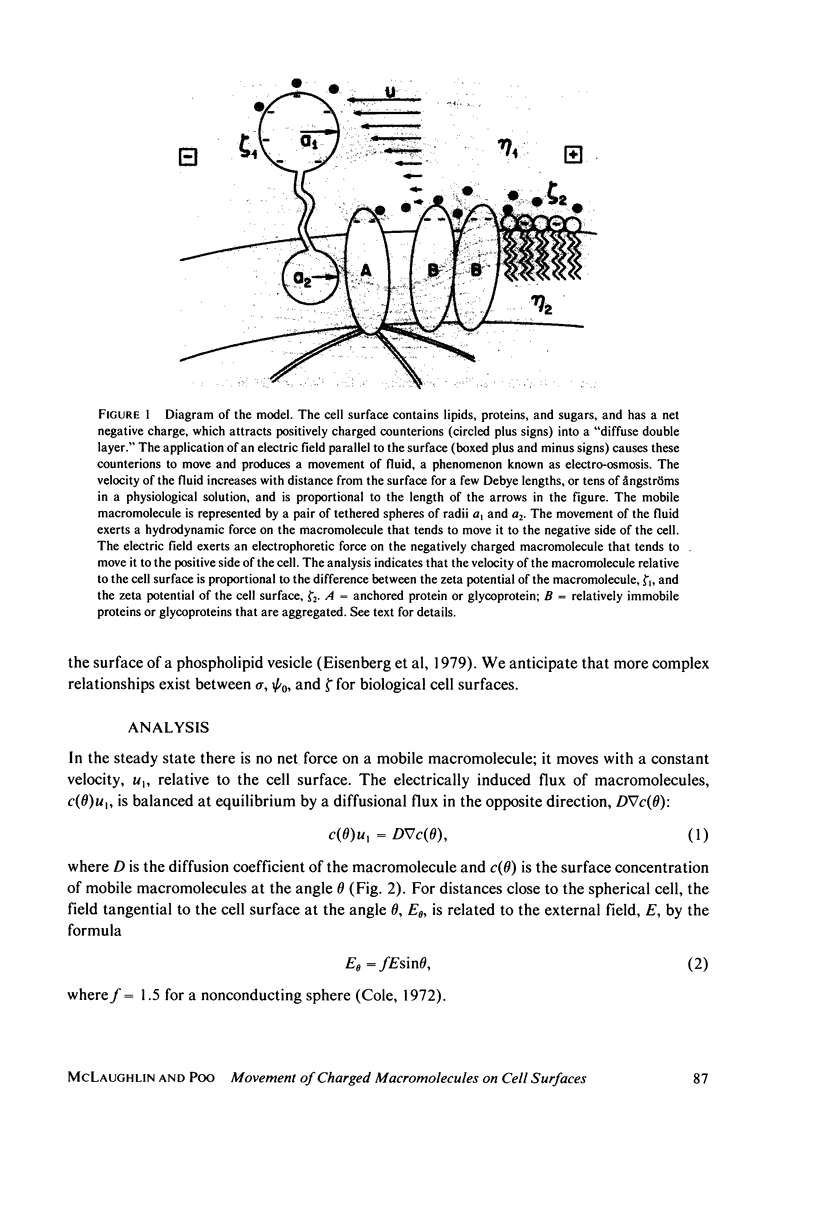
The surfaces of most cells bear a net negative charge. The imposition of an electric field parallel to the surface of the cell should produce, therefore, an electro-osmotic flow of fluid towards the cathodal side of the cell. Our analysis of a simple model of the cell surface indicates that a negatively charged mobile macromolecule will be swept by this electro-osmotic flow of fluid to the cathodal side of the cell if its zeta potential, zeta 1, is less negative than the zeta potential of the cell surface, zeta 2. Conversely, if zeta 2 is less negative than zeta 1, the negatively charged macromolecule will accumulate at the anodal side of the cell. Our experimental results demonstrate that concanavalin A (Con A) receptors on embryonic muscle cells normally accumulate at the cathodal side of the cell, but that they can be induced to accumulate at the anodal side of the cell by preincubating the myotubes either with neuraminidase, a treatment that removes negatively charged sialic acid residues, or with the lipid diI, a treatment that adds positive charges to the surface of the cell. Addition of the negatively charged lipid monosialoganglioside (GM1), on the other hand, enhances the accumulation of Con A receptors at the cathodal side of the cell.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EYLAR E. H., MADOFF M. A., BRODY O. V., ONCLEY J. L. The contribution of sialic acid to the surface charge of the erythrocyte. J Biol Chem. 1962 Jun;237:1992–2000. [PubMed] [Google Scholar]
- Eisenberg M., Gresalfi T., Riccio T., McLaughlin S. Adsorption of monovalent cations to bilayer membranes containing negative phospholipids. Biochemistry. 1979 Nov 13;18(23):5213–5223. doi: 10.1021/bi00590a028. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F. Control of development by ionic currents. Soc Gen Physiol Ser. 1979;33:199–231. [PubMed] [Google Scholar]
- Jaffe L. F. Electrophoresis along cell membranes. Nature. 1977 Feb 17;265(5595):600–602. doi: 10.1038/265600a0. [DOI] [PubMed] [Google Scholar]
- Poo M. M., Poo W. J., Lam J. W. Lateral electrophoresis and diffusion of Concanavalin A receptors in the membrane of embryonic muscle cell. J Cell Biol. 1978 Feb;76(2):483–501. doi: 10.1083/jcb.76.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M., Lam J. W., Orida N., Chao A. W. Electrophoresis and diffusion in the plane of the cell membrane. Biophys J. 1979 Apr;26(1):1–21. doi: 10.1016/S0006-3495(79)85231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M., Robinson K. R. Electrophoresis of concanavalin A receptors along embryonic muscle cell membrane. Nature. 1977 Feb 17;265(5595):602–605. doi: 10.1038/265602a0. [DOI] [PubMed] [Google Scholar]
- Sims P. J., Waggoner A. S., Wang C. H., Hoffman J. F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974 Jul 30;13(16):3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Woodruff R. I., Telfer W. H. Electrophoresis of proteins in intercellular bridges. Nature. 1980 Jul 3;286(5768):84–86. doi: 10.1038/286084a0. [DOI] [PubMed] [Google Scholar]
- Zagyansky Y. A., Jard S. Does lectin-receptor complex formation produce zones of restricted mobility within the membrane? Nature. 1979 Aug 16;280(5723):591–593. doi: 10.1038/280591a0. [DOI] [PubMed] [Google Scholar]